Introduction
Chronic hepatitis C with cirrhosis is a major risk
factor for hepatocellular carcinoma (HCC). It has been reported
that the annual incidence of HCC is ~7% in patients with hepatitis
C virus (HCV)-related liver cirrhosis (LC) (1). In patients with HCV-related LC,
achievement of a sustained virological response (SVR) following
interferon (IFN) therapy was found to be associated with a
reduction in liver-related mortality and with a lower risk of
liver-related complications and the occurrence of HCC (2–4). IFN
therapy has also been reported to reduce the severity of liver
tissue fibrosis and inflammation (5). The patients achieving SVR had the
greatest improvements in fibrosis and inflammation. The patients
who achieved HCV RNA suppression under the level of detection
during treatment, but who relapsed subsequent to treatment
discontinuation, also experienced improvements in fibrosis and
inflammation.
Pancytopenia is a common manifestation in cirrhotic
patients due to the increasing platelet pool in the enlarged
spleen, reduction of thrombopoietin production in the failing liver
and platelet destruction due to an immunological mechanism. The
presence of severe thrombocytopenia in patients with cirrhosis
associated with HCV infection limits the use of IFN therapy
(6–8). Therefore, management of
thrombocytopenia prior to antiviral treatment should be
considered.
Laparoscopic splenectomy (9,10),
partial splenic embolization (PSE) (11,12)
and administration of a thrombopoietin receptor agonist (13) are considered useful treatment
options for thrombocytopenia with chronic liver disease. The
results of antiviral therapy with thrombopoietin receptor agonists
remain under investigation. Splenectomy and PSE could be useful
treatments for thrombocytopenia, however, further investigation is
required to identify which treatment is better in terms of safety
and a successful outcome following antiviral treatment. Splenectomy
has a greater effect in raising the platelet count and has recently
been employed as a salvage therapy following PSE (14), making it highly reliable for
raising platelet counts. However, splenectomy in general is
considered to be associated with a potential risk of infection. A
meta-analysis of follow-up studies involving 19,680 patients who
underwent splenectomy demonstrated that the incidence of sepsis
among post-splenectomy adult patients was low and that a high
mortality rate was observed only among children (15). It appears that splenectomy is
reasonably safe in adult patients. We previously reported that
splenectomy improves liver function in patients with LC (16). Thus, splenectomy appears to be the
definitive treatment for hypersplenism and IFN therapy following
splenectomy is efficacious and safe (17–20).
However, there are only a few studies that have
examined the long-term effects of splenectomy and subsequent IFN
therapy in patients with HCV cirrhosis and thrombocytopenia. In the
present study, the effect of IFN therapy following splenectomy was
investigated in patients with HCV-related LC during long-term
follow-up.
Materials and methods
Patients
Between January 2005 and April 2010, 19 HCV-related
cirrhotic patients with hypersplenism underwent splenectomy and
were followed up for over 2 years at the Mie University School of
Medicine (Tsu, Mie, Japan). Patients were diagnosed as having LC
using a combination of laboratory tests, including ultrasonography
(US) and computed tomography (CT), and this was confirmed during
surgery. The eligibility criteria for the present study were
adequate liver function (Child-Pugh class A or B) and
thrombocytopenia (platelet count ≤8×104/mm3).
The reasons for splenectomy included difficulties in starting or
continuing IFN therapy due to thrombocytopenia. The clinical
characteristics of the patients prior to splenectomy are shown in
Table I. There were 13 patients
who did not have HCC, however, six patients had a history of HCC
treatment and had received therapy for HCC prior to starting
IFN.
 | Table IClinical features of hepatitis C
patients prior to splenectomy. |
Table I
Clinical features of hepatitis C
patients prior to splenectomy.
| Factor (n=19) | Values |
|---|
| Age (years) | 56±7 |
| Male/female | 11/8 |
| Child-Pugh score
(5/6/7/8) | 6/10/2/1 |
| Serum albumin
(g/dl) | 3.5±0.5 |
| Total bilirubin
(mg/dl) | 1.0±0.4 |
| Prothrombin time
(%) | 74.1±12.1 |
| WBC count
(/mm3) | 2945±841 |
| Platelet count
(/104mm3) | 5.2±1.5 |
| Treatment history for
hepatocellular carcinoma | 6/13 |
| Treatment history
with interferon | 6/13 |
| HCV genotype
(1/2) | 15/4 |
| HCV load (log
IU/ml) | 5.9±0.8 |
Splenectomy
Splenectomy was principally performed
laparoscopically, however, whether open surgery or laparoscopy was
selected depended on the size of the spleen and the presence of
complications. The patient was placed in the right semi-decubitus
position under general anesthesia and pneumoperitoneum was induced.
Dissection of the splenocolic ligament and parietal peritoneum
using the vessel sealing system (Ligasure Atlas™; Valleylab,
Boulder, CO, USA) was started from the lower pole and then
proceeded to the upper pole through the lateral side of the spleen.
