Introduction
Budd-Chiari syndrome (BCS) is a life-threatening
disorder characterized by hepatic venous outflow obstruction at any
level from the small hepatic veins to the cavoatrial junction
(1,2). According to possible sites of
obstruction, BCS may be divided into hepatic vein obstruction,
inferior vena cava obstruction and a mixture of hepatic vein and
inferior vena cava obstructions. Development of effective vascular
surgery and interventional treatment approaches for the treatment
of BCS associated with diffuse hepatic vein obstruction is
difficult (3,4). Multiple studies have shown that
portal vein-vena cava surgical shunt does not increase the survival
of BCS patients (5–8). Normal hepatic vein flow cannot be
recovered in patients with BCS associated with chronic diffuse
hepatic vein obstruction, therefore, balloon dilation and stenting
is not suitable for this type of BCS. Transjugular intrahepatic
portosystemic shunts (TIPS) are able to relieve liver congestion
and improve clinical symptoms but the occurrence of TIPS
dysfunction and hepatic encephalopathy remains high (9). In order to more effectively treat
these symptoms, it is necessary to establish an animal model of BCS
to facilitate the development of surgical or interventional
approaches. A mouse model of BCS was previously established by
ligation of the inferior vena cava at the proximal end (10,11).
However, this model used a surgical approach in which hepatic vein
flow disorder was caused by stenosis or occlusion of the inferior
vena cava near the right atrium, rather than by diffuse occlusion
of the hepatic vein. At present, there is no animal model in place
mimicking human BCS associated with diffuse hepatic vein occlusion.
The present study used an endovascular technique to establish a
reliable and reproducible canine model of this BCS type. This model
may be useful for future pathophysiological, surgical and
interventional treatment studies of BCS.
Materials and methods
Grouping of the animals
A total of 24 healthy adult mongrel canines between
1 and 3 years-old (1.95±0.65 years-old; male and female; weight,
18±2.3 kg; Experimental Animal Center of Xuzhou Medical College,
Xuzhou, China) were randomly divided into experimental (n=18) and
control (n=6) groups. The canines were preconditioned in the
laboratory (Experimental Animal Center of Xuzhou Medical College)
for several weeks and received standard dog chow containing ~25%
protein and 8% fat. The same diet was used postoperatively. Animal
handling complied with the regulations on the management of
laboratory animals of the Chinese Academy of Sciences and the study
was approved by the Ethics Committee of Xuzhou Medical College,
Xuzhou, China.
Procedure for model development
All animals were fasted 8 h prior to surgery and
skin preparation was performed on the right side of the neck.
General anesthesia was performed by intramuscular injection of 3%
sodium pentobarbital (1 ml/kg; Hongyun Long Biological Technology
Co. Ltd., Wuhan, China) without endotracheal intubation. Canines
were fixed in the supine position on the digital subtraction
angiography (DSA) examination bed (Innova 3100; GE Healthcare,
Little Chalfont, UK). The right side of the neck was disinfected
with iodophor. Each layer of skin was cut along the outer edge of
the right sternocleidomastoid to expose the right external jugular
vein. Puncture of the right external jugular vein was performed by
modified Seldinger technique (12). A 6F-catheter sheath was
delivered along a short guidewire and, under the guidance of DSA, a
5F angiographic catheter (TEMPO®4; Cordis Corporation,
East Bridgewater, NJ, USA) was delivered to the left, middle and
right hepatic veins for phlebography. A contrast agent (iohexol)
was injected at a flow rate of 3.0 m1/sec and a total volume of 6.0
ml. Images were captured at 4 frames/sec to show the hepatic vein
diameter and degree of reflux. The hepatic vein with maximum
diameter was selected as a target for placement of the exchange
guidewire. The balloon catheter (diameter, 8–12 mm; Cordis
Corporation) was delivered to the target hepatic vein along the
guidewire and the balloon was filled with contrast agent. Once
complete occlusion of blood flow in the hepatic vein had been
confirmed, an emulsion mixture (3–5 ml) of
N-butyl-cyanoacrylate (NBCA; Baiyun Medical Adhesive Co.
Ltd., Guangzhou, China) and lipiodol (2:1; Guerbet, Villepinte,
France) was injected into animals of the experimental group via the
balloon catheter. Injections were stopped upon observation of
vascular casting of the hepatic vein by X-ray. Subsequently, the
balloon catheter was removed and a 5F-angiographic catheter was
placed to indicate hepatic vein occlusion. In the control group,
3–5 ml normal saline was injected via balloon catheter and contrast
agent was injected to indicate hepatic vein patency. Following
surgery, the catheter and sheath were removed and the external
jugular vein pressed to ensure that no bleeding occurred.
