Introduction
Despite the overall decreasing incidence and
mortality rates observed over the last 50 years, gastric cancer
ranks as the fourth most common malignancy and the second leading
cause of cancer-related mortality worldwide (1). Approximately one million novel cases
of gastric cancer and >700,000 deaths resulting from it were
reported in 2008, with an overall five-year survival rate of
<20% (1). Lymphatic metastasis
and distal dissemination are the main causes of mortality.
Metastasis and recurrence following surgery are the main factors
affecting the therapeutic efficacy of gastric cancer. Regional
lymph nodes are the most common site of metastasis and lymph node
metastasis is a major prognostic factor in gastric cancer.
Understanding the mechanisms of lymphatic metastasis represents a
crucial step and may result in a novel therapeutic target in the
treatment of human gastric cancer.
Numerous pathways have been proposed to be involved
in the process of gastric cancer lymphatic metastasis. A number of
markers, such as epidermal growth factor receptor (EGFR) (2) and cyclooxygenase 2 (COX-2) (3), have been detected as predicted
targets of gastric cancer lymphatic metastasis. Certain studies
have indicated that survivin and vascular endothelial growth
factor-C (VEGF-C) may be involved in lymphatic metastasis of
gastric cancer (4,5).
Survivin is a protein of 142 amino acids and is the
smallest mammalian member of the inhibitor of apoptosis protein
family (6). It regulates two
essential cellular processes by inhibiting apoptosis and promoting
cell proliferation. Survivin is expressed at high levels during
fetal development, while it is rarely expressed in normal healthy
adult tissues. Overexpression of survivin has been observed in a
number of malignant tumors, including carcinomas of the lung,
breast, stomach, colon and ovary, as well as melanomas and
lymphomas (7). Expression of
survivin is also associated with worse therapy results, higher risk
of recurrences and resistance to chemotherapy (8,9).
Several studies have indicated that the expression of survivin is
significantly associated with the occurrence of lymphatic
metastasis, but the mechanism by which survivin controls lymphatic
metastasis remains unknown (10,11).
VEGF-C is a member of the VEGF family. Studies have
demonstrated that VEGF-C is critical in facilitating tumor
metastasis (12,13). VEGF-C induces lymphangiogenesis and
lymph node metastasis of tumors by activating VEGFR-3 in lymphatic
endothelial cells. It has been demonstrated that VEGF-C is strongly
expressed and is an important predictor of lymphangiogenesis and
prognosis in numerous types of cancer, including gastric carcinoma
(14,15).
There are few studies concerned with the functions
of survivin and VEGF-C and their clinical characteristics in
gastric cancer. In the present study, the correlation between the
expression of survivin and VEGF-C, and its association with
lymphangiogenesis in gastric cancer tissues was investigated. The
pathway by which survivin may affect gastric cancer lymphatic
metastasis was also predicted.
Material and methods
Patients and specimens
The study enrolled 195 patients (139 males and 56
females; age range, 30–81 years; average age, 58 years) who
underwent surgery (total or partial gastrectomy) for histologically
proven gastric carcinoma between 2006 and 2008 at the Department of
Surgical Oncology, First Hospital of China Medical University
(Shenyang, China). Tumor-node-metastasis staging was conducted
according to the American Joint Committee on Cancer classification,
and histological grading was performed according to World Health
Organisation criteria (16). The
clinical information obtained from the records and the
histopathology reports included patient age, first diagnosis, tumor
size and grade, and lymph node involvement. Follow-up data were
available from all patients, whom were assessed every six months
for five years or until they succumbed to the disease during those
five years. The study was approved by the Ethics Committee of China
Medical University (Shenyang, China) and written informed consent
was obtained from the patients family.
Tissue microarray construction
The paraffin tissue microarray (TMA; Outdo Biotech,
Co., Ltd., Shanghai, China) was constructed according to the
guidelines previously used by the National Cancer Institute’s
Tissue Array Research Programme. Each individual case was
represented by one tumor core (1 mm) and one peri-carcinoma core (1
mm) that was obtained from the original paraffin block (from the
archive of the Institute of Pathology, First Hospital of China
Medical University) according to its hematoxylin and eosin
(H&E) slides. These core needle biopsies were placed in
recipient paraffin array blocks at defined coordinates. The cores
were incubated in the paraffin block for 30 min at 37ºC to improve
adhesion between the cores and the paraffin of the recipient block.
The paraffin tissue microarray blocks were then cut into 5-μm
sections by an ultra-thin semi-automatic microtome (RM2235; Leica
Biosystems, Wetzlar, Germany).
