Introduction
Angiogenesis is the process by which novel blood
vessels form through the growth of existing blood vessels. It is a
complex process involving the proliferation, sprouting and
migration of endothelial cells, followed by pruning and remodeling
of the vascular network (1). The
endothelium is the main regulator of angiogenesis and is highly
responsive to factors in the extracellular environment. When the
balance of this physiological process is disturbed, it may result
in a variety of diseases, including cancer, thrombosis, diabetic
retinopathy and inflammatory disorders.
MicroRNAs (miRNAs) are a class of conserved
non-coding small RNAs, which mainly regulate gene expression
post-transcriptionally by targeting the 3′-untranslated regions
(3′UTRs) of mRNAs and participate in almost all cellular processes
(2). Recently, numerous studies
have demonstrated that miRNAs are also important in regulating
angiogenesis (3–5). Firstly, it was demonstrated that the
miRNA Dicer was important in angiogenesis. The results showed that
knocking down Dicer led to the formation of severely compromised
embryos and yolk sacs (6). A
further study demonstrated that miRNA participates in angiogenesis
in succession. Through the detection of miRNA expression levels in
human umbilical vein endothelial cells (HUVECs), it was revealed
that miR-21, miR-126, miR-221/222, the let-7 family, the miR-17~92
cluster and the miRNA-23~24 cluster were highly expressed in
endothelial cells (Ecs) (7–11).
Excluding the effects on the phenotype and angiogenesis of ECs, the
abnormal expression of certain miRNAs also correlated with tumor
angiogenesis, including the miR-17~92 cluster, miR-210, miR-221/222
and miR-296 (6,7,8,9,11–14).
The findings suggest that miRNA expression is
required for angiogenesis. At present, although certain miRNAs that
affect angiogenesis have been identified, the specific role of
miRNAs in angiogenesis remains to be elucidated.
In the present study, it was demonstrated that
overexpressing or inhibiting miR-137 was able to regulate the
viability and migration of HUVECs by targeting the ephrin type-A
receptor 7 (EPHA7). This finding may provide a greater
understanding of the process of angiogenesis.
Materials and methods
Cell culture and transfection
HUVECs were isolated following Nature Protocols
(15). Cells were propagated and
maintained in minimal essential medium (MEM)-α (Invitrogen Life
Technologies, Carlsbad, CA, USA) supplemented with 20% fetal bovine
serum (FBS) and antibiotics [1% penicillin (10,000
U/ml)/streptomycin (10 mg/ml) (Gibco, Milan, Italy] in a humidified
atmosphere at 37°C with 5% CO2. Plasmids or antisense
oligonucleotides (ASO) were transfected in antibiotic-free Opti-MEM
medium (Invitrogen Life Technologies) with lipofectamine™ 2000
reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions.
Vector construction
The miR-137 expression plasmid pcDNA3/pri-miR-137
was constructed. The DNA fragment carrying pre-miR-137 was
amplified from genomic DNA by PCR using the following specific
primers: miR-137-S, 5′-CGCGGATCCAGCAAGAGTTCTGGTGGC-3′; miR-137-AS,
5′-CCGGAATTCACACCCGAGGA AATGAAAAG-3′. The fragment was then cloned
into pcDNA3 between BamHI and EcoRI restriction sites.
To construct the EPHA7 expression vector, the coding
sequence of EPHA7 without the 3′UTR was obtained by PCR using the
following primers: EPHA7, forward
5′-CGGGATCCTACCCTAGAAGGAAGAGGTG-3′ and reverse
5′-CGGAATTCAATTCTGGGGTAGTTCATG-3′. The 3′UTR of EPHA7 containing
the miR-137 binding site was cloned into pcDNA3/enhanced green
fluorescent protein (EGFP). Similarly, the fragment of the EPHA7
3′UTR mutant, which contained a triple point mutation in the
miR-137 binding site, was also cloned into the pcDNA3/EGFP.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The MTT assay was used to determine relative cell
viability. Cell viability was detected 48 h after transfection in
96-well plates. The absorbance at 570 nm was detected using an
IQuant Universal Microplate Spectrophotometer (BioTek Instruments,
Inc., Winooski, VT, USA).