Subsequently, each layer of the incision was sutured and
disinfected. Antibiotics (penicillin, 800,000 U/day) and analgesics
(ketoprofen, 1 ml/5 kg) were used for a period of three days
postoperatively. The animals were then returned to their breeding
circle and their airways were kept open. Breathing and eating
patterns were monitored daily. Following surgery, the animals were
allowed unlimited exercise.
Sample collection and biochemical
testing
Under general anesthesia, peripheral venous blood
was collected from six canines in the control group (at 4 weeks
following surgery) and 18 canines from the experimental group (six
from each of the 4-, 6- and 8-week postoperative stages). The blood
samples were kept in procoagulant test tubes and centrifuged at 4°C
for 10 min (1,600 × g or 3,000 rpm). The serum was tested for
alanine aminotransferase, γ-glutamyl transpetidase, albumin,
pro-albumin, total bile acid, total bilirubin and
cholinesterase.
Color Doppler ultrasound evaluation
Portal venous hemodynamics, proper hepatic arterial
hemodynamics and sonographic appearance of liver parenchyma were
evaluated with color Doppler ultrasound and two-dimensional
gray-scale ultrasound using a curvilinear 3.5 MHz transducer (iU22;
Philips, Eindhoven, Netherlands). Canines were examined at supine
and lateral positions under general anesthesia. Diameter of main
portal vein (D), average peak velocity (V), diameter of proper
hepatic artery, peak systolic velocity (PSV) and end-diastolic peak
velocity (EDV) were measured over a period of 4–5 cardiac cycles.
Average values of these parameters were obtained from continuous
measurements taken three times. Resistance index (RI) and blood
flow volume (Q) were calculated according to the following
formulae: RI = (PSV - EDV)/PSV; Q = V × (D/2)2 × π × 60,
respectively.
Measurement of portal venous
pressure
The abdominal cavity was cut along the right quarter
of the costal arch. Portal venous pressure (PVP) was determined by
cannulation of the superior mesenteric vein. The unit of
measurement was cm H2O (1 cm H2O = 0.098
kPa).
Harvest of liver specimen
Gross anatomical changes were recorded following the
sacrifice of the animals by laparotomy. The target hepatic vein and
hepatic tissues from the drainage area of the target hepatic vein
were collected and fixed with 10% formalin. Hematoxylin and eosin
(H&E) staining was performed to analyze structural changes in
hepatic cells and the hepatic vein wall using an SZX16 microscope
(Olympus, Tokyo, Japan). The results were compared between
experimental and control groups.
Statistical analysis
The SPSS 16.0 package (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Data measurements are expressed
as mean ± standard deviation. One-way analysis of variance was used
to compare the data between different groups and P<0.05 was
considered to indicate a statistically significant difference.