Immunohistochemical staining
Specimens were immunostained with the standard
labeled streptavidin-biotin (LsAB) protocol. The 5-μm sections from
each paraffin block were deparaffinized and rehydrated. TMA slides
were heated in sodium citrate buffer (0.01 mol/l, pH 6.0) for 15
min in a microwave oven. Following cooling for 20 min and washing
in phosphate-buffered saline (PBS), endogenous peroxidase was
detected by incubating samples with PBS containing 10% normal goat
serum (Envision™+ kit; Dako, Glostrup, Denmark) for 30 min. The
sections were then incubated with each primary antibody overnight
at 4ºC. The primary antibodies were anti-survivin (mouse
monoclonal; 1:10; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) and anti-VEGF-C (rabbit polyclonal; 1:200; Abcam, Cambridge,
MA, USA). A further wash in PBS was followed by treatment with a
peroxidase-labeled polymer conjugated to goat anti-mouse or
anti-rabbit immunoglobulins (Envison™ + kit; Dako) as the secondary
antibody for 10 min at room temperature. The staining was
visualized with diaminobenzidine, followed by counterstaining with
hematoxylin. As a negative control, PBS was substituted for the
primary antibody.
Evaluation of immunohistochemical
staining
The immunohistochemical score, based on the German
immunoreactive score, was used for survivin and VEGF-C
immunohistochemical evaluation. Two pathologists, who were blinded
to the outcome data, independently evaluated the sections three
times. The staining of survivin and VEGF-C was then scored from 0
to 3, considering only the cytoplasmic reaction: A score of 0
required <10% of the cells to be stained, 1 required 11–20% of
cells to be stained, 2 required 21–50% of cells to have been
stained and 3 required 51–100% of cells to be stained.
Cell culture
The gastric cancer cell line, SGC-7901, was
purchased from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China) and cultured in
RPMI-1640 medium (Hyclone, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS) in a 5% CO2 humidified
atmosphere at 37ºC. Log phase cells were collected following
trypsin digestion by centrifugation for 5 min at 33.3 × g,
re-suspended in PBS and counted using a hemocytometer (Hausser
Scientific, Horsham, PA, USA).
Construction and transfection of
plasmid-shRNA
Plasmids containing survivin-shRNA and VEGF-C-shRNA
were constructed by Shanghai GenePharma Co., Ltd. (Shanghai,
China). First, 10 μg plasmid-survivin-shRNA and 10 μg
plasmid-VEGF-C-shRNA, respectively and 25 μl Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA) were mixed
together with 1350 μl RPMI-1640 medium (without FBS), and then the
mixture was transfected into SGC-7901 gastric cancer cells. The
mixture was added into a 25-cm2 culture flask that was
previously plated with 1×106 SGC-7901 gastric cancer
cells. The culture medium was replaced with complete RPMI-1640
medium 6 h following inoculation and the cells were collected after
a further 28 h. Protein and RNA were extracted for western blot and
qPCR analyses, respectively.
Western blot analysis
Proteins were separated by SDS-PAGE, transferred
onto nitrocellulose membranes and detected using the relevant
primary and secondary antibodies. Primary antibodies included:
Anti-survivin (mouse monoclonal, Santa Cruz Biotechnology, Inc.),
anti-VEGF-C (rabbit polyclonal, Abgent, San Diego, CA, USA) and
anti-β-actin (mouse monoclonal, Santa Cruz Biotechnology,
Inc.).
RNA isolation and qPCR
Total RNA was extracted using the guanidinium
thiocyanate-phenol-chloroform method (17). RNA yield and purity were determined
photometrically (BioPhotometer, Eppendorf, Hamburg, Germany), and
reverse transcription was performed. Survivin and VEGF-C were
amplified using qPCR. Primer subsequences were as follows: Forward:
5′-ACAGTCCATGCCATCACTGCC-3′ and reverse:
5′-GCCTGCTTCACCACCTTCTTG-3′ for β-actin; forward:
5′-TCATAGAGCTGCAGGGTGGATTGT-3′ and reverse:
5′-AGTAGGGTCCACAGCAGTGTTTGA-3′ for survivin; and forward:
5′-AACCTCCATGTGTGTCCGTC-3′ and reverse:
5′-TGGCAAAACTGATTGTTACTGG-3′ for VEGF-C. A total of 10 ng of
reverse-transcribed total RNA was used as the template, and the PCR
reaction contained 20 pmol/ml of each forward and reverse primer
and SYBR Premix Ex Taq II (Takara Bio, Inc., Dalian, China) in a
final volume of 20 μl. An ABI PRISM 7700 Sequence Detection system
(Applied Biosystems, Inc., Foster City, CA, USA) was used for the
amplification. Cycling conditions consisted of an initial
denaturation step at 95ºC for 10 min as a ‘hot start’, followed by
40 cycles of 95ºC for 15 sec, annealing temperature (72ºC) for 30
sec, and a final extension at 72ºC for 10 min. GAPDH was used in
each experiment as an endogenous control. The relative
quantification for a gene was expressed as fold changes over the
control group. Fold changes were calculated using the
2−ΔΔCt method.
