Introduction
At present, cardiovascular diseases are the leading
cause of mortality in Western countries and patients with
cardiovascular disease often require vascular reconstruction
(1). To replace malfunctioning or
diseased blood vessels, several reconstructive procedures have been
developed, including the transfer of healthy tissue from one site
of the donor to another site and the use of tissue engineered blood
vessels (1). Although high-flow
vessels with a large diameter (>6 mm) are successful,
autografts, allografts or synthetic prosthetic grafts are unable to
meet the clinical demand, particularly in low-flow small-diameter
vascular grafts, which are often associated with thrombosis,
aneurysm formation, calcification and severe inflammatory reactions
(2). Thus, the development of an
effective vascular grafts, characterized by a non-thrombogenic
surface and possessing biomechanical properties matching those of
native vessels, has become one of the main targets of tissue
engineering. Several polymers have been used to obtain blood vessel
substitutes (BVSs): Poly(dimethylsiloxane), poly(caprolactone),
poly(methyl methacrylate), poly-L-lactic acid (PLLA), polyglycolic
acid and poly(glycerol sebacate) (3).
In this context, polyvinyl alcohol (PVA), a linear
synthetic polymer derived from the hydrolysis of polyvinyl acetate,
has not yet been investigated. As a consequence of its water
solubility, PVA must be cross-linked by either physical or chemical
methods in order to form stable hydrogels whose mechanical
properties depend on the degree of the crosslinks which,
simultaneously, determine the water uptake and diffusional features
of the biomaterial (4). Due to its
biocompatibility and swelling properties, PVA has been approved by
the Food and Drug administration and is widely used to produce
medical devices, including drug delivery devices, hemodialysis
membranes and soft contact lenses (5). Furthermore, PVA hydrogels have been
evaluated as scaffolds for tissue engineering purposes,
particularly as soft tissue substitutes (4). Indeed, the three-dimensional network
of polymer chains allows the removal of wastes and the diffusion of
nutrients and oxygen, which are required for cell survival.
Previous studies have demonstrated that PVA
hydrogels possess mechanical properties similar to those of porcine
aortas (6) and human arteries
(7,8), thus suggesting their suitability for
the fabrication of BVSs. However, the highly hydrophilic nature of
PVA hinders cell adhesion, which has a major role in the outcome of
vascular grafts. Moreover, the in vivo formation of an
endothelial lining on the biomaterial is required to avoid platelet
adhesion and ultimately dictates the success of the reconstructive
surgery. Consequently, several approaches have been applied to
improve endothelial cell (EC) adhesion on PVA. Liu et al
(9) blended PVA with various
natural macromolecules and demonstrated that gelatin increased
in vitro EC proliferation. Furthermore, Vrana et al
(10) verified the influence of
shear stress conditions on proliferation and the expression of
adhesion molecules of ECs that were cultured on PVA/gelatin
cryogels. Enhancement of cell adhesion was also achieved by
blending PVA with chitosan (11),
a positively charged polysaccharide derived from shellfish and
through polyesterification of PVA with citric acid (12). However, few in vivo studies
have been performed to verify the effectiveness of these
biomaterials as vascular grafts (13,14).
In a previous study conducted by our group (15), a three-layered vessel substitute
(TLVS) was developed, which was composed of a core of PLLA coated
on both sides with a homogenate of decellularized bovine aorta.
TLVS possessed good mechanical properties and the extracellular
matrix (ECM) components improved the adhesion and growth of human
ECs and smooth muscle cells.
The present study aimed to design and develop BVSs
composed of either PVA or PVA coated with a lyophilized
decellularized vascular matrix (DVM). First of all, human umbilical
vein endothelial cell (HUVEC) adhesion and proliferation on BVSs
were assessed in vitro. BVSs were implanted into the
abdominal aorta of Sprague-Dawley rats and the outcomes of the
reconstructive surgery were evaluated after 12 months.