Migration assay
In the transwell assays, HUVECs were transfected
with pcDNA3/pri-miR-137, a control vector, miR-137-antisense
oligonucleotide (ASO) or control ASO, respectively. Following 48 h,
4×104 cells suspended in serum-free medium were plated
onto a gelatin-coated, 8.0-μm pore size polycarbonate membrane in
24-well plates (Corning Inc., Corning, NY, USA). The lower chamber
contained MEM-α medium with 20% FBS and 20 ng/ml vascular
endothelial growth factor (VEGF). Following 6 and 18 h, the
migrated cells were fixed with a crystal violet stain and images
were captured for counting.
In the scratch assay, once the cells reached 100%
confluence and formed a monolayer, a 200-μl pipette tip was used to
create a scratch on the cell monolayer. The plate was washed once
with 2% phosphate-buffered saline and replaced with 2% FBS medium.
Migration was quantified by measuring the cell recovery area.
Fluorescent reporter assays
pcDNA3/pri-miR-137, a control vector, miR-137-ASO
and control ASO were transfected into HUVECs in 48-well plates,
respectively and then with the reporter vector
pcDNA3/EGFP-EPHA7-UTR or pcDNA3/EGFP-EPHA7-MUT the following day.
The vector pDsRed2-N1 (Clontech Laboratories, Mountain View, CA,
USA), expressing red fluorescent protein (RFP), was spiked in and
used for normalization. Following 72 h, the cells were lysed with
RIPA lysis buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.2; 1% Triton
X-100 and 0.1% SDS). The intensities of EGFP and RFP fluorescence
were detected using a Fluorescence Spectrophotometer F-4500
(Hitachi, Tokyo, Japan).
Western blot analysis
Proteins from transfected HUVECs were extracted 72 h
post-transfection using RIPA buffer and protein expression was
analyzed by western blot analysis. GAPDH served as a loading
control. The proteins were electrotransferred onto supported
nitrocellulose membranes (Amersham, Uppsala, Sweden) by a semi-dry
transfer. The membranes were blocked in 5% skimmed milk in
Tris-buffered saline with Tween 20 (TBS-T) containing 0.05% Tween
20 at room temperature for 2 h, and then incubated at room
temperature for 2 h with antibodies diluted in 1% skimmed milk in
TBS-T, followed by incubating with the appropriate horseradish
peroxidase-linked secondary antibodies. The following antibodies
were used: rabbit anti-EPHA7, rabbit anti-GAPDH and goat
anti-rabbit (Santa Cruz Biotechnology Inc., Santa Cruz, CA,
USA).
Gelatin sponge-chorioallantoic membrane
(CAM) assay
The chick embryo CAM formed on day 4 of incubation
by fusion of the chorion and the allantois. Since it mediates gas
exchanges with the extraembryonic environment until hatching, it
has a thick capillary network that forms a continuous surface in
direct contact with the shell. Rapid capillary proliferation
continues until day 12; the mitotic index then decreases just as
rapidly and the vascular system attains its final arrangement on
day 18, immediately prior to hatching (15).
Statistical analysis
All experiments were repeated a minimum of three
times, statistical significance was determined using Student’s
t-test. In all figures, values are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-137 decreases the viability of
HUVECs
In order to determine the effects of miR-137 on
angiogenesis, HUVECs were used as a model for in vitro
study. Plasmids expressing the miR-137 precursor
(pcDNA3/pri-miR-137) were constructed and the 2′-Ome miR-137 ASO
was synthesized. The efficiency of either overexpression or
suppression of miR-137 in HUVECs was validated by quantitative (q)
PCR. qPCR results demonstrated that transfection with
pcDNA3/pri-miR-137 resulted in a 60% increase in miR-137 levels in
HUVECs, whereas miR-137 2′-Ome ASO resulted in a 70% reduction in
miR-137 levels compared with the control group (Fig. 1A). Transfection efficiency was
>90%, as determined using the pDsRed2-N1 or Cy5-oligomer (data
not shown). To explore the effects of human miR-137 on the
viability of HUVECs, the MTT assay was used to determine whether
miR-137 affects cell viability. As shown in Fig. 1B, when the level of miR-137 was
overexpressed, the viability of HUVECs decreased ~40%, whereas when
miR-137 was blocked, the cell viability was increased ~30% compared
with the control.