Statistical analysis
Kaplan-Meier analysis was applied to estimate
cancer-specific survival. Different groups were compared using the
log-rank test. Univariate analysis used the χ2 test or a
two-tailed t-test for statistical comparisons. Multivariate
analysis was conducted with the Cox regression model. Statistical
procedures were analyzed with SPSS software, version 16.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of survivin and VEGF-C
proteins
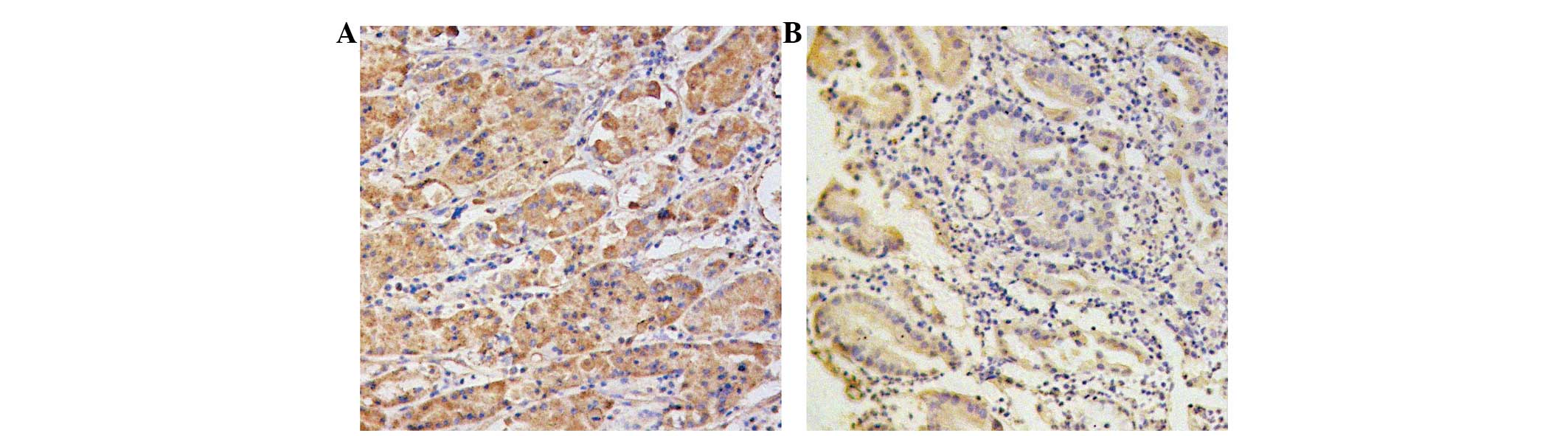
Cancer tissue exhibited positive cytoplasmic
staining for survivin (100 cells, 51.3%) and VEGF-C (108 cells,
55.4%). Gastric carcinoma and peri-carcinoma tissues positively
expressed the two proteins. The proteins showed as a yellow or
brown-yellow stain in the cytoplasm of the carcinoma cells
(Fig. 1). Survivin and VEGF-C were
expressed at significantly higher levels in patients with lymphatic
metastasis (P=0.008 and 0.001, respectively). Survivin and VEGF-C
were expressed at significantly higher levels in patients with
lymphatic metastasis (P=0.008 and 0.001, respectively). The
survivin expression levels were also significantly different in
tumors of different histological types (P=0.013), and the VEGF-C
expression levels were significantly different in patients with
different ages. However, there were no significant differences in
the expression levels of the two genes in Lauren’s classifications,
tumor locations and sizes (Table
I).
 | Table ICorrelation between VEGF-C and
survivin expression and clinicopathological factors of gastric
cancer. |
Table I
Correlation between VEGF-C and
survivin expression and clinicopathological factors of gastric
cancer.