Materials and methods
Reagents
All the chemicals and reagents used in the present
study were obtained from Sigma-Aldrich (St. Louis, MO, USA), with
the following exceptions: Phosphate-buffered saline (PBS) tablets
were purchased from Gibco Invitrogen Corp. (Paisley, UK), sodium
chloride from Fluka Chemie AG (Basel, Switzerland), Vectashield
Mounting medium for fluorescence with DAPI from Vector
Laboratories, Inc. (Burlingame, CA, USA), Movat pentachrome
staining kit from Bio-Optica (Milano, Italy), Milli-Q ultrapure
water from the Milli-Q Integral Water Purification System
(Millipore, Bedford, MA, USA), the mouse monoclonal anti-von
Willebrand Factor (vWF) antibody from Abcam (Cambridge, UK), the
goat anti-mouse immunoglobulin (IgG)-fluorescein isothiocyanate
(FITC; SC-2010) antibody from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA), the CellTiter 96® AQueous One Solution
Cell Proliferation Assay from Promega Corporation (Madison, WI,
USA), the Endothelial Cell Growth Medium MV2 from
PromoCell GmbH (Heidelberg, Germany).
Fabrication of PVA and PVA/DVM BVSs
Two types of BVSs were fabricated: one was composed
of only PVA and one was composed of a lyophilized decellularized
vessel matrix arranged as internal concentric layers to PVA.
PVA [molecular weight (MW) 146,000–186,000 g/mol; 16
g] and polyethylene glycol (PEG, MW 1,000 g/mol; 160 mg) were
dissolved into 84 ml distilled water at 95–100°C for ~2 h. To
fabricate PVA BVSs, the polymer solution was aspirated into
specific tubular moulds (2 mm in diameter) and then stainless steel
rods (10 cm in length and 1 mm in diameter) were fixed
concentrically into the moulds.
To produce PVA/DVM BVSs, tibial arteries were
harvested from calves. Following the removal of the surrounding
soft tissues, vessels were extensively washed and decellularized
using the modified Meezan’s method as previously described
(16). A single decellularization
cycle consisted of three steps: i) treatment with distilled water
for 72 h at 4°C; ii) incubation in 4% sodium deoxycholate solution
for 4 h at room temperature; and after washing, iii) treatment with
2,000 KU DNase-I in 1 M NaCl solution for 2 h at room temperature.
The decellularization protocol was repeated four times as
previously described (17).
Decellularized vessels (1 g) were cut into small pieces and
homogenized in 15 ml of cold 1.6 M acetic acid using an IKA T 10
Basic Ultra Turrax homogenizer (IKA®-Werke GmbH &
Co. KG, Staufen, Germany) (15). A
stainless steel rod (10 cm in length and 1 mm in diameter) was
pre-cooled at −70°C and dipped in decellularized vessel homogenate
several times until the layer deposited reached a thickness of ~1
mm. The deposited material was then lyophilized and the stainless
steel mandrel, covered by a spongy and dry layer of DVM, was dipped
into a 16% PVA solution and prepared as described above.
Both types of BVS were cross-linked through
freeze/thaw cycles. Moulds were put in a cold bath and frozen at
−20°C for 12 h, and then stored at −2/−5°C for 12 h. The two last
steps were repeated three times. BVSs were removed from the moulds
and sterilized in 80% (v/v) ethanol solution for 4 h under UV light
in a sterile hood. Scaffolds were then stored in PBS containing a
1% antibiotic and antimycotic solution at 4°C until use.