Following sufficient mobilization of the spleen, the splenic hilum
was stapled with linear staplers and the fractured spleen was
delivered from the extended wound.
Therapy for HCV
When platelet counts were increased and the general
condition of the patient had stabilized following splenectomy, IFN
therapy was initiated. The combination of peginterferon α-2b
(PEG-IFNα2b; PegIntron; Schering-Plough Pharmaceutical, Osaka,
Japan) and ribavirin (RBV; Rebetol; Schering-Plough Pharmaceutical)
was used. The dosage was determined using a weight-based regimen.
The treatment durations were 48 and 24 weeks for patients with HCV
genotypes 1 and 2, respectively. Dose reduction and discontinuation
measures were used in the event of cytopenia, other complications
and treatment ineffectiveness.
The effect of IFN therapy was classified according
to the elimination of HCV-RNA and the alanine aminotransferase
(ALT) value 6 months following the end of treatment. SVR was
defined as the persistent disappearance of HCV RNA following
therapy, the biochemical response (BR) was defined as normal ALT
values without elimination of HCV RNA for at least 6 months
following therapy and no response (NR) was defined as persistently
elevated or transiently normalized ALT levels without the loss of
HCV RNA.
For patients who did not attain SVR, the combination
therapy of PEG-IFNα2a + RBV or IFNβ + RBV was used as a follow-up
treatment aimed at achieving SVR. If this was not possible due to
problems in the past treatment history or blood cell counts, IFN
monotherapy was performed wherever possible (specifically, 90 μg of
PEG-IFNα2a or 180 μg of PEG-IFNα2a was used every 1 or 2 weeks or
3,000,000 units of natural IFN was used three times a week).
Follow-up of patients
Patients were followed up on a monthly basis
following the diagnosis of cirrhosis by monitoring hematological,
biochemical and virological data. Imaging studies were conducted
three or more times per year in the majority of patients using CT
or US. Angiography was performed only when there was a high
suspicion of HCC on CT or US.
The follow-up period was defined as the duration
between the date of splenectomy and either the date of mortality or
the latest date of confirmed survival.
Statistical analysis
Data values are expressed as the means ± standard