Results
Angiographic performance during
surgery
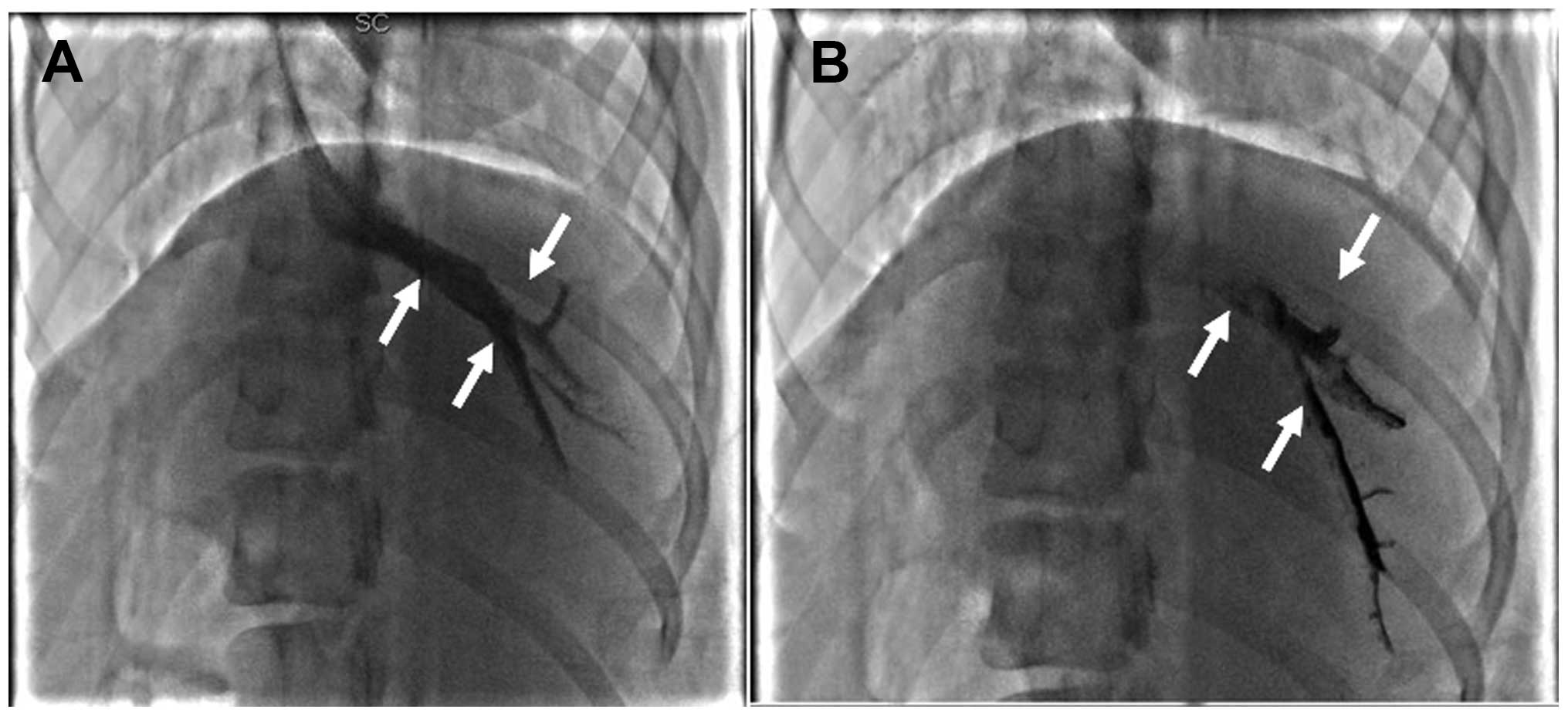
Hepatic venography indicated that the common trunk
of left hepatic and middle hepatic veins had the largest diameter.
This was therefore used as a target hepatic vein. Obstruction
occurred in the target hepatic vein of all 18 canines in the
experimental group (Figs. 1 and
2), indicating a 100% success rate
of model development. The hepatic vein in the control group was
unobstructed. There were no serious complications (e.g., pulmonary
embolism or mortality) in either group.
Liver function in the experimental and control
groups is shown in Table I.
 | Table ILiver function of canines in
experimental and control groups (mean ± SD; n=6). |
Table I
Liver function of canines in
experimental and control groups (mean ± SD; n=6).
| Parameter | Experimental
group | Control group |
|---|
|
|---|
| 4 weeks | 6 weeks | 8 weeks |
|---|
| ALT, U/l | 52.50±12.50a | 61.30±5.70a | 38.60±9.40a | 28.60±5.30 |
| GGT, U/l | 3.60±2.40 | 4.00±2.00 | 3.80±1.30 | 3.50±2.70 |
| PAB, g/l | 0.18±0.04a | 0.22±0.02a | 0.19±0.06a | 0.26±0.13 |
| ALB, g/l | 35.20±6.80 | 29.40±2.30a | 34.70±8.70 | 34.50±3.50 |
| TBIL, μmol/l | 0.90±0.50 | 1.20±0.20 | 1.40±0.50 | 1.10±0.30 |
| TBA, μmol/l | 0.85±0.35 | 0.90±0.25 | 1.15±0.45 | 0.93±0.47 |
| CHE, IU/l |
4,595.30±59.60a |
5,135.50±84.50a |
4,983.50±123.50a | 5,327.60±75.30 |
Clinical symptoms and signs of animals in
the experimental group
In the experimental group, anorexia and hepatomegaly
occurred in 18 canines following surgery. Ascites occurred in 3, 5
and 5 canines at 4, 6 and 8 weeks following surgery, respectively.
At 6 weeks post-surgery, one canine exhibited upper
gastrointestinal bleeding.
Color Doppler ultrasound
observations
In the experimental group, no color Doppler signal
was observed in the left and middle hepatic veins or in their
common trunk. Reverse flow signaling occurred in the distal segment
of the left and middle hepatic veins at 4, 6 and 8 weeks following
surgery. A heterogeneous hypoechoic mass was observed in the
occluded area of the liver. The color Doppler signal in the main
portal vein changed from hepatopetal to hepatofugal flow in two
canine BCS models. In 16 canine BCS models, the signal remained
hepatopetal but weakened markedly. In the control group, the patent
hepatic veins were confirmed by color Doppler ultrasound and the
results showed that the flow maintained normal hepatopetal
character in the main portal vein and the liver showed homogeneous
isoecho at week 4 following surgery. Altered hemodynamics of the
portal vein and proper hepatic artery are shown in Table II.
 | Table IIHepatic hemodynamics of canines in
experimental and control groups (mean ± SD; n=6). |
Table II
Hepatic hemodynamics of canines in
experimental and control groups (mean ± SD; n=6).