| VEGF-C protein
expression (n) | | Survivin protein
expression (n) | |
|---|
|
| |
| |
|---|
| Variables | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Patients (n) | 87 | 108 | | 95 | 100 | |
| Gender |
| Male | 59 | 80 | 0.337 | 70 | 69 | 0.470 |
| Female | 28 | 28 | | 25 | 31 | |
| Age (years) |
| ≤60 | 59 | 57 | 0.033 | 62 | 54 | 0.109 |
| >60 | 28 | 51 | | 33 | 46 | |
| Tumor location |
| Cardia | 12 | 12 | 0.492 | 9 | 15 | 0.443 |
| Body | 9 | 7 | | 9 | 7 | |
| Antrum | 66 | 89 | | 77 | 78 | |
| Tumor size
(cm) |
| ≤4 | 46 | 50 | 0.361 | 52 | 44 | 0.134 |
| >4 | 41 | 58 | | 43 | 56 | |
| Lauren’s
classification |
| Intestinal
type | 38 | 53 | 0.453 | 49 | 42 | 0.180 |
| Diffuse type | 49 | 55 | | 46 | 58 | |
| Histological
type |
|
Well-differentiated | 38 | 47 | 0.982 | 50 | 35 | 0.013 |
| Poorly
differentiated | 49 | 61 | | 45 | 65 | |
| Lymphovascular
invasion |
| Yes | 20 | 29 | 0.536 | 24 | 25 | 0.966 |
| No | 67 | 79 | | 71 | 75 | |
| T stage |
| T1 | 8 | 10 | 0.653 | 10 | 8 | 0.050 |
| T2 | 18 | 15 | | 22 | 11 | |
| T3 | 57 | 78 | | 61 | 74 | |
| T4 | 4 | 5 | | 2 | 7 | |
| N stage |
| N0 | 39 | 26 | 0.001 | 42 | 23 | 0.008 |
| N1 | 20 | 18 | | 19 | 19 | |
| N2 | 12 | 19 | | 12 | 19 | |
| N3 | 16 | 45 | | 22 | 39 | |
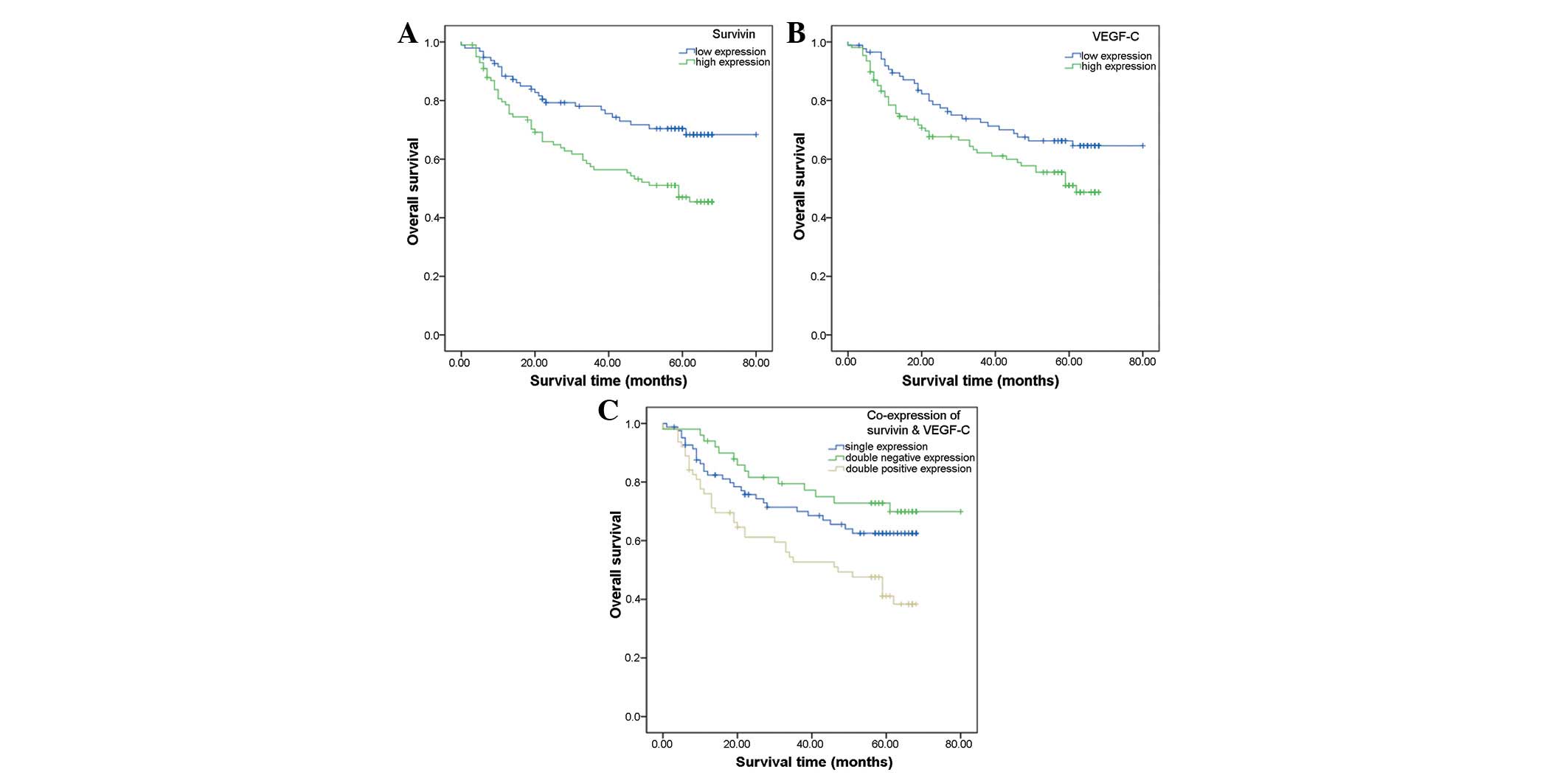
Survival analysis
Univariate prognostic analyses
According to the follow-up data, 78 of the 195
assessable cases did not survive. The overall survival (OS) rate
for patients was 60.0%. Analysis of the impact of survivin status
is shown in Fig. 2A. Among the
patients with low survivin expression levels, 27 cases had died and
the OS rate was 71.6%, whereas 51 cases had died in the group of
patients with high expression levels of survivin and the OS rate
was 49.0%. Patients with high survivin expression levels tended to
have a poorer prognosis than the patients with low survivin
expression levels (P=0.003, log-rank test). The OS rates of
patients with low and high VEGF-C expression levels were 66.7 and
54.6%, respectively. Kaplan-Meier curves of overall survival
stratified by VEGF-C status are shown in Fig. 2B. Patients with high VEGF-C
expression tended to have a poorer prognosis than patients with low
VEGF-C expression (P=0.039, log-rank test).
