Introduction
Citrobacter rodentium (C. rodentium)
is a predominantly mucosal enteric murine pathogen used in murine
models of enteropathogenic Escherichia coli (E. Coli)
and enterohemorrhagic E. coli infection in humans (1–3).
C. rodentium infection in the colon surface results in
epithelial hyperplasia and mucosal inflammation (4) and its colonization of the colon
reaches its peak 7–10 days post inoculation (DPI) (5), followed by weight loss and diarrhea
in association with crypt hyperplasia in mice and returns to normal
by 14–25 DPI (6). It is also well
established that C. rodentium infection induces significant
mucosal infiltration of lymphocytes, macrophages, neutrophils and
mast cells in the colon (7),
goblet cell loss (2) and local and
systemic inflammatory cytokines disorders (8,9).
Although innate cells that express Toll-like
receptors, including CD11b+ myeloid cells, are known to
play a major role in host defense against C. rodentium
infection (9–11), adaptive immune cells are also
involved in the eradication of C. rodentium infection
(12). The roles of B and T cells
with regard to protection against C. rodentium infection has
been previously characterized (13,14).
In the mice lacking antibodies production showed a higher
susceptibility to C. rodentium infection compared with the
wild-type mice. It is commonly accepted that T cells promote innate
immune responses mostly by providing pro-inflammatory cytokines,
however, in a previous study, T cells were observed to be involved
in B cell dependent C. rodentium antigen specific antibody
production and interacted with B cells directly (15). Pro-inflammatory cytokines,
including interleukin (IL)-17A, IL-21 and IL-17A-induced IL-22 were
shown to be crucial for the host defense against extracellular
bacteria (16) and these cytokines
were primarily secreted by Th17 subsets (17). In addition, it was previously shown
that segmented filamentous bacteria (SFB), another microorganism
that colonizes in the intestines, was sufficient to induce
functional Th17 cells in the lamina propria (18). However in the model of C.
rodentium infection, it was reported that IL-17A in this model
was produced by innate cell subsets, including innate lymphoid
tissue inducer cells (LTi cells), which was different from
intestinal Th17 cells. Whether C. rodentium infection in
mice increases CD4+ Th17 cell subset in the intestinal
tissue remains unclear.
To identify the functions of C. rodentium
infection in Th17 subset differentiation in the small intestine,
C. rodentium was inoculated into 5-week-old wild-type B6
mice and the percentage of Th17 cell subsets in various tissues was
observed. The results suggested that Th17 subset expression
specifically increased in Peyer’s patches (PP) but did not alter in
mesenteric draining lymph nodes. The mechanistic studies suggested
that the inflammatory cytokine IL-6 was required in Th17 cell
differentiation in PP as treatment with anti-IL-6 neutralizing
antibodies reduced the Th17 subset percentage and aggravated the
clinical manifestation of colitis. Furthermore, these intestinal
Th17 cells were functional in innate responses to C.
rodentium infection by inducing IL-22 production but not in
promoting immunoglobulin A (IgA) production in the small
intestine.
Materials and methods
Reagents and mice
C. rodentium strain DBS100 (catalog no.
51459) American Type Culture Collection (Manassas, VA, USA) was
cultured in LB medium for 6 h at 37°C with agitation. Following 6
h, the bacterial density was assessed using absorbance at an
optical density of 600 nm and confirmed by the plating of serial
dilutions.
Five-week-old mice were orally inoculated with
1×109 colony forming units (cfu) of C. rodentium
using a gavage needle. The body weights and the bacterial
concentrations in the feces were assessed for 3 weeks subsequent to
inoculation. PP and small intestine tissues were collected for
quantitative polymerase chain reaction (PCR) and FACS analysis.
Bacterial quantification
Fresh fecal pellets were collected from the mice and
dissolved in phosphate-buffered saline (PBS) at a concentration of
100 mg/ml. The bacterial colonies were quantified 24 h following
the start of the culture.
Serum samples
Whole blood was collected from mice infected with
C. rodentium. Serum samples were obtained by centrifugation
at 16,000 × g for 10 min and stored at −20°C until use in
subsequent experiments. Serum total IgA was detected using a mouse
total IgA ELISA kit (Tiangen, Beijing, China).