| Parameter | Experimental
group | Control group |
|---|
|
|---|
| 4 weeks | 6 weeks | 8 weeks |
|---|
| Diameter of PV,
cm | 0.97±0.05 | 0.99±0.13 | 0.91±0.17 | 1.10± 0.08 |
| Average peak velocity
of PV, cm/sec | 27.10±2.10a | 19.70±2.50a | 20.20±1.50a | 29.70±3.30 |
| BFV of PV,
ml/min |
1,301.60±73.60a | 989.40±42.20a |
1,187.90±35.20a | 1,561.70±64.20 |
| PVP, cm
H2O | 16.50±2.50a | 17.30±1.20a | 16.70±2.30a | 11.30±1.60 |
| Diameter of PHA,
cm | 0.30±0.02 | 0.28±0.03 | 0.31±0.02 | 0.31±0.03 |
| RI of PHA | 0.68±0.03a | 0.68±0.04a | 0.66±0.21a | 0.57±0.16 |
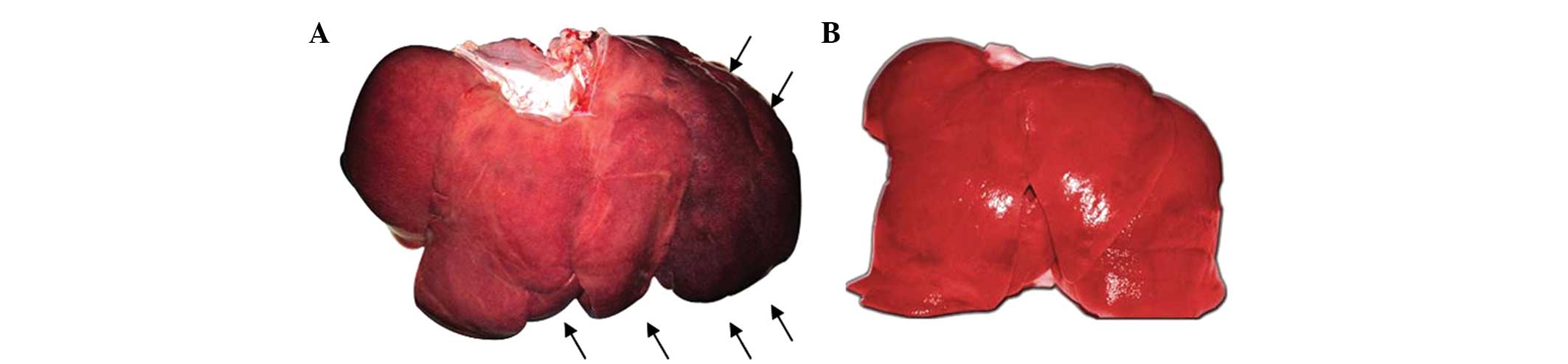
Gross anatomy in experimental and control
groups
In the experimental group, the left and middle
hepatic veins and the common trunk were filled with solidified
embolic agents at 4, 6 and 8 weeks following surgery and the lumen
was completely obstructed. The liver exhibited swelling at the
draining area and a color of pale red, dark red and near black was
noted at 4, 6 and 8 weeks following surgery, respectively (Fig. 3A). In the control group, the
hepatic vein was patent and the liver was bright red with a smooth
surface and sharp edge (Fig.
3B).
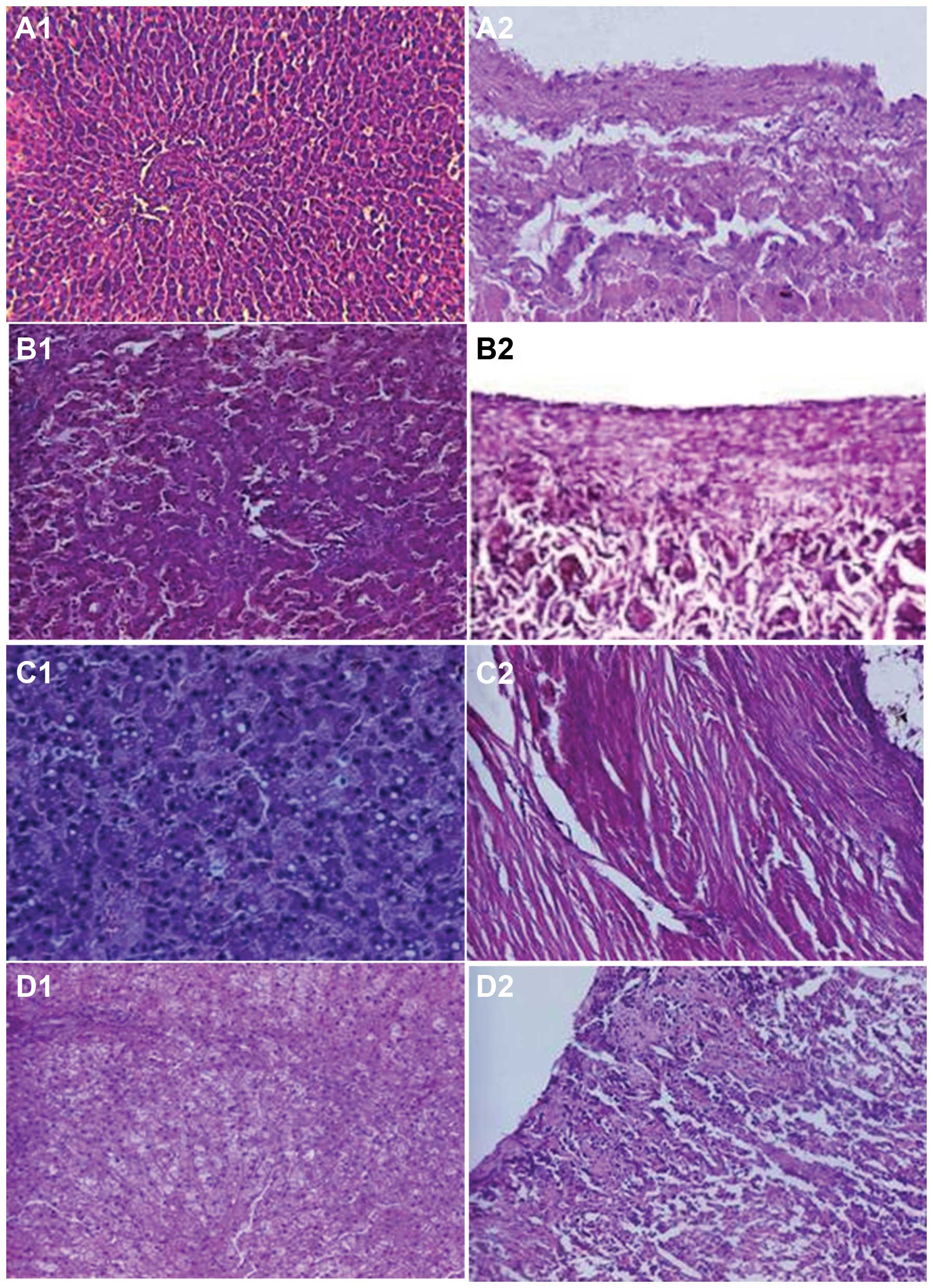
Changes in liver pathology in the
experimental and control groups
Four weeks after surgery, animals in the control
group exhibited normal hepatic cell structure and hepatic veins
(Fig. 4A1 and
A2). However, light microscopy revealed hepatic cell
congestion, edema, dilated centrilobular sinusoids, widened central
veins in the hepatic lobules, red blood cells situated in Disse’s
space and a large quantity of inflammatory cell infiltration (in
the tunica intima and tunica media of the hepatic vein without
significant thickening) in the experimental group at week 4