Cell cultures
Primary cultures of HUVECs were obtained by
enzymatic digestion of the umbilical vein endothelial layer with a
0.1% collagenase IV solution. The cells were seeded on Petri dishes
previously coated with 1 μg/ml fibronectin and cultured with
Endothelial Cell Growth Medium MV2 supplemented with 5%
fetal calf serum, 1 μg/ml ascorbic acid, 10 ng/ml human
fibroblastic growth factor-2, 5 ng/ml human epidermal growth
factor, 0.2 μg/ml hydrocortisone, 20 ng/ml
R3-insulin-like growth factor-1, 0.5 ng/ml vascular
endothelial growth factor (endothelial MV2 medium kit)
and 1% antibiotic solution, containing 10 ng/ml streptomycin
sulfate, 250 ng/ml amphotericin-B and 100 U/ml penicillin. Cultures
were incubated at 37°C in a humidified atmosphere. HUVECs were used
until the fourth passage and harvested at 80% confluence.
Proliferation assay
The
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
inner salt (MTS) dye assay was used to evaluate cell proliferation.
HUVECs (3×105 cells/cm2) were previously
seeded on either PVA or PVA/DVM BVSs and deposited onto 12-well
plates. Cultures grown on tissue culture-treated polystyrene plates
were used as a positive control. Twenty-four, 48 and 92 h after
seeding, cell proliferation was determined using the CellTiter
96® AQueous One Solution Cell Proliferation assay
according to the manufacturer’s instructions. Briefly, cells were
treated with 10% MTS for 4 h. Optical density of purple formazan
produced in living cells was measured at 490 nm using a Microplate
autoreader EL 13 (Bio-Tek Instruments, Winooski, VT, USA). The
means of three experiments were expressed as the number of cells.
The linearity of absorbance of formazan ranging from
3×103 to 2×104 cells was established by
determining the linear coefficient (0.986). All results were
expressed as the mean ± standard deviation of three separate
experiments. Statistical comparison was performed by analysis of
variance, followed by the Student’s t-test. Alternatively, cultures
were fixed with 10% formalin. Samples were then examined after
critical-point drying and gold sputtering was applied prior to
scanning electron microscopy (SEM; Stereoscan-205 S; Cambridge
Instruments, Cambridge, MA, USA).
In vivo experiments
BVSs were inserted to repair a surgically created
defect in the abdominal aorta of 14 male Sprague-Dawley rats
weighing 250–300 g and aged 6 months. Animals were divided in two
groups according to the implanted graft: Group 1 (n=7) received PVA
BVSs and Group 2 (n=7) received PVA/DVM BVSs. The protocol was
approved by the Institutional Animal Care Committee of the
University of Padova (Padova, Italy). Under halothane anesthesia,
the abdominal area was shaved and aseptically prepared using
povidone-iodine (Betadine). The muscles were exposed with a 3 cm
abdominal incision and the abdominal aorta was exposed and
isolated. After clamping the vessel, a segment of aorta (1 cm in
length) was excised and the scaffold (2 mm in diameter and 2 cm in
length) was anastomized proximally and distally end-to-end using
continuous 10.0 polypropylene sutures. No anticoagulants and
antibiotics were administered. The animals implanted with PVA/DVM
BVSs died 3–4 days after surgery, whereas the rats implanted with
PVA BVSs were sacrificed after 12 months. The implants were
recovered and each sample was divided into two pieces: one was
frozen in liquid nitrogen and cut into 10-μm thick cryosections for
histological analysis, one was fixed with 10% formalin for SEM and
processed as described above.
Cryostat sections were fixed in cold acetone for 5
min. The sections were then stained using the Movat pentachrome
stain kit according to the manufacturer’s instructions. Samples
were examined under an optical microscope (Leica DM2000; Leica
Microsystems, Wetzlar, Germany). Alternatively, aspecific sites
were blocked with 10% goat serum in PBS for 45 min at room
temperature. Samples were incubated with monoclonal anti-vWF
antibody (1:100 in 3% goat serum) at 4°C overnight and then
incubated with the secondary antibody, goat anti-mouse IgG-FITC
(1:200 in 1.5% goat serum) for 1 h at room temperature. Slices were
mounted with mounting medium with DAPI. Negative controls were
obtained by omitting the primary antibody.