deviation. Pre-splenectomy and 5 years post-splenectomy data were
compared using a paired t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Splenectomy
Splenectomy was safely performed in all the patients
without major complications with the exception of portal
thrombosis, which occurred in several patients, but did not affect
liver function when treated appropriately. Significant increases in
platelet counts and leukocyte counts were observed following
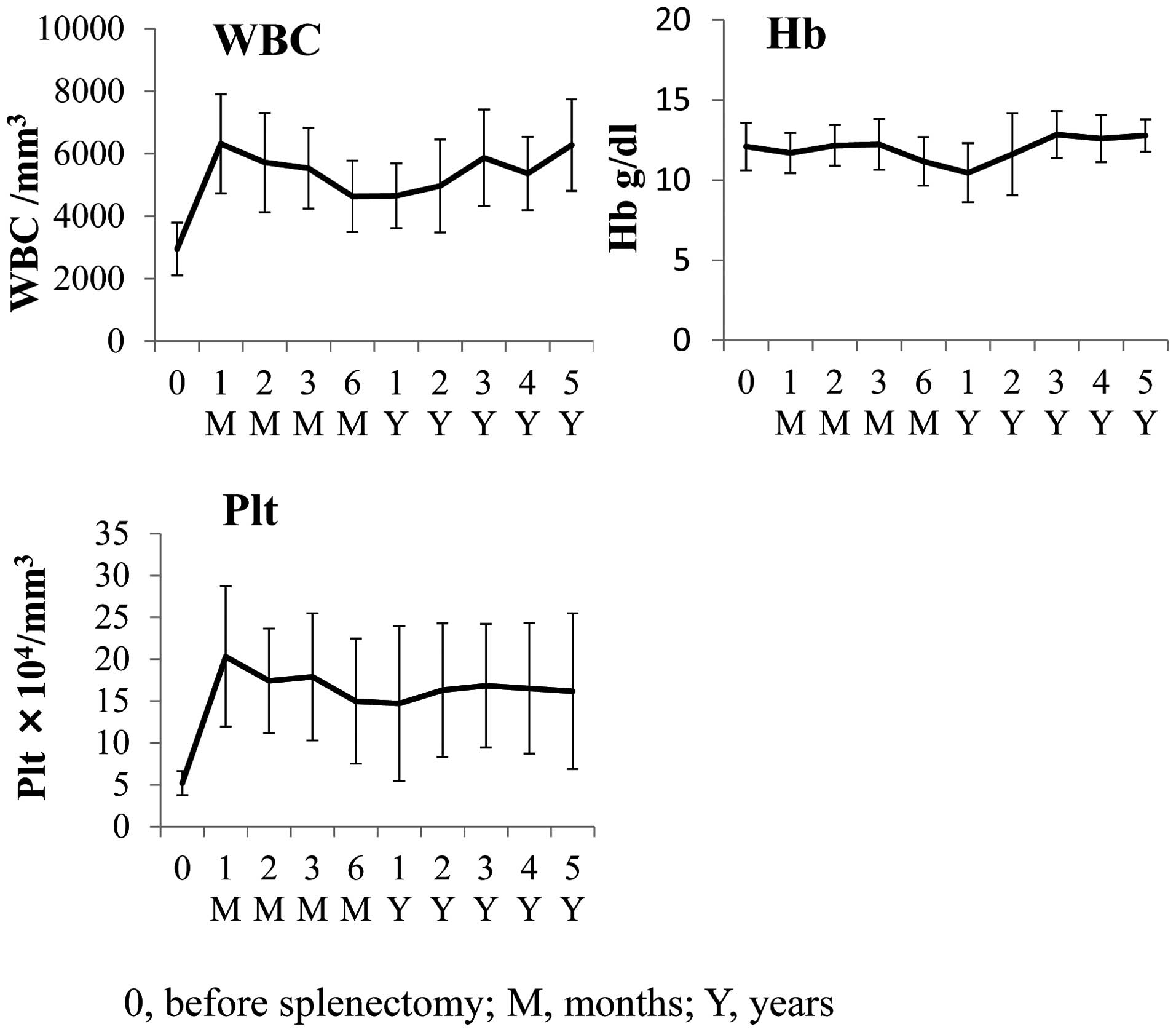
splenectomy. The mean platelet count at 4 weeks following
splenectomy was 20.3×104/mm3 (P<0.0001).
The mean leukocyte count at 4 weeks following splenectomy was
6.3×103/mm3 (P<0.0001). The mean
hemoglobin (Hb) prior to and following splenectomy remained almost
identical at 12.1 g/dl prior to and 11.7 g/dl following (not
significant; Fig. 1).
Response to IFN therapy
IFN therapy was initiated in all patients. Table II shows the effect of IFN therapy
following splenectomy. SVR was not observed in patients with
genotype 1, but was observed in 75% of patients with genotype 2.
Out of 15 patients with genotype 1, five discontinued IFN therapy
as they exhibited certain side effects, including interstitial
pneumonitis, HCV-associated glomerulonephritis or leukopenia. All
the patients with genotype 2 were able to continue IFN therapy.
 | Table IIIFN therapy following
splenectomy. |
Table II
IFN therapy following
splenectomy.
| Factor | Genotype 1
(n=15) | Genotype 2
(n=4) |
|---|
| Continued | 10 | 4 |
| Discontinued | 5 | 0 |
| SVR | 0 | 3 |
| Relapse | 1 | 1 |
| BR | 2 | 0 |
| NR | 9 | 0 |
In the group not achieving SVR, a switch to
PEG-IFNα2a + RBV or to long-term IFN monotherapy was considered to
achieve SVR. In the group with no previous HCC, 4 patients received
90 μg of PEGIFNα2a every other week, 1 patient received 180 μg of
PEG-IFNα2a weekly, 2 patients received IFNβ + RBV cotherapy, 2
patients received PEG-IFNα2a + RBV cotherapy and 2 patients
received 3,000,000 units of natural IFNα three times a week. In the
group with previous HCC, only 1 patient received PegIFNα2a + RBV
cotherapy. IFN could not be used in the other patients due to HCC
recurrence.
Changes in hematological and biochemical
parameters following splenectomy
The mean leukocyte and platelet counts increased in
patients following splenectomy (Fig.