miR-137 suppresses the migration of
HUVECs
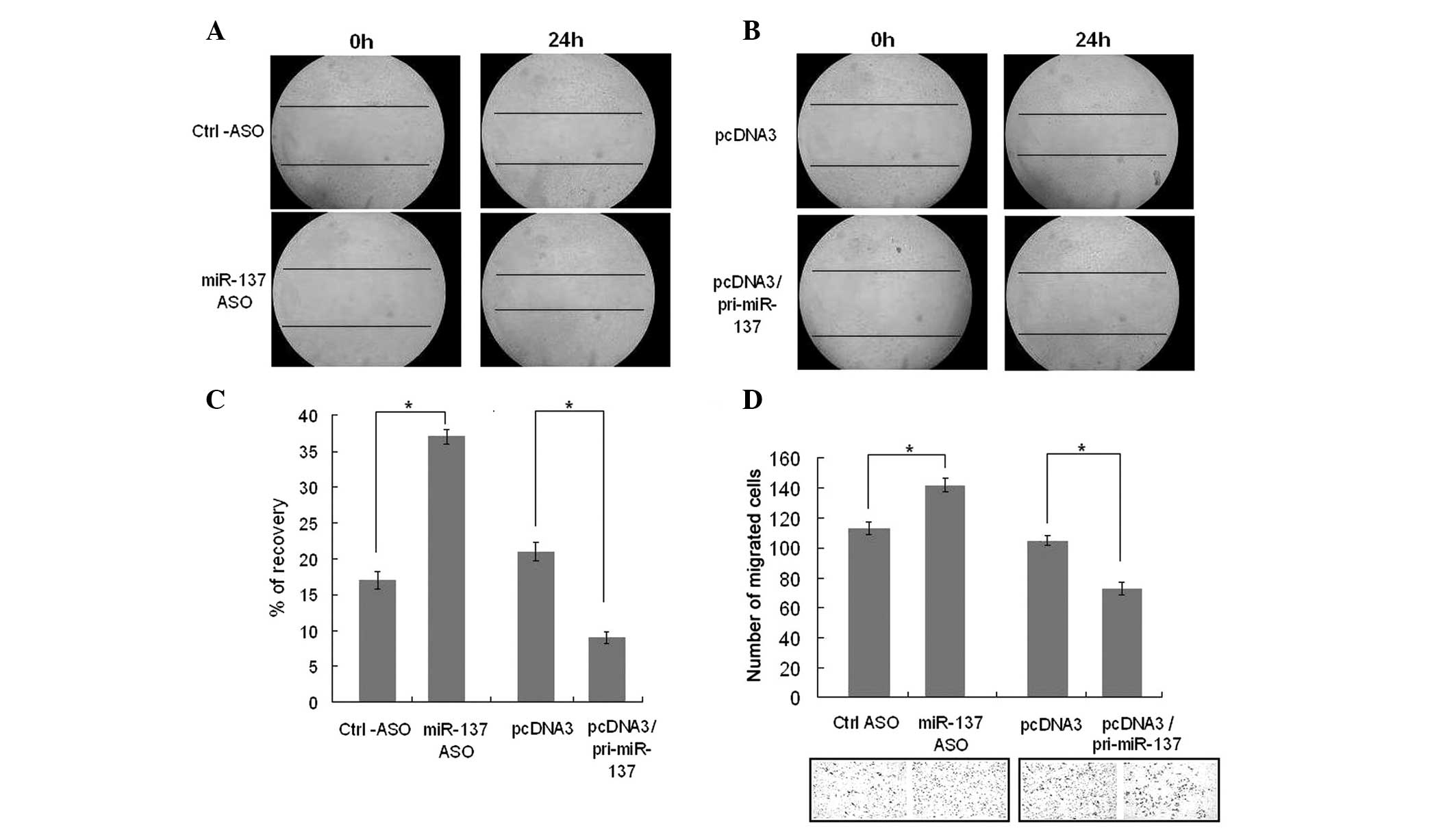
A transwell assay and scratch assay were used to
determine the effects of miR-137 on cell migration (Fig. 2). HUVECs were transfected with
pcDNA3/pri-miR-137, a control vector, miR-137-ASO or control ASO.