following surgery (Fig. 4B1
and B2). At week 6, hepatic cells showed swelling
with lipid degeneration and a number of neutrophils, Kupffer cells
and macrophages (Fig.
4C1). The hepatic vein tunica intima showed marginal
thickening and the circular smooth muscle of the tunica media had
become thickened with an increased number of layers (Fig. 4C2). At week 8 following
surgery, the majority of hepatic cells exhibited balloon-like
changes with sporadic atrophy of multiple hepatic cells, necrosis,
lightly stained cytoplasm, enlarged nucleolus, increased number of
polyploid nuclei cells and reduced inflammatory infiltration
compared with that at week 6 (Fig.
4D1). The hepatic vein intima exhibited significant
thickening with hyperplasia of the tunica media and elastic fibers
(Fig. 4D2).
Discussion
The present study successfully established a canine
BCS model by obstruction of the common trunk of the left and middle
hepatic veins and intravenous injection of an NBCA-lipiodol
mixture. Hepatomegaly, varying degrees of hepatic dysfunction and
altered hemodynamics of the portal vein (e.g., weakened hepatopetal
color Doppler signal, decreased portal blood volume and increased
PVP) were observed in 18 canine BCS models at 4–8 weeks following
surgery. Ascites was observed in 13 of 18 canine models. Therefore,
all canine models in the present study exhibited the classic
symptoms and pathology of BCS.
Balloon obstruction of hepatic veins in humans has
been successfully achieved through the right femoral vein, right
and left femoral veins, the right internal jugular vein and
percutaneous liver pathways (13).
In contrast to humans, the external jugular vein in canines has a
greater diameter (4.5–5.0 mm) than the internal jugular vein.