In the patients with co-expression of survivin and
VEGF-C, the OS rate was lower than that in the patients with single
or no expression of survivin and VEGF-C (P=0.003) (Fig. 2C). This result indicated that
patients with co-expression of survivin and VEGF-C tended to have
the poorest prognosis compared with patients with single or no
expression.
Multivariate analysis and Cox
proportional hazard regression model
In Cox regression for OS rate, including patient
age, lymph node metastasis, histological differentiation, T stage,
and survivin and VEGF-C expression, only lymph node metastasis
(P<0.001; hazard ratio, 1.425; 95% confidence interval,
1.328–1.529) and T stage (P<0.001; hazard ratio, 1.340; 95%
confidence interval, 1.181–1.522) remained as independent
prognostic factors. Neither of the two genes was an independent
prognostic factor (survivin: P=0.684; hazard ratio, 1.181;
confidence interval, 0.894–1.076; VEGF-C: P=0.116; hazard ratio,
1.215; confidence interval, 0.935–1.650).
Expression levels of survivin and
VEGF-C
According to the immunohistochemical staining
results, there was a significant difference between the levels of
survivin and VEGF-C expression (P<0.05) (Table II). When the levels of survivin
expression increased from degree zero to three, the levels of
VEGF-C expression increased correspondingly. The Pearson
coefficient of contingency was C=0.514, which implies there is a
true correlation between survivin and VEGF-C expression.
 | Table IICorrelation between survivin and
VEGF-C expression in study patients (n=95). |
Table II
Correlation between survivin and
VEGF-C expression in study patients (n=95).
| VEGF-C (0) (n) | VEGF-C (1+)
(n) | VEGF-C (2+)
(n) | VEGF-C (3+)
(n) | P-value |
|---|
| Survivin (0) | 29 | 14 | 21 | 17 | 0.038 |
| Survivin (1+) | 16 | 17 | 9 | 3 | |
| Survivin (2+) | 13 | 12 | 16 | 9 | |
| Survivin (3+) | 3 | 2 | 8 | 6 | |
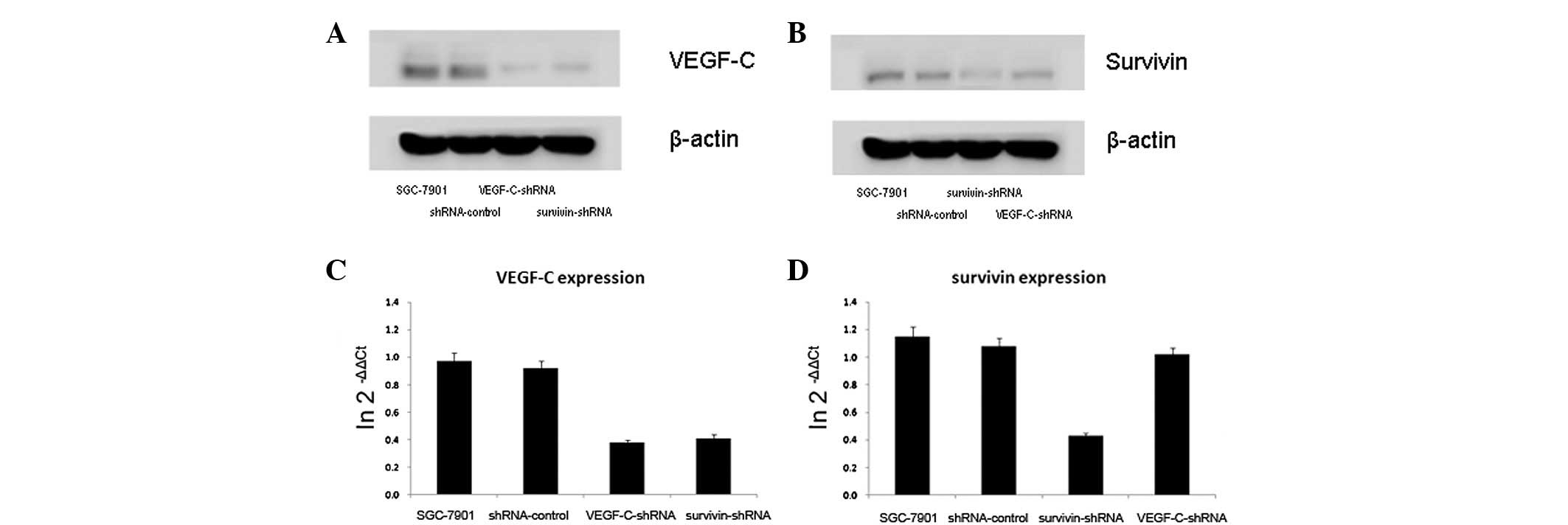
In the SGC-7901 gastric cancer cells, when survivin
was downregulated, VEGF-C was downregulated, as shown by the
western blot analysis, and when survivin was upregulated, VEGF-C
was upregulated correspondingly. qPCR demonstrated that the levels
of VEGF-C mRNA positively correlated with the levels of survivin
mRNA. However, the results did not reveal whether the levels of
survivin expression may be regulated by VEGF-C expression using
western blot and qPCR analysis (Fig.