1). Over 5 years, the mean platelet number increased from
5.2×104 to 16.8×104/mm3
(P<0.01) and the mean leukocyte number increased from
2.9×103 to 6.3×103/mm3
(P<0.001). The mean hemoglobin was virtually unchanged, from
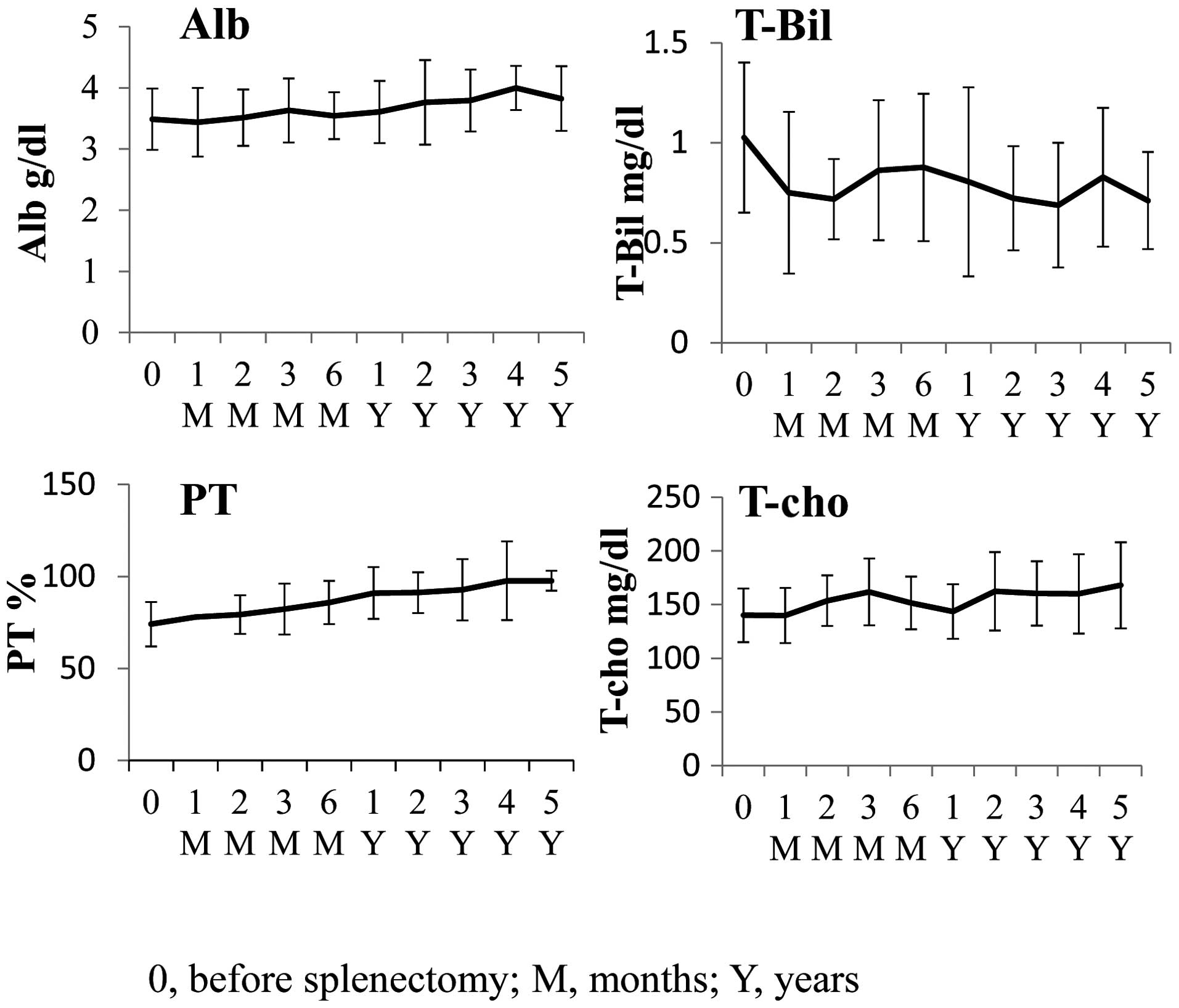
12.1 to 12.8 g/dl (not significant). The levels of albumin (Alb),
total cholesterol (T-cho), total bilirubin (T-Bil) and prothrombin
time (PT) improved following splenectomy. The mean Alb demonstrated
an upward trend from 3.5 to 3.8 g/dl, the mean T-Bil demonstrated a
downward trend from 1.0 to 0.7 mg/dl, the mean PT demonstrated an
upward trend from 74.1 to 97.7% and the mean T-cho increased from
140 to 168 mg/dl (P=0.0421), 5 years following splenectomy
(Fig. 2). These results indicated
that, at the very least, there was no deterioration in liver
function.