Intracellular cytokine staining
The intracellular expression of IL-17-producing T
cells was analyzed using a Cytofix/Cytoperm kit Plus (with
GolgiStop; BD Biosciences, San Jose, CA, USA), according to the
manufacturer’s instructions. In brief, lymphocytes obtained from
spleens, MLNs or PP were incubated with 50 ng/ml PMA, 5 μM calcium
ionophore A23187 (both from Sigma-Aldrich, St. Louis, MO, USA) and
GolgiStop at 37°C for 4 h. Surface staining was performed with
anti-CD4-PerCP/Cy5.5 (BioLegend, San Diego, CA, USA) for 20 min at
4°C, the cells were permeabilized with Cytofix/Cytoperm solution
for 20 min at 4°C and intracellular cytokine staining was performed
with anti-IL-17A-Alexa Fluor 647 (BD Biosciences).
Flow cytometry
The Abs used for flow cytometry included
anti-CD4-PerCP/Cy5.5, anti-TCRb-FITC (BioLegend) and anti-IL-17A-PE
(eBioscience, San Diego, CA, USA), according to the manufacturer’s
instructions. Data were obtained using a FACS LSR II (BD
Biosciences) and analyzed using FlowJo software (Tree Star, Inc.,
Ashland, OR, USA).
Quantitative PCR
Total RNA was extracted with TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Reverse transcription was performed
with a High Capacity cDNA Reverse Transcription kit (Applied
Biosystems, Tokyo, Japan) according to the manufacturer’s
instructions. Quantitative PCR was performed with SYBR-Green PCR
Master Mix (Applied Biosystems) according to the manufacturer’s
instructions. The reaction conditions consisted of 40 cycles of
two-stage PCR consisting of denaturation at 95°C for 15 sec and
annealing at 60°C for 1 min following an initial denaturation step
of 95°C for 10 min. The primer sequences used were: mouse
interferon-γ (IFN-γ), forward: 5′-TCAAGTGGCATAGATGTGGAAGAA-3′ and
reverse: 5′-TGGCTCTGCAGGATTTTCATG-3′; IL-17A, forward:
5′-ATCAGGACGCGCAAACATG-3′ and reverse: 5′-TGATCGCTGCTGCCTTCAC-3′;
and mouse β-actin, forward: 5′-AGAGGGAAATCGTGCGTGAC-3′ and reverse:
5′-CAATAGTGATGACCTGGCCGT-3′. To allow comparisons of mRNA
expression levels, the real-time PCR data were analyzed with the
ΔΔCt method and normalized to the amount of β-actin cDNA as an
endogenous control.
Fecal IgA
Fresh fecal pellets were collected from the mice and
dissolved in PBS at a concentration of 100 mg/ml. Fecal IgA was
examined with a mouse IgA-detecting kit (Tiangen).
Statistical analysis
Data are expressed as mean ± SE and statistical
significance was analyzed using the Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
C. rodentium infection induces
inflammatory bowel disease (IBD) in mice
C. rodentium infection induced IBD in mice is
a commonly used animal model to study innate cell responses and
inflammatory signaling in intestinal inflammation in vivo.
To examine the importance of T cells in IBD caused by C.
rodentium, wild-type female B6 mice of various ages were
infected with C. rodentium at 4×109 cfu/mouse. A
survival curve, body weight loss and clinical manifestation of
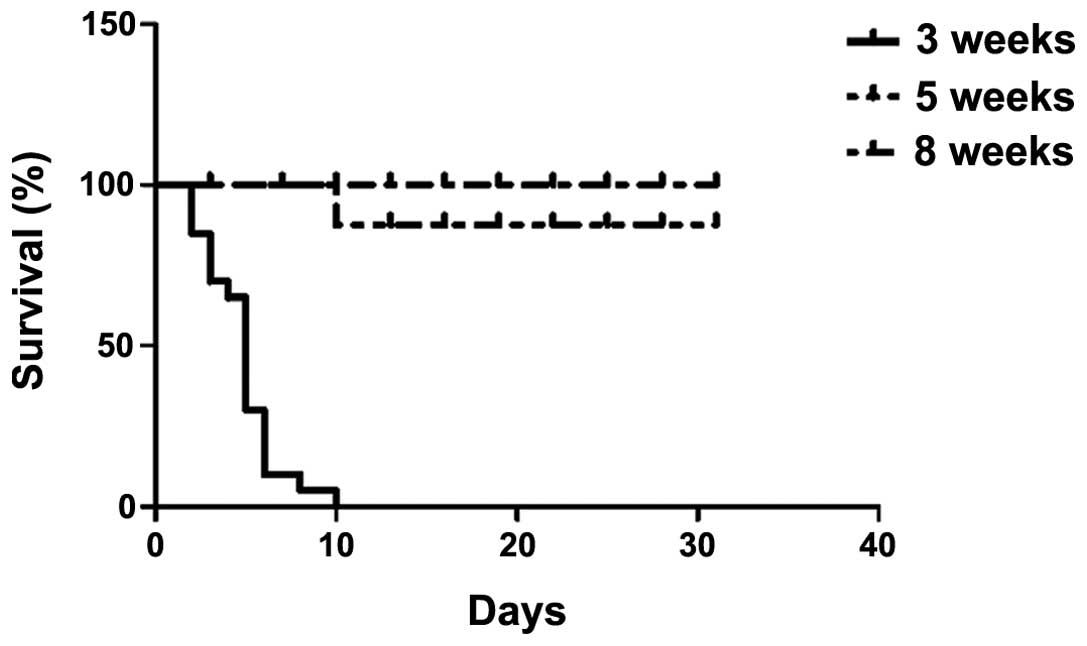
colitis were monitored during a course of 3 weeks following
infection and young mice (3 weeks) were observed to be highly
susceptible to C. rodentium infection as they cannot survive
colitis infection (Fig. 1). C.