Results
Morphological analysis
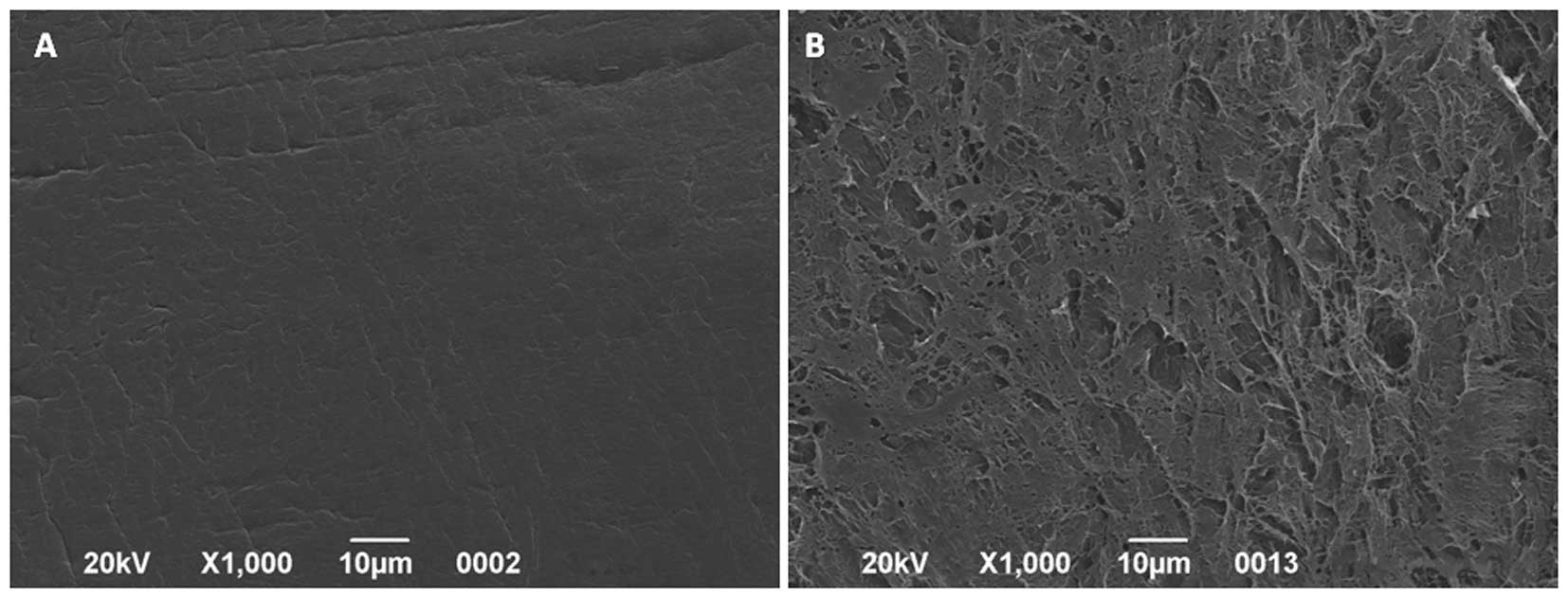
Fig. 1 shows the
structure of PVA/DVM BVSs. The external side (Fig. 1A), composed of PVA, appeared
smooth, whereas the internal side (Fig. 2B), coated with lyophilized DVM,
exhibited a porous structure and bundles of crimped fibers.