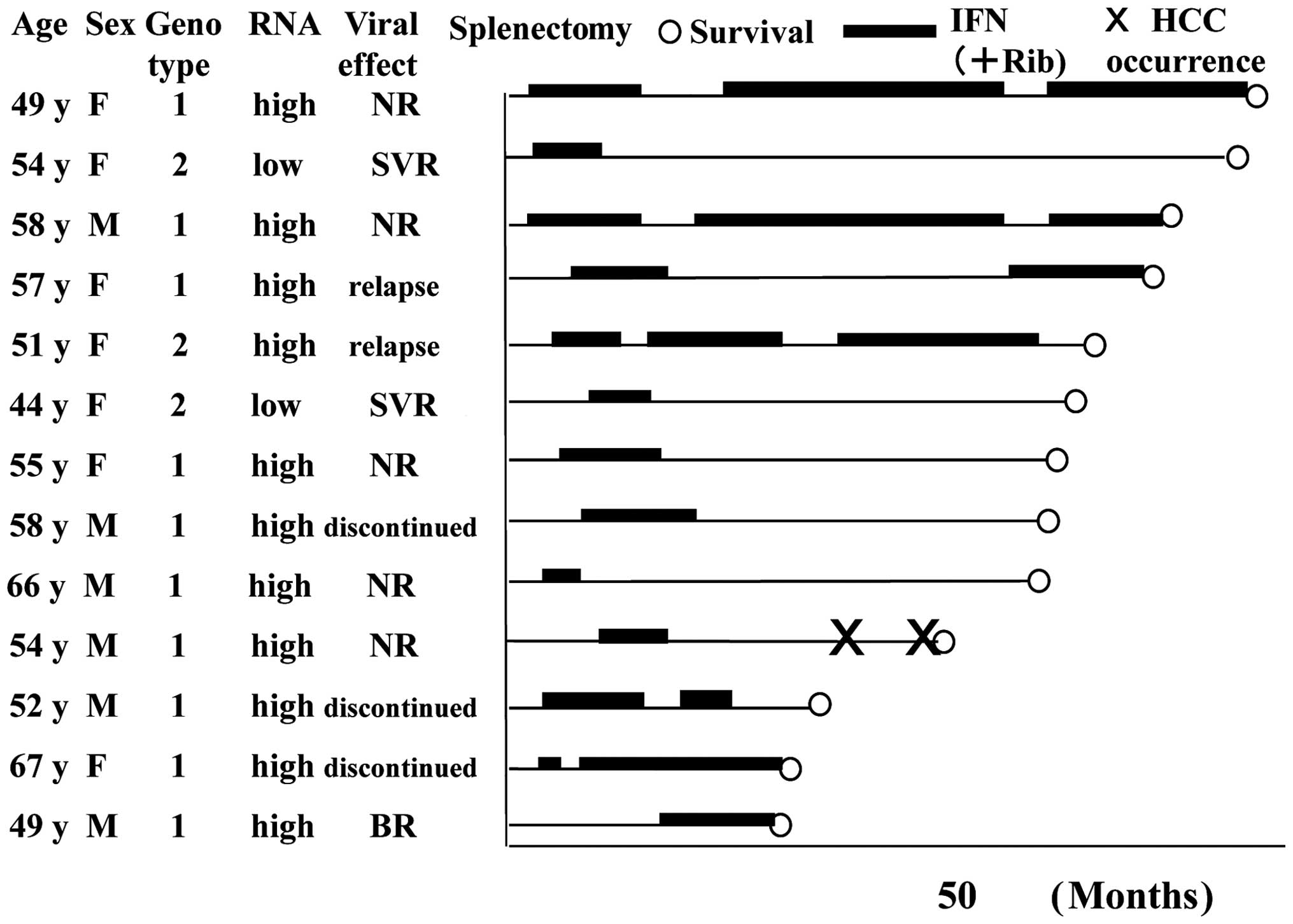
Hepatocarcinogenesis and mortality
following splenectomy and IFN combination therapy
Fig. 3 shows the
clinical course of the 13 patients without previous HCC. The mean
follow-up period in the group without HCC was 4.8 years. Wherever
possible, IFN therapy was also administered to patients who did not
achieve SVR. New HCC occurred in only one patient during the
observation period and all patients survived during this
period.
The mean follow-up period was 4.8 years in the group
with a history of HCC treatment. HCC recurrence was observed in
four patients, however, it was treatable in all the patients.
Curative treatment by radiofrequency ablation (RFA) with
transcatheter arterial chemoembolization (TACE) in two cases was
possible in all cases of HCC recurrence if it was the first
recurrence. Three patients had a second recurrence, two of whom
were treated by TACE + RFA and one was relieved by partial
resection following TACE treatment. One patient had a third
recurrence and developed distant metastasis, for which treatment
with sorafenib was initiated and TACE + RFA was performed on the
liver. There were no cases of liver failure and all patients were
Child-Pugh class A for liver fuction at the final follow-up. All
patients survived the follow-up period.
Discussion
In the present study, HCV-related cirrhotic patients
with hypersplenism underwent splenectomy to facilitate the
initiation and completion of IFN therapy. Since LC can cause
life-threatening conditions, including liver failure and HCC within
a few years, it is evident that treatment of such a severe disease
is an important medical objective. Although elusive, SVR in
patients with chronic hepatitis C and advanced liver disease
remains a significant objective. Previous studies (2,5) have
demonstrated that in patients receiving treatment, particularly
those whose virus is permanently eradicated, the progression of
fibrosis may be arrested and the cirrhosis reversed. Despite the
less favorable histological benefit in non-responder patients,
certain patients also demonstrated a reduction in the histological
activity index and fibrosis (5).
In addition, evidence suggests that antiviral therapy decreases the
rates of liver-related complications and mortality.
The present cases had severe leukopenia and
thrombocytopenia. Significant increases in leukocyte and platelet
counts were observed following splenectomy. These results are
consistent with those obtained in other studies (9,10,17–20).