3).
Discussion
Survivin is expressed in nearly all human
malignancies as well as embryonic and fetal tissues, but is almost
undetectable in normal adult tissues (17). Overexpression of survivin in cancer
invariably provides a survival advantage in tumor cells. As a
result, survivin has been considered as a potential tumor marker
and an important therapeutic target (18). Studies on different types of tumors
have indicated that the expression of survivin correlates with
lymphatic metastasis (19–21). However, few studies have focused on
the mechanisms by which survivin protein expression is regulated in
gastric cancer. The results of the present study suggest that
survivin may influence gastric cancer lymphatic metastasis and
distal invasion through VEGF-C.
VEGF-C, a key factor for angiogenesis, interacts
with the VEGF-3 receptor to promote hyperplasia of lymphatic
vessels. A number of studies have indicated that the upregulation
of VEGF-C promotes tumor lymphatic vessel formation and increases
lymph node metastasis. VEGF-C is vital for the lymphangiogenic
process, supported by evidence from transgenic and gene deletion
animal models through the VEGF-C/VEGFR-3 axis (23,24).
‘VEGF-C has been demonstrated to be highly expressed in gastric
carcinoma, and has a negative influence on prognosis and a positive
correlation with lymph node metastasis (15,24).
The results of the present study demonstrated that primary gastric
carcinoma tissue exhibited elevated expression levels of VEGF-C,
and there was a significant association between VEGF-C expression
and lymphatic metastasis.
In the present study, survivin and VEGF-C were
expressed at higher levels in patients with lymphatic metastasis.
The survivin expression levels were also statistically different in
tumors of differing histological type and T stage. Patients with
high survivin or VEGF-C expression levels tended to have a poorer
prognosis. Moreover, in comparison with patients that had low
expression levels of survivin and VEGF-C (S-C-), the patients with
high expression levels of survivin and VEGF-C (S+C+) had a poorer
OS rate. These results suggest that gastric cancer co-expressing
survivin and VEGF-C has higher levels of angiogenesis and
lymphangiogenesis, and higher risk of invasion and metastasis.
Therefore, the clinical significance of one factor (survivin or
VEGF-C alone) may be affected by the expression of the other factor
in the same patient. The combined analysis of associated factors
may be of clinical significance for patients with gastric
cancer.
To further investigate the biological co-effects of
survivin and VEGF-C in gastric cancer, the mRNA and protein
expression levels of survivin and VEGF-C were analyzed in the
SGC7901 human gastric cancer cell line. Survivin and VEGF-C were
highly expressed at the mRNA and protein levels. The proliferation
and invasion of gastric cancer cells in vitro were
significantly suppressed following silencing of survivin and VEGF-C
by plasmids with survivin-shRNA and VEGF-C-shRNA. Moreover, the
levels of VEGF-C expression were controlled by survivin, whereby
the levels of survivin expression were not regulated by VEGF-C.
Thus, there must be a hypothetical mechanism for how survivin
controls the expression of VEGF-C.
Studies have demonstrated that phosphoinositide
3-kinase (PI3K)/Akt activation is involved in the upregulation of
survivin (25,26). The survivin mRNA expression levels
are correlated with Akt activation, suggesting that Akt may be a
downstream target (27). It has
been demonstrated that the levels of survivin expression are
regulated by the direct effect of PI3K alone via the PI3K/Akt
signaling pathway in breast cancer cells (28). By contrast, VEGF-C expression is
also correlated with the PI3K/Akt pathway in numerous types of
cancer cells (29,30). It is possible that survivin may
regulate VEGF-C expression through the PI3K/Akt pathway.
COX-2 activation is highly correlated with survivin
expression in certain types of tumor cells (31,32)
and, as an important regulator of apoptosis, COX-2 is able to
upregulate the expression levels of survivin in cancer cells.