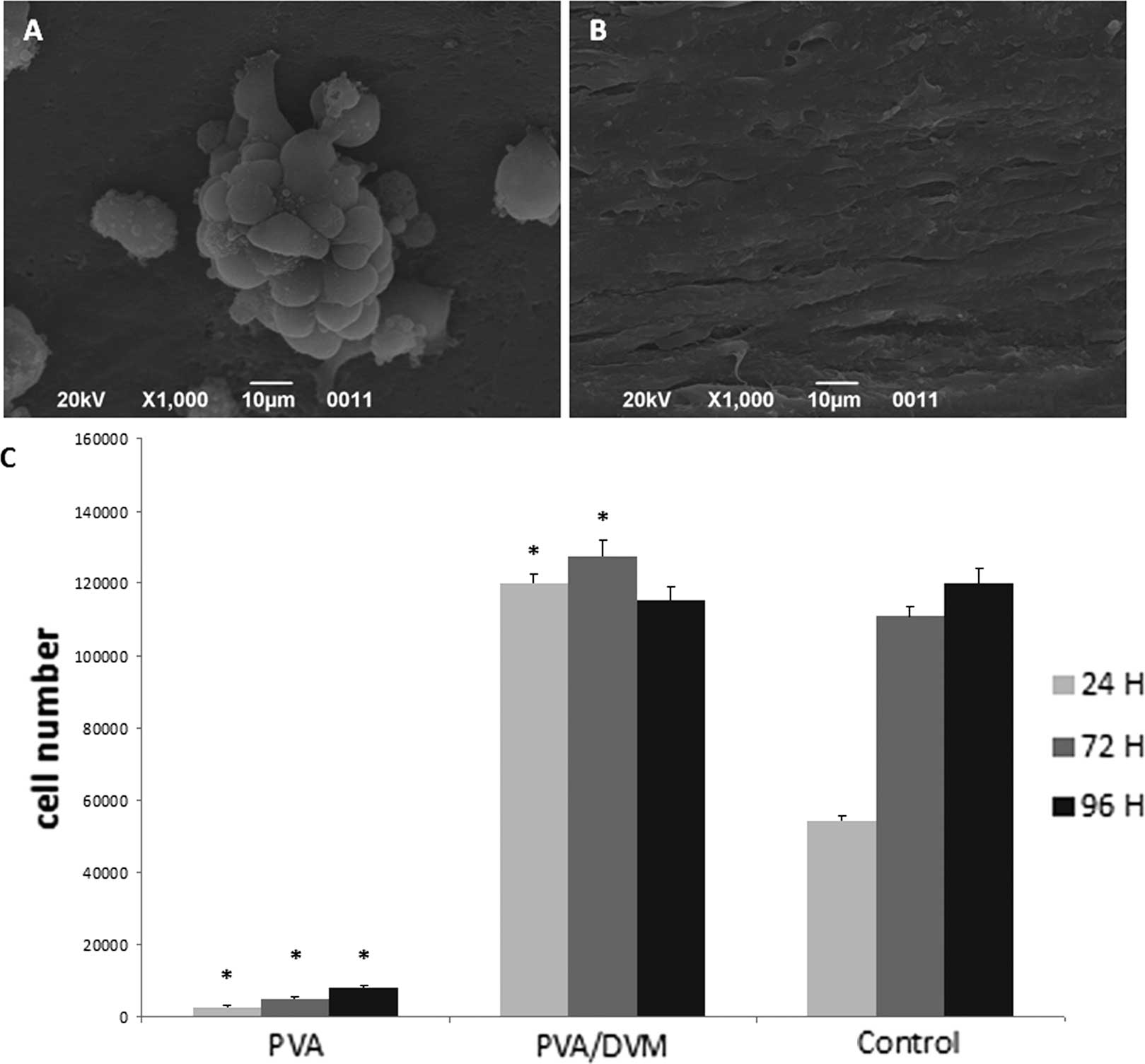
Only a few clusters of rounded cells were visible on
PVA (Fig. 2A) 96 h after seeding.
By contrast, HUVECs formed a confluent monolayer on the internal
side of PVA/DVM after 24 h (Fig.
2B).
Cell proliferation assay
The results of the cell proliferation assays
supported the findings of the morphological analysis (Fig. 2C). The cell number in cultures on
PVA/DVM did not increase from 24 to 96 h and were significantly
higher than those determined in the corresponding control cultures
grown on tissue culture-treated polystyrene plates. By contrast,
the number of cells detected on PVA was significantly lower in
comparison to those counted in the PVA/DVM and the control
groups.