Steady improvement of leukocyte and platelet counts is beneficial
for subsequent antiviral treatment. PSE can be an effective
treatment for thrombocytopenia with hypersplenism. Splenectomy and
PSE could be useful treatments for thrombocytopenia, however,
further investigation is required to identify which treatment is
better in terms of safety and a successful outcome following
antiviral treatment. In cases of severe thrombocytopenia, we
hypothesize that splenectomy is more beneficial from the
perspective of increasing platelet counts for antiviral therapy. In
cirrhotic patients with severe thrombocytopenia, splenectomy may be
a better supportive intervention than PSE (20). Splenectomy can be effective as
salvage treatment even if relapsed thrombocytopenia occurs
following PSE (14). Platelets
have been found to increase from 5.4×104/mm3
prior to splenectomy to 18.4×104/mm3
thereafter (21), whereas
following PSE, an increase from a mean value of 4.5×104
to 11.6×104/mm3 has been reported, although
this was with infarcted splenic volume (12). Splenectomy is superior in terms of
the level of the increase in platelet count and the low level of
relapsed thrombocytopenia following platelet increase. For this
reason, splenectomy is expected to be more reliable in raising
platelet counts in cases of severe thrombocytopenia and it is
highly likely that this increase may be sufficient for IFN
therapy.
The results of IFN therapy in the present study are
discussed below. As shown in Table
I, IFN could not be initiated prior to splenectomy. IFN therapy
was initiated in all the patients following splenectomy. SVR was
not observed in patients with genotype 1, however, it was observed
in three patients (75%) with genotype 2. Due to severe side
effects, including interstitial pneumonitis, HCV-associated
glomerulonephritis or leukopenia, five patients with genotype 1
discontinued IFN therapy. All the patients with genotype 2 were
able to continue IFN therapy. These data suggest that in patients
with genotype 2 in particular, splenectomy did improve the
effectiveness of IFN therapy. However, in subjects infected with
genotype 1, the result was far less satisfactory. The presence of
advanced fibrosis and cirrhosis has always presented an obstacle to
obtaining viral eradication during antiviral therapy (22,23).
The results of therapy differ according to the different clinical
stages of cirrhosis (24). As the
severity of liver disease deteriorates, the rate of SVR decreases
and viral eradication is often hampered by the occurrence of
adverse events. The development or exacerbation of cytopenia in
patients with cirrhosis may increase the risks of infection,
bleeding, anemia-related fatigue and poor stamina or exercise
intolerance, eventually causing the patient to withdraw from the
treatment program. Furthermore, when HCV-related LC patients with
portal hypertension were treated with the PEG-IFN + RBV cotherapy,
14 out of 51 patients had to cease treatment within 24 weeks due to
adverse events (25). In the
present study, numerous patients had severe fibrosis or portal
hypertension, as is reflected in the platelet counts, and the
figure for SVR was low, particularly in genotype 1 patients. The
relatively poor SVR rate in patients with genotype 1 mirrors other
studies (26). The rate of SVR
with PEG-IFN + RBV ranged from 10 to 44% for HCV genotypes 1/4 and
33 to 72% for HCV genotypes 2/3 in compensated cirrhosis (26). This treatment is thus highly useful
in genotype 2 patients.
As well as increased leukocyte and platelet counts,
the present subjects also demonstrated long-term improvement in
liver function. Improved liver function following splenectomy has
been previously reported (16). In
the present study, liver function was likely to improve 5 years
following splenectomy. The reasons for the improvement in liver
function following splenectomy included: i) the resolution of
portal hypertension; ii) the removal of hepatic regeneration
inhibition factors in the spleen; iii) the alleviation of the
prehepatic bilirubin burden due to a decrease in sites of
erythrocyte destruction and iv) the enhancement of hepatic
regeneration promotion factors. This discussion focuses on the
removal of hepatic regeneration inhibition factors and the
enhancement of hepatic regeneration promotion factors. Several
studies have focused on the removal of hepatic regeneration
inhibition factors. For example, in LC rats, TGF-β derived from the
spleen and hepatic stellate cells inhibited liver regeneration and
splenectomy improved liver regeneration and prevented the
progression of liver fibrosis (27,28).
In LC with portal hypertension, hepatic stellate cells are
activated (29) and TGF-β is
overproduced as a result of splenic cell activation (27). It is likely that splenectomy
improves liver regeneration due to loss of the TGF-β signal from
the spleen and the downregulation of TGF-β from hepatic stellate
cells due to the alleviation of portal hypertension.
Focus has been given to liver regeneration factors,
including evidence that splenectomy leads to enhanced liver
regeneration via the liver regeneration initiator TNF-α (30). Studies have demonstrated that
platelets have marked effects in promoting liver regeneration
(31). A review (32) presented experimental evidence
demonstrating that platelets accelerate liver regeneration,
involving three different mechanisms: i) the direct effect on
hepatocytes, where platelets translocate to the space of Disse and
release growth factors through direct contact with hepatocytes; ii)
the co-operative effect with liver sinusoidal endothelial cells,
where the dense concentration of sphingosine-1-phosphate in
platelets induces excretion of interleukin-6 from liver sinusoidal
endothelial cells and iii) the collaborative effect with Kupffer
cells, where the functions of Kupffer cells are enhanced by
platelets.