Furthermore, this vein is more superficially located, being covered
only by nuchal muscle. The carotid vein has a larger diameter but
is located in a deeper position, which is not conducive to surgical
intervention. Therefore, the external jugular vein was selected and
a 100% rate of technical success was achieved. An NBCA-lipiodol
mixture has previously been used as the embolic agent for the
treatment of symptomatic polycystic liver disease, intramuscular
active hemorrhage and tumors (14–16).
However, use of the NBCA-lipiodol mixture for the development of
BCS animal models has not yet been reported. Attention to the speed
and time of injection of the embolic agent is required. To prevent
the occurrence of reflux, the balloon should be filled and complete
obstruction of the target hepatic vein confirmed by angiography,
prior to injection of the embolic agent. Immediate removal of the
balloon catheter following occurrence of vascular casting in the
target hepatic vein may prevent adhesion between the embolic agent
and the balloon. The majority of studies use a ratio of 1:3–1:5 for
the NBCA-lipiodol mixture (15,16).
However, according to the canine hepatic vein diameter and flow
rate, a ratio of 2:1 was used in the present study for the
NBCA-lipiodol mixture. A high percentage of NBCA results in rapid
coagulation and adhesion with the balloon, while a low percentage
causes slow coagulation, incomplete obstruction of the hepatic
vein, reflux and pulmonary embolism. All animals in this study
showed no adverse effects or serious complications of pulmonary
embolism and mortality, indicating that a ratio of 2:1 for the
NBCA-lipiodol mixture is suitable for establishment of a BCS model.
The NBCA-lipiodol embolic agent has the advantage of rapid
coagulation in the vessels, easy filling of the hepatic vein, tight
connection with the hepatic vein wall and notable angiographic
performance. Furthermore, the degree of hepatic vein obstruction
may be easily controlled using an NBCA-lipiodol mixture as the
embolic agent, which ensures that pulmonary embolism does not occur
frequently. Therefore, obstruction of hepatic veins by a
combination of balloon occlusion and NBCA-lipiodol as the embolic
agent provides a novel, reliable and reproducible approach to
developing a BCS animal model.
Canines possess 4–6 hepatic veins, which is
different to the human liver anatomical structure. Dual-phase CT
angiography of normal canine hepatic vasculature has demonstrated
that several large hepatic vein branches, which drain the left
lobes of the liver, anastomose to form a main trunk (17). In the present study, intraoperative
angiography and postoperative anatomy confirmed that canine left
and middle hepatic veins had a common trunk with the largest
diameter among the hepatic veins and mainly collects venous blood
from the left hepatic lobe, left central lobe, lobus quadratus and
the majority of the right central lobe. By contrast, the right
hepatic vein is small and only collects venous blood from the right
dorsal part of the right hepatic lobe. Complete obstruction of all
major hepatic veins may result in acute liver failure and mortality
(18). However, the main
characteristic of BCS is obstruction of the hepatic vein outflow
tract. BCS is caused by occlusion of one, two or three of the major
hepatic veins (right, middle and left) and/or occlusion of the
inferior vena cava (19). The
present study used the common trunk of left and middle hepatic
veins as the site of occlusion and achieved severe obstruction of
the outflow tract consistent with a diagnosis of BCS. Akiyoshi
et al (11) used a surgical
approach to ligate the inferior vena cava between the diaphragm
muscle and liver in mice and observed severe hepatic congestion and
mild fibrosis following one week of surgery. Following two weeks of
surgery, this fibrosis had extended from the centrilobular zone to
the midlobular zone (11). In the
present study, significant damage to liver function was observed at
weeks 4 and 6 following surgery, but had partially recovered by
week 8. Additionally, liver swelling and congestion, hepatocyte
degeneration and necrosis tended to be worse between 4–8 weeks
following surgery. Typical histopathology observed with obstruction
of the human hepatic venous outflow tract includes hepatic
congestion and fibrosis and loss of liver cells (20). Histopathology of the canine BCS
model in this study was similar to that of the mouse BCS model and
to human BCS. Previous studies have shown that necrotic hepatocytes
are gradually replaced by fibrosis, eventually resulting in
cirrhosis (18,21). The development of cirrhosis from
hepatic fibrosis takes a relatively long time. For instance, it
takes 2–15 years (average, 8.7 years) for congestive liver fibrosis
of the hepatic vena cava disease (a form of BCS) to advance to
cirrhosis (22). It was
hypothesized that, with time, cirrhosis may also develop
following hepatocyte necrosis in the canine BCS model of the
present study.