HUVECs were allowed to migrate through gelatin-coated filters 48 h
post-transfection in response to 20% FBS and 20 ng/ml VEGF for 6 or
18 h. When transfected with pcDNA3/pri-miR-137, cells were
significantly suppressed, while blocking miR-137 led to increased
cell migration (Fig. 2D). The same
results were demonstrated in the scratch assay (Fig. 2A and B). The average recovery
percentage of the wounded portion was measured and calculated. When
miR-137 was overexpressed, the recovery of the cell area was less
than that of the control and when miR-137 was knocked down, the
recovery of the cell area was greater than that of the control.
(Fig. 2C). These findings
indicated that miR-137 suppresses the migration of HUVECs.
EPHA7 is the target gene of miR-137
In the novel miRNA pathway, miRNA produces a marked
effect by negatively regulating its target gene. TargetScan was
used to predict the target gene of miR-137 and it was discovered
that EPHA7 has a putative miR-137 binding site in its 3′UTR
(Fig. 3A). To confirm whether
EPHA7 was the direct target gene of miR-137 and was negatively
regulated by it, a vector of the 3′UTR of EPHA7 was constructed and
was cloned into the pcDNA3 vector downstream with an EGFP gene. The
reporter vector or a pcDNA3/EGFP control vector was transfected
into HUVECs. The intensity of EGFP fluorescence in
reporter-vector-transfected cells was lower than that in the
control group demonstrating that endogenous miRNAs are able to