Carcinogenesis was also rare in patients with
previous HCC who did not achieve SVR. This was possibly due to two
mechanisms, one of which is the cancer inhibition effect due to the
reduction of portal pressure. The other mechanism was due to the
use of IFN therapy. Although the extent of the decrease in portal
pressure following splenectomy was not measured in the present
study, previous studies have reported lower portal pressure
following splenectomy. Ripoll et al demonstrated that the
hepatic venous pressure gradient predicts the development of HCC
independently of the severity of cirrhosis (33). It is thus possible that decreased
portal pressure and decreased carcinogenesis are involved.
The use of IFN therapy, including long-term IFN
monotherapy, also appears to have benefits in patients not
achieving SVR. For example, the hepatitis C antiviral long-term
treatment against cirrhosis (HALT-C) trial was designed to confirm
the effect of PEG-IFN half-dose administration to patients who did
not achieve SVR in previous IFN therapy. Extended analysis of the
HALT-C cohort demonstrated that patients with cirrhosis who
received PEG-IFN treatment had a reduced incidence of HCC (34).
The effect on blood biochemistry persisted over the
long term. The long-term persistence of this effect was possibly
due to splenectomy as well as antiviral therapy. In SVR patients
and those with no virological response, very few patients with no
previous history of HCC developed HCC and all achieved long-term
survival. Although recurrence was not suppressed in patients with
previous HCC, liver function was maintained and repeat treatment
was possible. The present was a retrospective study, however,
despite its limitations, further development of these treatments is
anticipated. In HCV-related LC with severe thrombocytopenia,
splenectomy and subsequent IFN therapy is potentially useful in
improving the inhibition of fibrosis, suppressing HCC and improving
survival rates. However, future investigations using new antiviral
therapy, including protease inhibitors are crucial in order to
overcome the low rate of SVR in genotype 1 patients.
References
|
1
|
Yoshida H, Shiratori Y, Moriyama M, et al:
Interferon therapy reduces the risk for hepatocellular carcinoma:
national surveillance program of cirrhotic and noncirrhotic
patients with chronic hepatitis C in Japan. Ann Intern Med.
131:174–181. 1999. View Article : Google Scholar
|
|
2
|
Bruno S, Stroffolini T, Colombo M, et al:
Sustained virological response to interferon-α is associated with
improved outcome in HCV-related cirrhosis: a retrospective study.
Hepatology. 45:579–587. 2007.
|
|
3
|
Ikeda K, Saitoh S, Arase Y, et al: Effect
of interferon therapy on hepatocellular carcinogenesis in patients
with chronic hepatitis C: A long-term observation study of 1,643
patients using statistical bias correction with proportional hazard
analysis. Hepatology. 29:1124–1130. 1999. View Article : Google Scholar
|
|
4
|
Tanaka H, Tsukuma H, Kasahara A, et al:
Effect of interferon therapy on the incidence of hepatocellular
carcinoma and mortality of patients with chronic hepatitis C: a
retrospective cohort study of 738 patients. Int J Cancer.
87:741–749. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Poynard T, McHutchison J, Manns M, et al:
Impact of pegylated interferon alfa-2b and ribavirin on liver
fibrosis in patients with chronic hepatitis C. Gastroenterology.
122:1303–1313. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahmed F and Jacobson IM: Management of
hematologic side effects: impact on compliance and efficacy. Cur
Hepat Rep. 4:56–60. 2005. View Article : Google Scholar
|
|
7
|
Shiffman ML, Ghany MG, Morgan TR, et al:
Impact of reducing peginterferon alfa-2a and ribavirin dose during
retreatment in patients with chronic hepatitis C. Gastroenterology.
132:103–112. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McHutchson JG, Manns M, Patel K, et al:
Adherence to combination therapy enhances sustained response in
genotype-1-infected patients with chronic hepatitis C.
Gastroenterology. 123:1061–1069. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kercher KW, Carbonell AM, Heniford B, et
al: Laparoscopic splenectomy reverses thrombocytopenia in patients
with hepatitis C cirrhosis and portal hypertension. J Gastrointest
Surg. 8:120–126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai YQ, Zhou J, Chen XD, et al:
Laparoscopic splenectomy is an effective and safe intervention for
hypersplenism secondary to liver cirrhosis. Surg Endosc.
25:3791–3797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murata K, Shiraki K, Takase K, et al: Long
term follow-up for patients with liver cirrhosis after partial
splenic embolization. Hepatogastroenterology. 43:1212–1217.
1996.PubMed/NCBI
|
|
12
|
Hayashi H, Beppu T, Masuda T, et al:
Predictive factors for platelet increase after partial splenic
embolization in liver cirrhosis patients. J Gastroenterol Hepatol.