Hepatic vein obstruction leads to the elevation of
sinusoidal blood pressure and portal vein pressure and ultimately
portal vein hypertension. Hiraki et al (23) showed that maximum peak velocity of
the portal vein was reduced and Doppler signals disappeared
following temporary occlusion of the right hepatic vein in humans,
indicating that portal vein hemodynamics change shortly following
hepatic vein obstruction. In the present study, average peak
velocity of the main portal vein and portal blood flow volume
decreased significantly at 4–8 weeks following surgery, which
indicated an increased resistance of the portal vein. With time,
BCS patients may form intrahepatic venous and portosystemic
collateral circulations, which partially alleviate elevated portal
vein pressure (24–26). Compensatory mechanisms of portal
veins were also observed in the mouse BCS model (10). However, in the present study, the
portal vein pressure was altered significantly following 4, 6 or 8
weeks of hepatic vein obstruction. Upper gastrointestinal bleeding
caused by esophageal varices was observed in one canine BCS model
at 6 weeks following surgery. Results of the present study
demonstrate that embolization of the largest hepatic vein in
canines has a significant effect on portal vein hemodynamics.
In a previous study, average peak velocity of the
right hepatic artery was found to decrease marginally for 15–30 sec
following the onset of hepatic vein occlusion, prior to increasing
rapidly by 1.5–2.0 times at 75–90 sec. Once the hepatic vein became
unobstructed, average peak velocity began to decrease (23). However, in the present study,
hepatic vein occlusion was permanent and the RI of the proper
hepatic artery increased following 4–8 weeks of hepatic vein
obstruction. The increase in RI following surgery may be explained
by an elevation in peripheral vascular resistance, which is caused
by an increase in sinusoidal pressure due to liver congestion and
hepatic cell necrosis.
One limitation of this study was that there was only
obstruction of the hepatic vein with the largest diameter (the
common trunk of left and middle hepatic veins), while the other
hepatic veins remained unobstructed. Future studies must
investigate the obstruction of the remaining hepatic veins
following a given time period for obstruction of the largest
hepatic vein. This is likely to allow recovery of liver function
and formation of a compensatory collateral circulation in order to
avoid fulminant hepatic failure and mortality observed in human
acute hepatic vein obstruction (27,28).
In addition, the long-term histopathological and hemodynamic
changes following the development of a BCS model also require
further study.
In conclusion, the present study successfully
established a canine BCS model using a balloon and NBCA-lipiodol
mixture to obstruct hepatic veins. This model is reliable,
reproducible and produces pathophysiological changes consistent
with human hepatic vein obstruction. In addition, compared with the
BCS model developed by surgical procedures, endovascular
obstruction is advantageous due to minimal trauma, fewer incidences
of complications and a greater ease of implementation. Due to
diffuse hepatic vein occlusion, this canine BCS model may also be
useful to studies regarding angiogenesis (e.g., endothelial
progenitor cells, vascular endothelial growth factor), surgery and
interventional radiology.
Acknowledgements
This study was financially supported by grants from
Jiangsu Province (333 Project; no. BRA2011221), Xuzhou Municipal
Science and Technology Bureau (grant no. XF11C097) and Xuzhou
Medical College Dean Special Talent Fund (grant no. 2011KJZ27).
References
|
1
|
Janssen HL, Garcia-Pagan JC, Elias E,
Mentha G, Hadengue A and Valla DC; European Group for the Study of
Vascular Disorders of the Liver. Budd-Chiari syndrome: a review by
an expert panel. J Hepatol. 38:364–371. 2003. View Article : Google Scholar
|
|
2
|
Valla DC: Primary Budd-Chiari syndrome. J
Hepatol. 50:195–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horton JD, San Miguel FL, Membreno F, et
al: Budd-Chiari syndrome: illustrated review of current management.
Liver Int. 28:455–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cura M, Haskal Z and Lopera J: Diagnostic
and interventional radiology for Budd-Chiari syndrome. Radio
Graphics. 29:669–681. 2009.PubMed/NCBI
|
|
5
|
Plessier A and Valla DC: Budd-Chiari
syndrome. Semin Liver Dis. 28:259–269. 2008. View Article : Google Scholar
|
|
6
|
Zeitoun G, Escolano S, Hadengue A, et al:
Outcome of Budd Chiari syndrome: a multivariate analysis of factors
related to survival including surgical portosystemic shunting.
Hepatology. 30:84–89. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Darwish Murad S, Valla DC, de Groen PC, et
al: Determinants of survival and the effect of portosystemic
shunting in patients with Budd-Chiari syndrome. Hepatology.
39:500–508. 2004.PubMed/NCBI
|
|
8
|
Langlet P, Escolano S, Valla D, et al:
Clinicopathological forms and prognostic index in Budd-Chiari
syndrome. J Hepatol. 39:496–501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia-Pagán JC, Heydtmann M, Raffa S, et
al: TIPS for Budd Chiari syndrome: long-term results and
prognostics factors in 124 patients. Gastroenterology. 135:808–815.