rodentium was capable of inducing IBD in the mice between 5 and
8 weeks old, however, the older mice (8 weeks) exhibited less
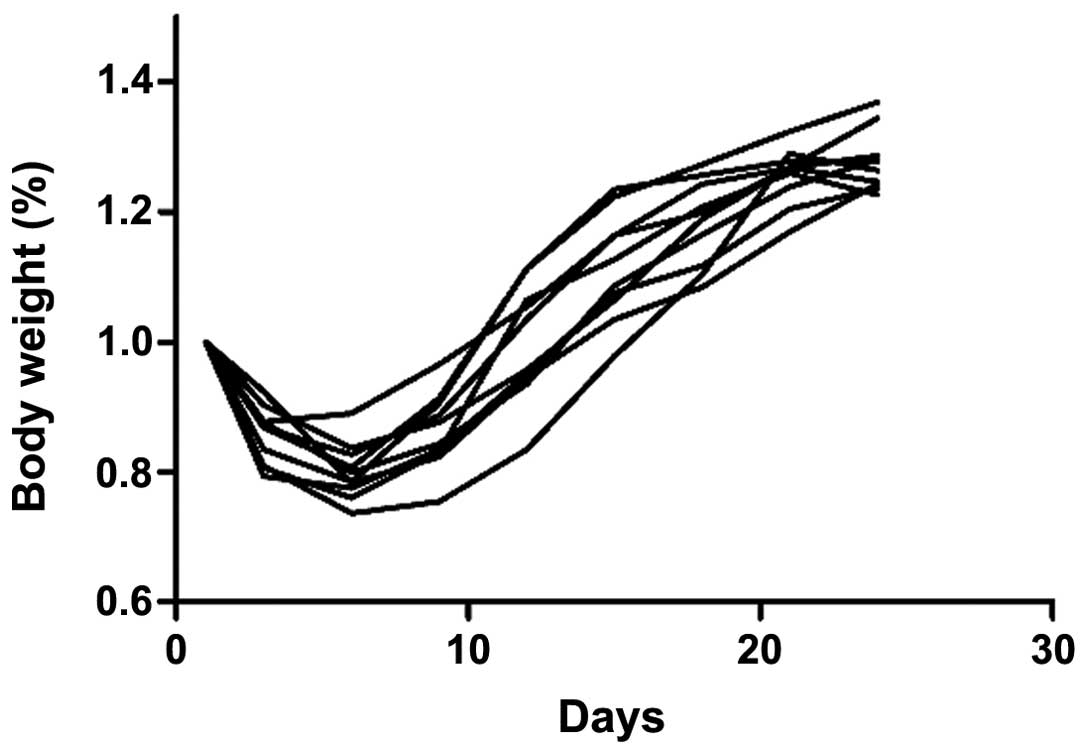
susceptibility compared with the 5-week-old mice (Fig. 2), as they lost significantly less
body weight (Fig. 3).
Considering that innate immune cells were immature
in newborn mice and the newborn mice did not have sufficient
amounts of IgA antibody secretion on the surface of the intestine,
which was significant in the host against microbe infection
(14), female mice of 5 weeks age
were selected for the present study. The body weight continued to
decrease in the first 10 days following C. rodentium
infection and visible colonic inflammation, including diarrhea and
loss of appetite was observed. Loss of appetite was observed in the
first 1 week following C. rodentium infection. Bacterial
counts in fecal pellets showed that the amount of C.
rodentium reached the peak at day 7 and returned to normal 2
weeks following infection (Fig.