VEGF-C expression is also regulated by COX-2. The COX-2 inhibitor
mediates VEGF-C to block lymphangiogenesis and lymph node
metastasis (33). It has also been
proven that COX-2 is usually overexpressed with VEGF-C in breast,
prostate and gastric cancer (34–36).
Thus, COX-2 may be important in linking survivin and VEGF-C
expression.
In the present study, following incubation in
vitro with VEGF-C, SGC-7901 cells showed a significantly
increased invasive ability. Moreover, the invasiveness of the
gastric cancer cells significantly decreased when the survivin
expression levels were downregulated. Thus, survivin may be
important in enhancing the invasiveness of tumor cells initially
caused by VEGF-C. The results demonstrate that VEGF-C protein is
able to promote gastric cancer cell invasion and proliferation and
suggest that the expression of VEGF-C in human gastric cancer cells
may be regulated by survivin. They also indicate that
VEGF-C-mediated tumor proliferation and metastases may be used in
therapeutics by targeting survivin.
In conclusion, this study showed that survivin and
VEGF-C were expressed in human gastric cancer cells, and that they
were significantly associated with lymphatic metastasis. Patients
with high expression levels of survivin and VEGF-C exhibited
significantly less favorable survival rates compared with patients
with low expression levels of those two genes. However,
multivariate analysis demonstrated that neither of the two genes
was an independent prognostic determinant. Survivin may promote
VEGF-C expression through the PI3K/Akt pathway or COX-2 gene
expression. Survivin may be a regulator of VEGF-C expression in
gastric cancer cells. Moreover, the study also provides a possible
therapeutic method for the inhibition of VEGF-C-mediated tumor
growth and lymphatic metastases by using a survivin-specific
inhibitor.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iida A, Hirose K, Arai M, Yamaguchi A and
Nakagawara G: Relationships among the expression of epidermal
growth factor receptor, proliferating cell nuclear antigen labeling
index, and lymph node metastasis in gastric cancer. Oncology.
52:189–195. 1995. View Article : Google Scholar
|
|
3
|
Murata H, Kawano S, Tsuji S, et al:
Cyclooxygenase-2 overexpression enhances lymphatic invasion and
metastasis in human gastric carcinoma. Am J Gastroenterol.
94:451–455. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yonemura Y, Endo Y, Fujita H, et al: Role
of vascular endothelial growth factor C expression in the
development of lymph node metastasis in gastric cancer. Clin Cancer
Res. 5:1823–1829. 1999.PubMed/NCBI
|
|
5
|
Miyachi K, Sasaki K, Onodera S, et al:
Correlation between survivin mRNA expression and lymph node
metastasis in gastric cancer. Gastric Cancer. 6:217–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srinivasula SM and Ashwell JD: IAPs:
what’s in a name? Mol Cell. 30:123–135. 2008.
|
|
7
|
Altieri DC: The molecular basis and
potential role of survivin in cancer diagnosis and therapy. Trends
Mol Med. 7:542–547. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang TT, Qian XP and Liu BR: Survivin:
potential role in diagnosis, prognosis and targeted therapy of
gastric cancer. World J Gastroenterol. 13:2784–2790.
2007.PubMed/NCBI
|
|
9
|
Chandele A, Prasad V, Jagtap JC, Shukla R
and Shastry PR: Upregulation of survivin in G2/M cells and
inhibition of caspase 9 activity enhances resistance in
staurosporine-induced apoptosis. Neoplasia. 6:29–40. 2004.
View Article : Google Scholar
|
|
10
|
Da CL, Xin Y, Zhao J and Luo XD:
Significance and relationship between Yes-associated protein and
survivin expression in gastric carcinoma and precancerous lesions.
World J Gastroenterol. 15:4055–4061. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiaoyuan C, Longbang C, Jinghua W, et al:
Survivin: a potential prognostic marker and chemoradiotherapeutic
target for colorectal cancer. Ir J Med Sci. 179:327–335. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mandriota SJ, Jussila L, Jeltsch M, et al:
Vascular endothelial growth factor-C-mediated lymphangiogenesis
promotes tumour metastasis. EMBO J. 20:672–682. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawakami M, Yanai Y, Hata F and Hirata K:
Vascular endothelial growth factor C promotes lymph node metastasis
in a rectal cancer orthotopic model. Surg Today. 35:131–138. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stacker SA, Achen MG, Jussila L, Baldwin
ME and Alitalo K: Lymphangiogenesis and cancer metastasis. Nat Rev
Cancer. 2:573–583. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shida A, Fujioka S, Kobayashi K, et al:
Expression of vascular endothelial growth factor (VEGF)-C and -D in
gastric carcinoma. Int J Clin Oncol. 11:38–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dikken JL, van de Velde CJ, Gönen M, et
al: The New American Joint Committee on Cancer/International Union
Against Cancer staging system for adenocarcinoma of the stomach:
increased complexity without clear improvement in predictive
accuracy. Ann Surg Oncol. 19:2443–2451. 2012. View Article : Google Scholar
|
|
17
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pennati M, Folini M and Zaffaroni N:
Targeting survivin in cancer therapy. Expert Opin Ther Targets.