22:1638–1642. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McHutchison JG, Dusheiko G, Shiffman ML,
et al: Eltrombopag for thrombocytopenia in patients with cirrhosis
associated with hepatitis C. N Engl J Med. 357:2227–2236. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hashimoto N, Akahoshi T, Tomikawa M, et
al: Value of laparoscopic splenectomy as salvage treatment for
relapsed thrombocytopenia after partial splenic arterial
embolization. Dig Surg. 27:515–520. 2010. View Article : Google Scholar
|
|
15
|
Bisharat N, Omari H, Lavi I, et al: Risk
of infection and death among post-splenectomy patients. J Infect.
43:182–186. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murata K, Ito K, Yoneda K, et al:
Splenectomy improves liver function in patients with liver
cirrhosis. Hepatogastroenterology. 55:1407–1411. 2008.PubMed/NCBI
|
|
17
|
Morihara D, Kobayashi M, Ikeda K, et al:
Effectiveness of combination therapy of splenectomy and long-term
interferon in patients with hepatitis C virus-related cirrhosis and
thrombocytopenia. Hepatol Res. 39:439–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ikezawa K, Naito M, Yumida T, et al:
Splenectomy and antiviral treatment for thrombocytopenic with
chronic hepatitis C virus infection. J Viral Hepat. 17:488–492.
2010.PubMed/NCBI
|
|
19
|
Akahoshi T, Tomikawa M, Korenaga D, et al:
Laparoscopic splenectomy with peginterferon and ribavirin therapy
for patients with hepatitis C virus cirrhosis and hypersplenism.
Surg Endosc. 24:680–685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomikawa M, Akahoshi T, Sugimachi K, et
al: Laparoscopic splenectomy may be a superior intervention for
cirrhotic patients with hypersplenism. J Gastroenterol Hepatol.
25:397–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshida D, Nagao Y, Tomikawa M, et al:
Predictive factors for platelet count after laparoscopic
splenectomy in cirrhotic patients. Hepatol Int. Sep 30–2011.(Epub
ahead of print).
|
|
22
|
Pawlotsky JM: Therapy of hepatitis C: from
empiricism to eradication. Hepatology. 43(Suppl 1): S207–S220.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeuzem S: Heterogeneous virologic response
rates to interferon based therapy in patients with chronic
hepatitis C: who responds less well? Ann Intern Med. 140:370–381.
2004.PubMed/NCBI
|
|
24
|
Piccinino F and Coppola N: Antiviral
treatment of HCV-related cirrhosis. Dig Liver Dis. 39(Suppl 1):
S96–S101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Di Marco V, Almasio PL, Ferraro D, et al:
Peg-interferon alone or combined with ribavirin in HCV cirrhosis
with portal hypertension: a randomized controlled trial. J Hepatol.
47:484–491. 2007.
|
|
26
|
Vezali E, Aghemo A and Colombo M: A review
of the treatment of chronic hepatitis C virus infection in
cirrhosis. Clin Ther. 32:2117–2138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura T, Sakata R, Ueno T, et al:
Inhibition of transforming growth factor β prevents progression of
liver fibrosis and enhances hepatocyte regeneration in
dimethylnitrosamine-treated rats. Hepatology. 32:247–255. 2000.
|
|
28
|
Akahoshi T, Hashizume M, Tanoue K, et al:
Role of the spleen in liver fibrosis in rats may be mediated by
transforming growth factor β-1. J Gastroenterol Hepatol. 17:59–65.
2002.PubMed/NCBI
|
|
29
|
Okada Y, Tsuzuki Y, Hokari R, et al:
Pressure loading and ethanol exposure differently modulate rat
hepatic stellate cell activation. J Cell Physiol. 215:472–480.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murata K, Shiraki K, Sugimoto K, et al:
Splenectomy enhances liver regeneration through tumor necrosis
factor (TNF)-α following dimethylnitrosamine-induced cirrhotic rat
model. Hepatogastroenterology. 48:1022–1027. 2001.
|
|
31
|
Murata S, Ohkohchi N, Matsuo R, et al:
Platelets promote liver regeneration in early period after
hepatectomy in mice. World J Surg. 31:808–816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi K, Murata S, Ohkohchi N, et al:
Novel therapy for liver regeneration by increasing the number of
platelets. Surg Today. Nov 21–2012.(Epub ahead of print).
|
|
33
|
Ripoll C, Groszmann RJ, Garcia-Tsao G, et
al: Hepatic venous pressure gradient predicts development of
hepatocellular carcinoma independently of severity of cirrhosis. J
Hepatol. 50:923–928. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lok AS, Everhart JE, Wright EC, et al:
Maintenance peginterferon therapy and other factors associated with
hepatocellular carcinoma in patients with advanced hepatitis C.
Gastroenterology. 140:840–849. 2011. View Article : Google Scholar : PubMed/NCBI
|