4). However, there was no significant difference observed
between the male and female groups (data not shown).
C. rodentium infection increased the Th17
subset specifically in PP
The pro-inflammatory cytokine IL-17A is critical for
host response to the invasion of the immune system by extracellular
pathogens (19,21) by increasing chemokine production in
various tissues to recruit innate cells, including monocytes and
neutrophils to the site of local infection. In the model of C.
rodentium infection, it was reported that C.
rodentium-induced colitis triggers strong IL-17A secretion in
the small intestine in mice (17).
Recent studies suggested that IL-17A was primarily secreted by LTi
cells and ‘innate’ Th17 cells (16,20).
However, whether C. rodentium infection was capable of
increasing the classic Th17 subset in vivo remains
unclear.
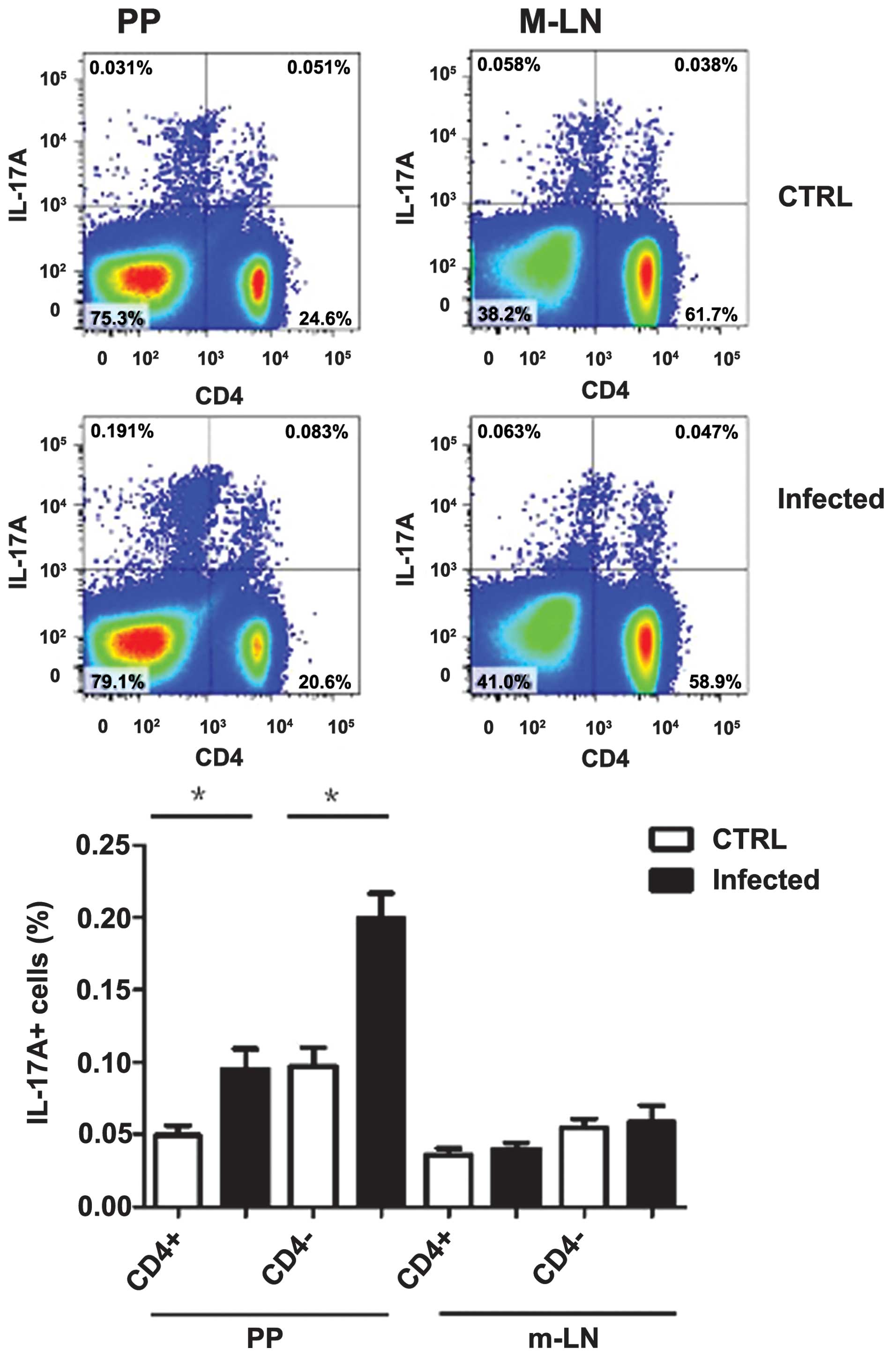
To confirm intestinal Th17 differentiation in
colitis, 5-week-old female B6 wild-type mice were infected with
C. rodentium (4×109 cfu/mouse) or inactivated
C. rodentium as the control group. Mice were sacrificed 7
days following infection and Th17 cells group were examined using
intercellular staining of the cytokine IL-17A. IL-17A producing
CD4+ T cells group were observed to increase in PP, but
no significant change in mesenteric draining lymph nodes was
observed. Notably, the IL-17-producing non-T cell-group
(TCRb-CD4−) also increased in PP and did not change in
the m-LN group (Fig. 5) and these
innate cells produced increased IL-17A compared with
CD4+ Th17 cells, which was consistent with a previous
report (16). However, the innate
cells cannot protect the host from C. rodentium infection,
as it was observed that these mice lack T cells and with functional
innate cells, including Rag1 deficient mice, exhibited a higher
susceptibility to IBD compared with the wild-type mice. In
addition, the mice with colitis also exhibited increased PP and
shorter length of the small intestine when compared with the
control group (data not shown).