negatively regulate EGFP expression by targeting its 3′UTR
(Fig. 3B). HUVECs were then
transfected with the reporter vector along with the miR-137
expression vector or ASO. The results demonstrated that ectopic
expression of miR-137 was able to reduce the intensity of EGFP
fluorescence and blocking miR-137 was able to enhance EGFP
expression levels (Fig. 3C).
However, when the EPHA7 3′UTR of the miR-137 binding site has three
base mutations, the intensity of EGFP fluorescence was not
significantly changed by either overexpressing or blocking miR-137
(Fig. 3C). These results suggested
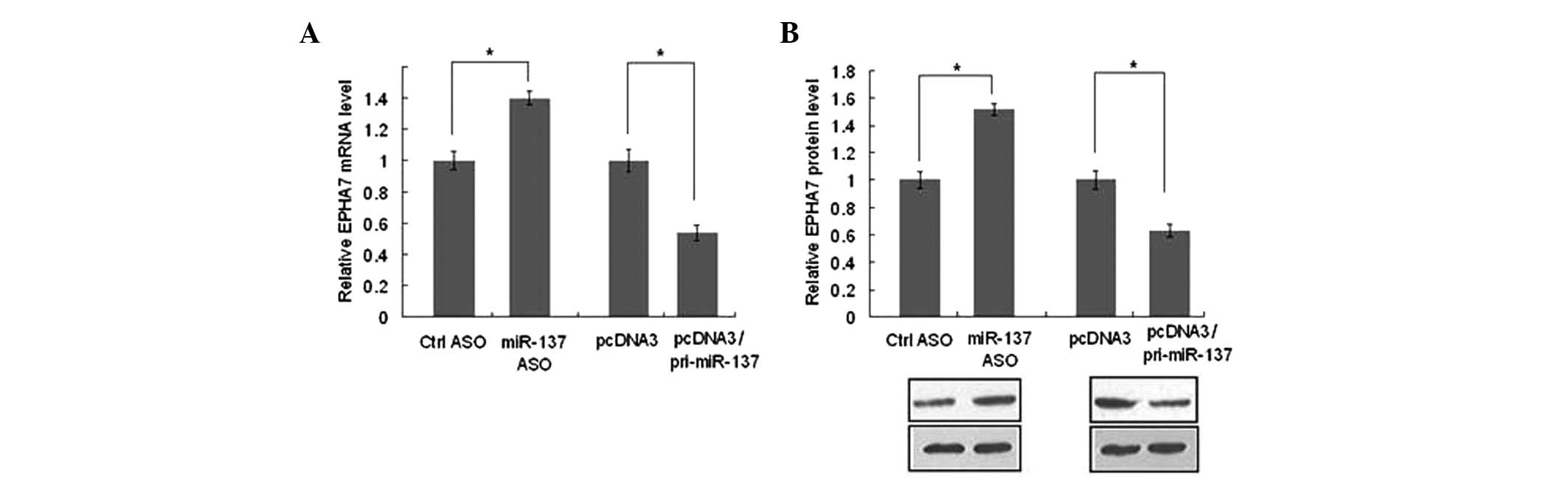
that EPHA7 was the direct target gene of miR-137. qPCR and western
blot analysis were used to further confirm how miR-137 regulates
EPHA7 gene expression. When miR-137 was overexpressed, compared
with the control groups, the levels of EPHA7 mRNA (Fig. 4A) and protein (Fig. 4B) decreased by ~35%. Conversely,
blocking miR-137 increased the mRNA (Fig. 4A) and protein (Fig. 4B) levels of EPHA7 by ~45%. These
results indicate that miR-137 is able to negatively regulate the
expression of EPHA7 at a post-transcriptional level, which
coincides with the results from the EGFP reporter assay.
 | Figure 3EPHA7 is the target gene of miR-137.
(A) As the TargetScan predicted, the EPHA7 3′UTR has a miR-137
binding site. The EPHA7 3′UTR mutant contains a mutant miR-137
binding site. The grey base underlined indicates the mutated