SEM analysis and immunofluorescence
Sprague-Dawley rats received either PVA or PVA/DVM
BVSs as abdominal aorta interposition grafts (2 cm). All animals
who received PVA/DVM BVSs died 3–4 days after surgery. As shown in
Fig. 3C, grafts were totally or
partially occluded by thrombi and the luminal surface presented a
network of fibrin fibres (Fig.
3D). By contrast, all rats implanted with PVA BVSs survived
surgery without signs of infection or implant rejection. After 12
months, the outcome was evaluated by means of SEM,
immunofluorescence and Movat staining. PVA grafts (Fig. 3E) were patent and both sides were
wrapped by regenerating tissue continuous to that of native
vessels. Although the implants appeared thicker than the wall of
the host aorta (Fig. 3A), the
inner diameters of all PVA grafts were similar to those of the
original vessels. Furthermore, compared with the native artery
(Fig. 3B), the luminal surface of
the grafts was completely covered by cells orientated along the
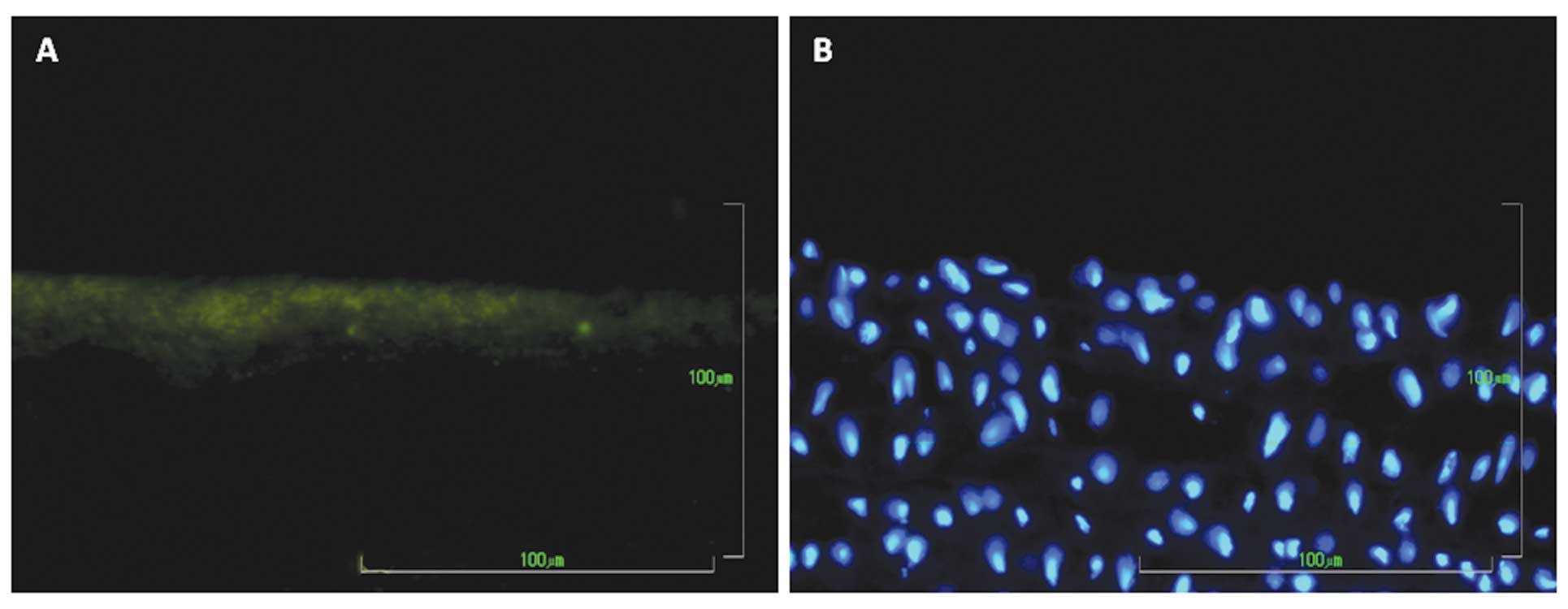
blood flow in longitudinal beamed structures (Fig. 3F). These cells were immunoreactive
to anti-vWF antibody, confirming their endothelial phenotype
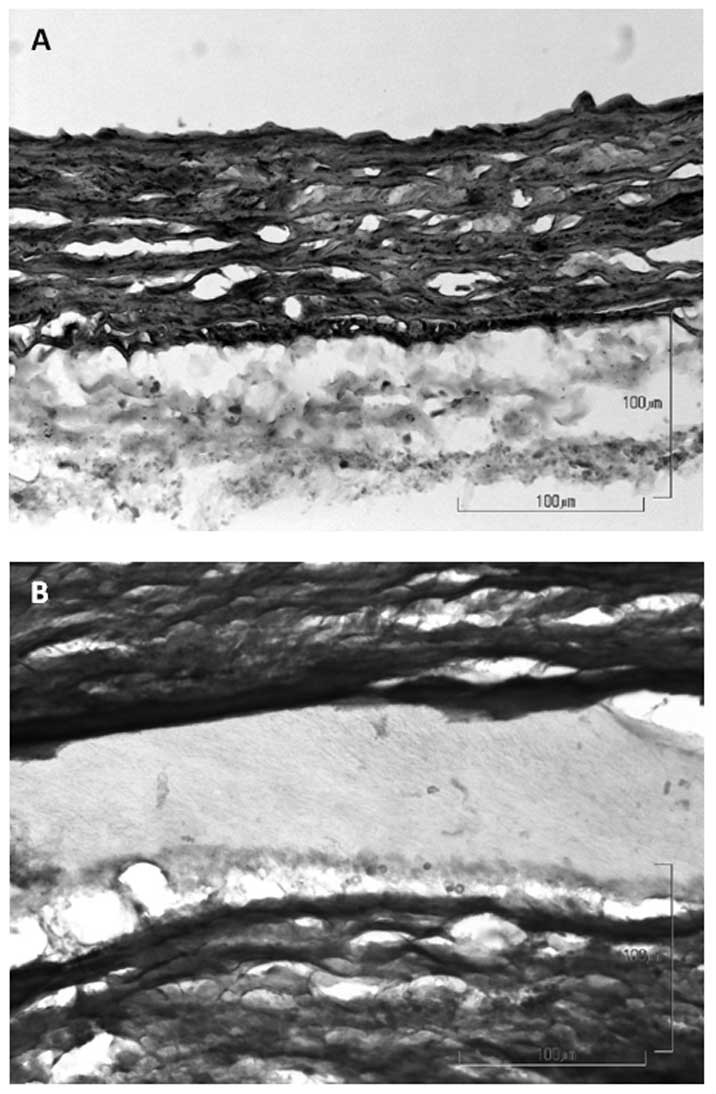
(Fig. 4A). Movat staining
demonstrated that the tissue layer covering the luminal side of PVA
(Fig. 5B) resembled the structure
of the native aorta (Fig. 5A).
However, no cells were visible inside the polymer.
Discussion
To date, the development of functionally and
biologically compatible BVSs has gained increasing attention due to
the augmented morbidity of cardiovascular diseases in Western
countries. Thus, the fabrication of suitable vascular grafts is one
of the main targets of tissue engineering. Since Weinberg and Bell
(18) reported the construction of
blood vessels using collagen, cultured bovine aortic ECs, smooth
muscle cells and adventitial fibroblasts in vitro,
considerable progress in this field has been achieved. Several
approaches have emerged in the last two decades, including ECM
protein and nanostructured synthetic polymer-based grafts and
scaffold-free cell transplantable constructs (3). Although venous grafts have entered
clinical trials (19), the
achievement of suitable arterial substitutes, particularly those of
small diameters (<6 mm) for coronary artery bypass procedures or
peripheral arterial revascularization, remains a challenge. The
ideal BVS must possess mechanical properties that are able to
sustain a high-pressured pulsatile blood flow. Furthermore, BVSs
must promote cell growth, inhibit thrombogenesis, avoid
inflammation and neointimal proliferation.