IL-6 is required in intestinal Th17
differentiation in C. rodentium-induced colitis model
The IL-6 cytokine was required in Th17
differentiation and activation in vitro and in vivo.
Early induction of IL-6 secretion was critical in the host response
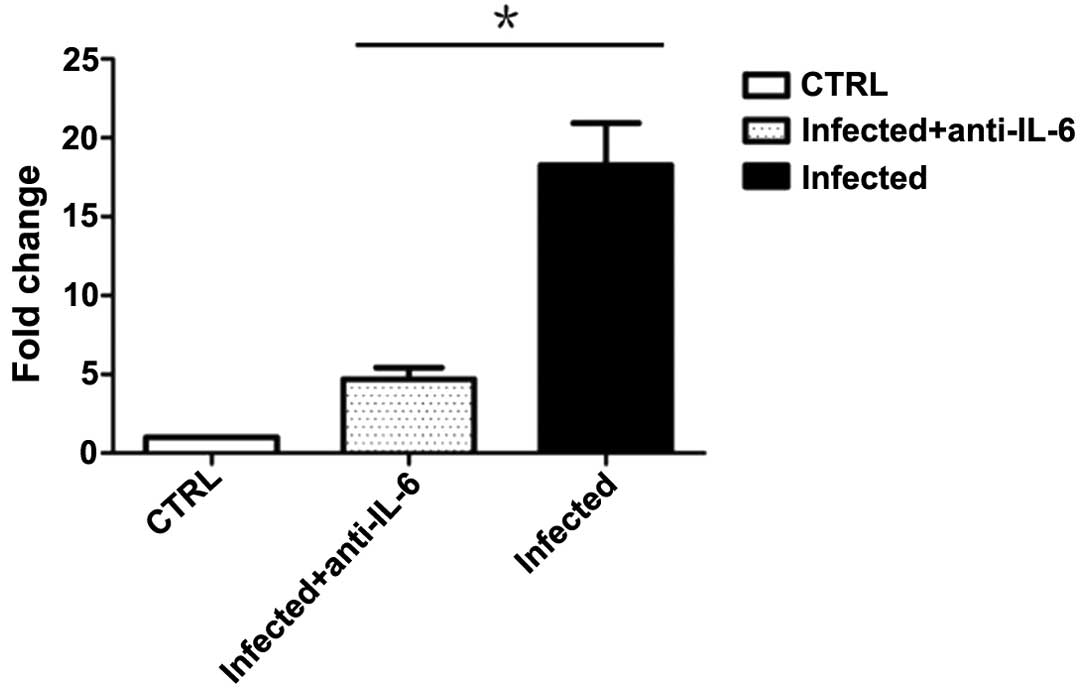
to enteric microbial infection. C. rodentium-infected female
B6 wild-type mice were treated with anti-IL-6 neutralizing
antibodies or control IgG to block IL-6 signaling 0 and 3 days
following infection. These mice were sacrificed 7 days following
infection and cells from PP were collected for intercellular
cytokine analysis. The results suggest that treatment with
anti-IL-6 neutralizing antibodies was capable of decreasing
IL-17A-producing CD4+ T cells in PP (Fig. 6). However, the percentage of Th17
cells in PP remained higher than that in mice treated with
inactivated C. rodentium. Similarly, anti-IL-6 neutralizing
antibody-treated mice exhibited more severe clinical symptoms
compared with the control IgG-treated group. However, results of
the statistical analysis of clinical scores and length of the small
intestine revealed no significant difference (data not shown).