nucleotides. (B) HUVECs were transfected with pcDNA3/EGFP or
pcDNA3/EGFP-EPHA7-UTR. pDsRed2-N1 expressing RFP was also spiked in
for normalization. The fluorescence value in the control group was
set to 1. (C) HUVECs were transfected with either
pcDNA3/EGFP-EPHA7-UTR reporter vector or pcDNA3/EGFP-EPHA7-MUT
mutant vector, along with pcDNA3/pri-miR-137, control vector,
miR-137-ASO or control ASO as indicated. The fluorescence value in
the control group was set to 1. *P<0.05. HUVECs,
human umbilical vein endothelial cells; ASO, antisense
oligonucleotides; EPHA7, ephrin type-A receptor 7; 3′UTR, 3′
untranslated region; RFP, red fluorescent protein; EGFP, enhanced
green fluorescence protein; miR, microRNA. |
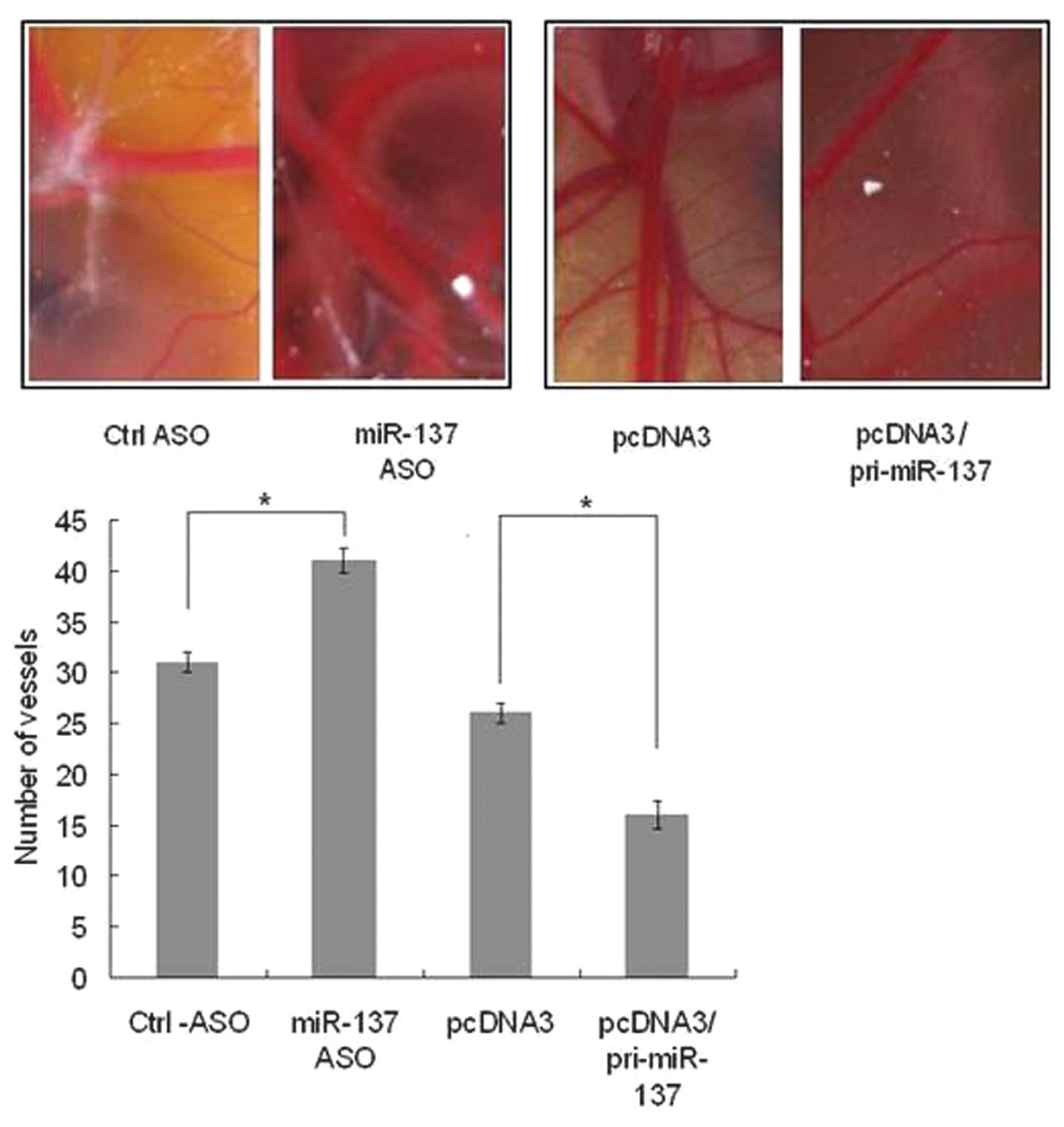
miR-137 inhibits angiogenesis in
vivo
The gelatin sponge-chorioallantoic membrane assay
was used in order to investigate the function of miR-137 in
angiogenesis. pcDNA3/pri-miR-137, a control vector, miR-137-ASO or
control ASO were transfected in the chick embryo chorioallantoic
membrane model according to Nature Protocols (15). Following 10 days, the number of
vessels were observed and counted. It was demonstrated that when
miR-137 was blocked, the vessels were more abundant than in the
control (Fig. 5A), whereas when
miR-137 was overexpressed, the vessels were less abundant than i
the control (Fig. 5B). This result
demonstrated that miR-137 is able to suppress angiogenesis in
vivo.