12:463–476. 2008. View Article : Google Scholar
|
|
19
|
Al-Joudi FS, Iskandar ZA, Hasnan J, et al:
Expression of survivin and its clinicopathological correlations in
invasive ductal carcinoma of the breast. Singapore Med J.
48:607–614. 2007.PubMed/NCBI
|
|
20
|
Kim YH, Kim SM, Kim YK, Hong SP, Kim MJ
and Myoung H: Evaluation of survivin as a prognostic marker in oral
squamous cell carcinoma. J Oral Pathol Med. 39:368–375.
2010.PubMed/NCBI
|
|
21
|
Su L, Wang Y, Xiao M, Lin Y and Yu L:
Up-regulation of survivin in oral squamous cell carcinoma
correlates with poor prognosis and chemoresistance. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 110:484–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryan BM, O’Donovan N and Duffy MJ:
Survivin: a new target for anti-cancer therapy. Cancer Treat Rev.
35:553–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Makinen T, Jussila L, Veikkola T, et al:
Inhibition of lymphangiogenesis with resulting lymphedema in
transgenic mice expressing soluble VEGF receptor-3. Nat Med.
7:199–205. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wirzenius M, Tammela T, Uutela M, et al:
Distinct vascular endothelial growth factor signals for lymphatic
vessel enlargement and sprouting. J Exp Med. 204:1431–1440. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding S, Li C, Lin S, et al: Distinct roles
of VEGF-A and VEGF-C in tumour metastasis of gastric carcinoma.
Oncol Rep. 17:369–375. 2007.PubMed/NCBI
|
|
26
|
Papapetropoulos A, Fulton D, Mahboubi K,
et al: Angiopoietin-1 inhibits endothelial cell apoptosis via the
Akt/survivin pathway. J Biol Chem. 275:9102–9105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mesri M, Morales-Ruiz M, Ackermann EJ, et
al: Suppression of vascular endothelial growth factor-mediated
endothelial cell protection by survivin targeting. Am J Pathol.
158:1757–1765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao P, Meng Q, Liu LZ, You YP, Liu N and
Jiang BH: Regulation of survivin by PI3K/Akt/p70S6K1 pathway.
Biochem Biophys Res Commun. 395:219–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Asanuma H, Torigoe T, Kamiguchi K, et al:
Survivin expression is regulated by coexpression of human epidermal
growth factor receptor 2 and epidermal growth factor receptor via
phosphatidylinositol 3-kinase/AKT signaling pathway in breast
cancer cells. Cancer Res. 65:11018–11025. 2005. View Article : Google Scholar
|
|
30
|
Tsutsui S, Matsuyama A, Yamamoto M, et al:
The Akt expression correlates with the VEGF-A and -C expression as
well as the microvessel and lymphatic vessel density in breast
cancer. Oncol Rep. 23:621–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiang L, Xie G, Ou J, Wei X, Pan F and
Liang H: The extra domain A of fibronectin increases VEGF-C
expression in colorectal carcinoma involving the PI3K/AKT signaling
pathway. PLoS One. 7:e353782012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song IH, Kim DW, Shin KC, et al:
Down-regulation of survivin in growth inhibition of hepatoma cells
induced by a selective cyclooxygenase-2 inhibitor. Korean J
Hepatol. 14:351–359. 2008.(In Korean).
|
|
33
|
Jang JW: Anti-tumor mechanisms and
regulation of survivin by selective cyclooxygenase-2 inhibitor.
Korean J Hepatol. 14:305–308. 2008.(In Korean).
|
|
34
|
Iwata C, Kano MR, Komuro A, et al:
Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via
reduction of lymphangiogenesis. Cancer Res. 67:10181–10189. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bhattacharjee RN, Timoshenko AV, Cai J and
Lala PK: Relationship between cyclooxygenase-2 and human epidermal
growth factor receptor 2 in vascular endothelial growth factor C
up-regulation and lymphangiogenesis in human breast cancer. Cancer
Sci. 101:2026–2032. 2010. View Article : Google Scholar
|
|
36
|
Di JM, Zhou J, Zhou XL, et al:
Cyclooxygenase-2 expression is associated with vascular endothelial
growth factor-C and lymph node metastases in human prostate cancer.
Arch Med Res. 40:268–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Ji J, Yuan F, et al:
Cyclooxygenase-2 expression is associated with VEGF-C and lymph
node metastases in gastric cancer patients. Biomed Pharmacother.
59(Suppl 2): S285–S288. 2005. View Article : Google Scholar : PubMed/NCBI
|