IL-17A production in non-T cells was investigated in
this model and no significant difference was observed between
anti-IL-6 neutralizing treatment and the control group, which
suggesting that IL-6 was not required in the activation of
IL-17A-producing innate cells.
Intestinal Th17 cells promotes IL-22 and
IgA production in the host defense against C. rodentium
infection
IL-22 is a multi-functional cytokine with diverse
biological activities, including tissue repair and pathogen
defense in the model of IBD in mice. In addition, it was previously
reported that IgA production by B cells was dependent on T-helper
cells (15). To determine whether
these increasing intestinal Th17 cells were involved in colitis,
mRNA expression levels of IL-22 were examined in the small
intestine tissue and IgA in the feces in anti-IL-6 neutralizing
antibodies or IgG control-treated mice 7 days following C.
rodentium infection. Results suggest that anti-IL-6
neutralizing antibody-treated mice exhibited a lower level of IL-22
expression in the small intestine (Fig. 7), however, the IL-22 protein was
not detected in the small intestine tissue lysis and intestinal
lavage fluid (data not shown). There was no significant difference
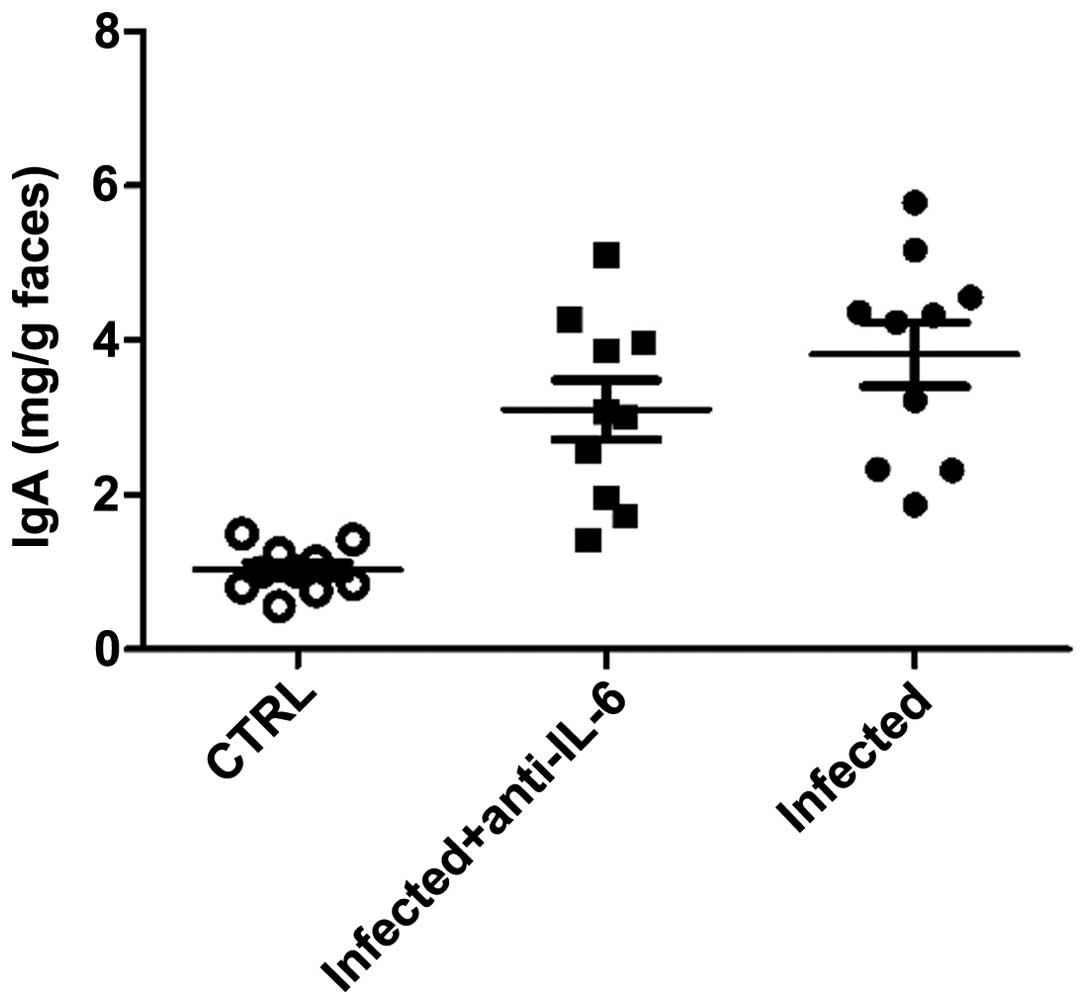
in IgA levels in feces between anti-IL-6 neutralizing
antibody-treated mice and the control group (Fig. 8), suggesting that intestinal Th17
cells was involved in the defense against C. rodentium
infection by promoting IL-22 expression in the small intestine.