Discussion
Increasing evidence has indicated that miRNAs may be
important in physiological or pathological angiogenesis (3). In the present study, the role of
miR-137 in the angiogenic properties of HUVECs was demonstrated.
Knockdown of miR-137 was able to significantly increase cell
viability and migration, and ectopic expression of miR-137 was able
to decrease cell viability and migration. From these results, it
was shown that miR-137 is an important antiangiomiR in
angiogenesis. Next, EPHA7 was identified to be the direct target
gene of miR-137 that may participate in this process. EPHA7 belongs
to the Eph/ephrin signaling pathway, which is essential for the
patterning of multiple tissues and cell types, including vascular
endothelial cell assembly, cell migration, mesenchymal cell
condensation, vascular bed formation, tumor neovascularization and
the closure of the external genitalia (16–24).
EPHA7 is highly conserved in vertebrates and is widely expressed in
embryonic tissues, particularly in the developing central nervous
system (25). In the present
study, it was demonstrated that EPHA7 was negatively regulated by
miR-137, and that miR-137 by targeting EPHA7 regulated the
migration and angiogenesis of Ecs. It has been demonstrated that
miR-137 is able to act as a tumor suppressor in uveal melanoma cell
proliferation through the downregulation of MITF and CDK6 (26). Whether miR-137 is able to suppress
tumor angiogenesis and thus inhibit tumor growth by targeting EPHA7
remains uncertain and requires further investigation.
In conclusion, the results of the present study
indicated that miR-137 targets EPHA7 and regulates the behavior of
ECs in vitro and in vivo, including cell viability,
migration and angiogenesis. The identification of the antiangiomiR
miR-137 and its target gene EPHA7 in HUVECs may aid our
understanding of the molecular mechanisms underlying
angiogenesis.
References
|
1
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69:4–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eulalio A, Huntzinger E and Izaurralde E:
Getting to the root of miRNA-mediated gene silencing. Cell.
132:9–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suarez Y and Sessa WC: MicroRNAs as novel
regulators of angiogenesis. Circ Res. 104:442–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donnem T, Fenton CG, Lonvik K, Berg T,
Eklo K, Andersen S, Stenvold H, AI-Shibli K, AI-Saad S, Bremnes RM
and Busund LT: MicroRNA signatures in tumor tissue related to
angiogenesis in non-small cell lung cancer. Plos One. 7:e296712012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamasaki K, Nakasa T, Miyaki S, Yamasaki
T, Yasunaqa Y and Ochi M: Angiogenic microRNA-210 is present in
cells surrounding osteonecrosis. J Orthop Res. 30:1263–1270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang WJ, Yang DD, Na S, Sandusky GE, Zhang
Q and Zhao G: Dicer is required for embryonic angiogenesis during
mouse development. J Biol Chem. 280:9330–9335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuehbacher A, Urbich C, Zeiher AM and
Dimmeler S: Role of Dicer and Drosha for endothelial microRNA
expression and angiogenesis. Circ Res. 101:59–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suárez Y, Fernández-Hernando C, Pober JS
and Sessa WC: Dicer dependent microRNAs regulate gene expression
and functions in human endothelial cells. Circ Res. 100:1164–1173.
2007.PubMed/NCBI
|
|
9
|
Fasanaro P, D’Alessandra Y, Di Stefano V,
Melchionna R, Romani S, Pompilio G, Capogrossi MC and Martelli F:
MicroRNA-210 modulates endothelial cell response to hypoxia and
inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol
Chem. 283:15878–15883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harris TA, Yamakuchi M, Ferlito M, Mendell
JT and Lowenstein CJ: MicroRNA-126 regulates endothelial expression
of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA.
105:1516–1521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poliseno L, Tuccoli A, Mariani L,
Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S and
Rainaldi G: MicroRNAs modulate the angiogenic properties of HUVECs.