2008.PubMed/NCBI
|
|
10
|
Darwish Murad S, Dom VA, Ritman EL, et al:
Early changes of the portal tract on microcomputed tomography
images in a newly-developed rat model for Budd-Chiari syndrome. J
Gastroenterol Hepatol. 23:1561–1566. 2008.PubMed/NCBI
|
|
11
|
Akiyoshi H and Terada T: Centrilobular and
perisinusoidal fibrosis in experimental congestive liver in the
rat. J Hepatol. 30:433–439. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pancholy SB, Sanghvi KA and Patel TM:
Radial artery access technique evaluation trial: randomized
comparison of Seldinger versus modified Seldinger technique for
arterial access for transradial catheterization. Catheter
Cardiovasc Interv. 80:288–291. 2012. View Article : Google Scholar
|
|
13
|
de Baere T, Deschamps F, Briggs P, et al:
Hepatic malignancies: percutaneous radiofrequency ablation during
percutaneous portal or hepatic vein occlusion. Radiology.
248:1056–1066. 2008.
|
|
14
|
Wang MQ, Duan F, Liu FY, Wang ZJ and Song
P: Treatment of symptomatic polycystic liver disease: transcatheter
super-selective hepatic arterial embolization using a mixture of
NBCA and iodized oil. Abdom Imaging. 38:465–473. 2013. View Article : Google Scholar
|
|
15
|
Yoo DH, Jae HJ, Kim HC, Chung JW and Park
JH: Transcatheter arterial embolization of intramuscular active
hemorrhage with N-butyl cyanoacrylate. Cardiovasc Intervent Radiol.
35:292–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loewe C, Schindl M, Cejna M, Niederle B,
Lammer J and Thurnher S: Permanent transarterial embolization of
neuroendocrine metastases of the liver using cyanoacrylate and
lipiodol: assessment of mid- and long-term results. Am J
Roentgenol. 180:1379–1384. 2003. View Article : Google Scholar
|
|
17
|
Zwingenberger AL and Schwarz T: Dual-phase
CT angiography of the normal canine portal and hepatic vasculature.
Vet Radiol Ultrasound. 45:117–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoekstra J and Janssen HL: Vascular liver
disorders (I): diagnosis, treatment and prognosis of Budd-Chiari
syndrome. Neth J Med. 66:334–339. 2008.PubMed/NCBI
|
|
19
|
Mine T: Is hepatic vena cava disease an
endemic type of the Budd-Chiari syndrome? Hepatol Res. 37:170–171.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka M and Wanless IR: Pathology of the
liver in Budd-Chiari syndrome: portal vein thrombosis and the
histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and
large regenerative nodules. Hepatology. 27:488–496. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cazals-Hatem D, Vilgrain V, Genin P, et
al: Arterial and portal circulation and parenchymal changes in
Budd-Chiari syndrome: a study in 17 explanted livers. Hepatology.
37:510–519. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shrestha SM: Liver cirrhosis and
hepatocellular carcinoma in hepatic vena cava disease, a liver
disease caused by obstruction of inferior vena cava. Hepatol Int.
3:392–402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hiraki T, Kanazawa S, Mimura H, et al:
Altered hepatic hemodynamics caused by temporary occlusion of the
right hepatic vein: evaluation with Doppler US in 14 patients.
Radiology. 220:357–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Küşük NO, Ozkan E, Aras G and Kir KM:
Visualization of collaterals in budd-chiari syndrome with Tc-99m
MDP bone scintigraphy and Tc-99m HMPAO-labeled leukocyte
scintigraphy. Clin Nucl Med. 28:236–237. 2003.PubMed/NCBI
|
|
25
|
Steingruber IE, Bodner G, Czermak B,
Hochleitner B and Jaschke W: Color-coded doppler sonographic
demonstration of intrahepatic venous collaterals in Budd-Chiari
syndrome. Ultraschall Med. 22:55–59. 2001.(In German).
|
|
26
|
Karaosmanoglu D, Karcaaltincaba M, Akata
D, Ozmen M and Akhan O: CT, MRI, and US findings of incidental
segmental distal hepatic vein occlusion: a new form of Budd-Chiari
syndrome? J Comput Assist Tomogr. 32:518–522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shih KL, Yen HH, Su WW, Soon MS, Hsia CH
and Lin YM: Fulminant Budd-Chiari syndrome caused by renal cell
carcinoma with hepatic vein invasion: report of a case. Eur J
Gastroenterol Hepatol. 21:222–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amitrano L, Guardascione MA, Schiavone EM,
et al: Hepatic vein thrombosis leading to fulminant hepatic failure
in a case of acute non-promyelocytic myelogenous leukemia. Blood
Coagul Fibrinolysis. 17:59–61. 2006. View Article : Google Scholar : PubMed/NCBI
|