Discussion
A Th17 cell subset was identified in multiple
auto-immune disease models in mice and humans, however, the
differentiation and activation of these cells remains unclear. It
was reported that the Th17 subset in intestinal lamina propria was
dependent on commensal microbes with specialized properties since,
in germ-free (GC) mice, Th17 cells failed to develop (15,22).
In addition, in the study of SFB mono-colonized mice, SFB was shown
to sufficiently direct the accumulation of Th17 cells in the
intestinal lamina propria by unknown mechanisms.
C. rodentium is a predominantly mucosal
enteric murine pathogen that is widely used in murine models of
intestinal colitis. These models are primarily mediated by
pro-inflammatory cytokine IL-17A. It is commonly accepted that
LTi-associated innate cells and γΔT cells are the major sources in
C. rodentium-infected Rag1-deficient mice (23,24),
however, Th17 cells and their roles in C. rodentium-infected
wild-type mice may be different from Rag1-deficient mice.
Elucidating the sources of IL-17A and the differentiation and
activation of Th17 cells in intestine is essential for
understanding the etiology of IBD.
In the current study, IL-17A-producing Th17 cell
subsets in C. rodentium infection-induced colitis model were
investigated and the Th17 cell subset was observed to increase
specifically in PP. Although IL-17A are produced by a number of
types of cells, including intestinal endothelium cells and
CD4+ lymphoid tissue inducer cells, Th17 cells were
hypothesized to play a major role in IBD as decreasing Th17
percentage in PP led to high susceptibility to C. rodentium
infection in mice. Notably, the innate cells secreted increased
IL-17A compared with Th17 cells in this model, but were unable to
protect the host from the disease.
The inflammatory cytokine IL-6 is required in Th17
cell differentiation and activation in vitro and a large
amount of IL-6 secretion may be detected in intestinal tissues.
Therefore, IL-6 was hypothesized to be required in C.
rodentium infection-induced colitis model. In the current
model, treatment with anti-IL-6 neutralizing antibodies in C.
rodentium-infected mice were efficient to decrease the Th17
subset in PP and aggravate the symptoms of the disease.
Furthermore, the Th17 cells were considered to have a physical
function in inducing IL-22 secretion in intestinal tissue according
to the quantitative PCR data, however, IL-22 protein was not
observed in the intestinal surface and feces. The results also
suggested that Th17 cells had no effect on IgA production by B
cells, although it was recently reported that Th17 cells in PP was
responsible for the induction of T cell-dependent IgA (25).
Acknowledgements
This study was supported by a Youth Innovation Fund
of the First Affiliated Hospital of Zhengzhou University and grants
from the National Grand Program on Key Infectious Disease (no.
2008ZX1002-005-6) and the National Natural Science Foundation of
China (no. 81141106).
References
|
1
|
Mundy R, MacDonald TT, Dougan G, Frankel G
and Wiles S: Citrobacter rodentium of mice and man. Cell
Microbiol. 7:1697–1706. 2005. View Article : Google Scholar
|
|
2
|
Luperchio SA and Schauer DB: Molecular
pathogenesis of Citrobacter rodentium and transmissible
murine colonic hyperplasia. Microbes Infect. 3:333–340. 2001.
|
|
3
|
Borenshtein D, McBee ME and Schauer DB:
Utility of the Citrobacter rodentium infection model in
laboratory mice. Curr Opin Gastroenterol. 24:32–37. 2008.
|
|
4
|
Kim YG, Kamada N, Shaw MH, Warner N, Chen
GY, Franchi L and Núñez G: The Nod2 sensor promotes intestinal
pathogen eradication via the chemokine CCL2-dependent recruitment
of inflammatory monocytes. Immunity. 34:769–780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson E and Barthold SW: The
ultrastructure of transmissible murine colonic hyperplasia. Am J
Pathol. 97:291–313. 1979.PubMed/NCBI
|
|
6
|
Morgan ET, Goralski KB, Piquette-Miller M,
et al: Regulation of drug-metabolizing enzymes and transporters in
infection, inflammation, and cancer. Drug Metab Dispos. 36:205–216.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neurath MF, Weigmann B, Finotto S, et al:
The transcription factor T-bet regulates mucosal T cell activation
in experimental colitis and Crohn’s disease. J Exp Med.