Blood. 108:3068–3071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Würdinger T, Tannous BA, Saydam O, Skog J,
Grau S, Soutschek J, Weissleder R, Breakefield XO and Krichevsky
AM: miR-296 regulates growth factor receptor overexpression in
angiogenic endothelial cells. Cancer Cell. 14:382–393.
2008.PubMed/NCBI
|
|
13
|
le Sage C, Nagel R, Egan DA, Schrier M,
Mesman E, Mangiola A, Anile C, Maira G, Mercatelli N, Ciafrè SA,
Farace MG and Agami R: Regulation of the p27(Kip1) tumor suppressor
by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J.
26:3699–3708. 2007.PubMed/NCBI
|
|
14
|
Bonauer A, Carmona G, Iwasaki M, et al:
MicroRNA-92a controls angiogenesis and functional recovery of
ischemic tissues in mice. Science. 324:1710–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ribatti D, Nico B, Vacca A and Presta M:
The gelatin sponge-chorioallantoic membrane assay. Nat Protoc.
1:85–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang HU, Chen ZF and Anderson DJ:
Molecular distinction and angiogenic interaction between embryonic
arteries and veins revealed by ephrin-B2 and its receptor Eph-B4.
Cell. 93:741–753. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogawa K, Pasqualini R, Lindberg RA, Kain,
Freeman AL and Pasquale EB: The ephrin-A1 ligand and its receptor,
EphA2, are expressed during tumor neovascularization. Oncogene.
19:6043–6052. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stadler HS, Higgins KM and Capecchi MR:
Loss of Eph-receptor expression correlates with loss of cell
adhesion and chondrogenic capacity in Hoxa13 mutant limbs.
Development. 128:4177–4188. 2001.PubMed/NCBI
|
|
19
|
Chan J, Mably JD, Serluca FC, Chen JN,
Goldstein NB, Thomas MC, Cleary JA, Brennan C, Fishman MC and
Roberts TM: Morphogenesis of prechordal plate and notochord
requires intact Eph/ephrinB signaling. Dev Bio. 234:470–482. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dravis C, Yokoyama N, Chumley MJ, Cowan
CA, Silvany RE, Shay J, Baker LA and Henkemeyer M: Bidirectional
signaling mediated by ephrin-B2 and EphB2 controls urorectal
development. Dev Biol. 271:272–290. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davy A, Aubin J and Soriano P: Ephrin-B1
forward and reverse signaling are required during mouse
development. Genes Dev. 18:572–583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marquardt T, Shirasaki R, Ghosh S, Andrews
SE, Carter N, Hunter T and Pfaff SL: Coexpressed EphA receptors and
ephrin-A ligands mediate opposing actions on growth cone navigation
from distinct membrane domains. Cell. 121:127–139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Egea J, Nissen UV, Dufour A, Sahin M,
Greer P, Kullander K, Mrsic-Flogel TD, Greenberg ME, Kiehn O,
Vanderhaeghen P and Klein R: Regulation of EphA 4 kinase activity
is required for a subset of axon guidance decisions suggesting a
key role for receptor clustering in Eph function. Neuron.
47:515–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taneja R, Thisse B, Rijli FM, Thisse C,
Bouillet P and Chambon P: The expression pattern of the mouse
receptor tyrosine kinase gene MDK1 is conserved through evolution
and requires Hoxa-2 for rhombomere-specific expression in mouse
embryos. Dev Biol. 177:397–412. 1996. View Article : Google Scholar
|
|
25
|
Ciossek T, Millauer B and Ulrich A:
Identification of alternatively spliced mRNAs encoding variants of
MDK1, a novel receptor tyrosine kinase expressed in the murine
nervous system. Oncogene. 10:97–108. 1995.
|
|
26
|
Chen X, Wang J, Shen H, Lu J, Li C, Hu DN,
Dong XD, Yan D and Tu L: Epigenetics, microRNAS, and
carcinogenesis: functional role of microRNA-137 in uveal melanoma.
Invest Ophthalmol Vis Sci. 52:1193–1199. 2011. View Article : Google Scholar : PubMed/NCBI
|