195:1129–1143. 2002.PubMed/NCBI
|
|
8
|
Nenci A, Becker C, Wullaert A, et al:
Epithelial NEMO links innate immunity to chronic intestinal
inflammation. Nature. 446:557–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gibson DL, Ma C, Bergstrom KS, Huang JT,
Man C and Vallance BA: MyD88 signalling plays a critical role in
host defence by controlling pathogen burden and promoting
epithelial cell homeostasis during Citrobacter
rodentium-induced colitis. Cell Microbiol. 10:618–631. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gibson DL, Ma C, Rosenberger CM, et al:
Toll-like receptor 2 plays a critical role in maintaining mucosal
integrity during Citrobacter rodentium-induced colitis. Cell
Microbiol. 10:388–403. 2008.PubMed/NCBI
|
|
11
|
Lebeis SL, Bommarius B, Parkos CA, Sherman
MA and Kalman D: TLR signaling mediated by MyD88 is required for a
protective innate immune response by neutrophils to Citrobacter
rodentium. J Immunol. 179:566–577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eckmann L: Animal models of inflammatory
bowel disease: lessons from enteric infections. Ann N Y Acad Sci.
1072:28–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vallance BA, Deng W, Knodler LA and Finlay
BB: Mice lacking T and B lymphocytes develop transient colitis and
crypt hyperplasia yet suffer impaired bacterial clearance during
Citrobacter rodentium infection. Infect Immun. 70:2070–2081.
2002. View Article : Google Scholar
|
|
14
|
Shiomi H, Masuda A, Nishiumi S, et al:
Gamma interferon produced by antigen-specific CD4+ T
cells regulates the mucosal immune responses to Citrobacter
rodentium infection. Infect Immun. 78:2653–2666. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bry L and Brenner MB: Critical role of T
cell-dependent serum antibody, but not the gut-associated lymphoid
tissue, for surviving acute mucosal infection with Citrobacter
rodentium, an attaching and effacing pathogen. J Immunol.
172:433–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sonnenberg GF, Monticelli LA, Elloso MM,
Fouser LA and Artis D: CD4(+) lymphoid tissue-inducer cells promote
innate immunity in the gut. Immunity. 34:122–134. 2011.
|
|
17
|
Ishigame H, Kakuta S, Nagai T, et al:
Differential roles of interleukin-17A and -17F in host defense
against mucoepithelial bacterial infection and allergic responses.
Immunity. 30:108–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ivanov II, Atarashi K, Manel N, et al:
Induction of intestinal Th17 cells by segmented filamentous
bacteria. Cell. 139:485–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiricozzi A, Guttman-Yassky E,
Suarez-Fariñas M, et al: Integrative responses to IL-17 and TNF-α
in human keratinocytes account for key inflammatory pathogenic
circuits in psoriasis. J Invest Dermatol. 131:677–687. 2011.
|
|
20
|
Geddes K, Rubino SJ, Magalhaes JG, et al:
Identification of an innate T helper type 17 response to intestinal
bacterial pathogens. Nat Med. 17:837–844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miossec P, Korn T and Kuchroo VK:
Interleukin-17 and type 17 helper T cells. N Engl J Med.
361:888–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O’Dell JR, Elliott JR, Mallek JA, et al:
Treatment of early seropositive rheumatoid arthritis: doxycycline
plus methotrexate versus methotrexate alone. Arthritis Rheum.
54:621–627. 2006.PubMed/NCBI
|
|
23
|
Sartor RB: Microbial influences in
inflammatory bowel diseases. Gastroenterology. 134:577–594. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buonocore S, Ahern PP, Uhlig HH, Ivanov
II, Littman DR, Maloy KJ and Powrie F: Innate lymphoid cells drive
interleukin-23-dependent innate intestinal pathology. Nature.
464:1371–1375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirota K, Turner JE, Villa M, Duarte JH,
Demengeot J, Steinmetz OM and Stockinger B: Plasticity of TH17
cells in Peyer’s patches is responsible for the induction of T
cell-dependent IgA responses. Nat Immunol. 14:372–379. 2013.
|