In this context, previous studies have reported that
PVA hydrogels possess mechanical properties resembling those of
human arteries (7,8). King et al (8) demonstrated that this biomaterial can
be used as a vessel wall mimicking material in anatomically
realistic flow phantoms. In vitro and in vivo
experiments have also shown that the PVA surfaces were
athrombogenic (13,14). However, PVA does not warrant cell
adhesion and proliferation. Herein, to improve the compatibility
with vascular cells, the internal surface of tubular devices
composed of PVA cryogels were coated with lyophilized DVM. As
expected, the in vitro results of the present study showed
that the PVA surface was unable to support cell adhesion and
growth. By contrast, the presence of ECM macromolecules greatly
enhanced the adhesion of HUVECs, allowing the formation of a
confluent monolayer 24 h after seeding. A previous study conducted
by our group demonstrated that the DVM contained types I and VI
collagen, which mediated cell adhesion through the RGD motif
(15). Furthermore, the
decellularization process may have maintained growth and angiogenic
factors, including basic fibroblast growth factor and transforming
growth factor-β, as previously detected in skeletal muscle
acellular matrices (20,21). Thus, the presence of
tissue-specific proteins and growth factors may make DVM a more
permissive environment for vascular cells, with regard to the
addition of single bioactive molecules to the biomaterials
(22,23).
In the present study, when PVA/DVM BVSs were
implanted into the abdominal aorta of Sprague-Dawley rats, DVM was
found to be a highly thrombogenic surface, which subsequently
resulted in the mortality of all animals 3–4 days after surgery.
Obstruction of the grafts occurred due to the formation of a fibrin
network on the luminal side and activation of the coagulative
pathway. These results clearly indicated the need to mask ECM
proteins in order to avoid platelet adhesion and thrombi formation.
In this context, recellularizing scaffolds with host ECs prior to
implantation or treatment of the inner lining with heparin may be a
useful approach (24). By
contrast, all animals receiving PVA BVSs survived until the time of
sacrification (12 months). Miyake et al (14) reported that the patency of PVA
tubes implanted in the carotid artery of rats was 80% after 1 week
and 70% after 1 month. To make thromboresistant woven Dacron
artificial vessels, Tamura et al (13) coated them with either PVA-silica or
heparinized PVA-silica. One year after implantation into the
abdominal aorta of adult mongrel dogs, the percentage of patent
grafts ranged from ~57 to 67%. Herein, although neither
anticoagulants nor fibrinolitic agents were administered, all PVA
BVSs remained patent. Microscopic examination identified a
continuous EC coverage of the luminal surface of the explanted PVA
grafts whose inner diameter was similar to that of the native
vessel. Despite the lack of cells inside the polymer, PVA acted as
a guide for tissue regeneration and the host tissue was grown on
both sides of the graft, which resulted in a neovascular wall whose
architecture resembled that of the original vessel.
In conclusion, the present study demonstrated that
an effective and long-term replacement of tracts of the abdominal
aorta can be achieved using tubes composed of PVA cryogels. This
biomaterial, coupling biocompatible and non-thrombogenic features
with the capability to withstand high-flow rates, may be considered
as a promising tool for the fabrication of artificial vessels.
Further studies are required to monitor the local tissue responses
of the host at time-points prior to 12 months.
Acknowledgements
The authors would like to thank Dr Ilenia Zanusso
for advice on the micrographs.
References
|
1
|
Nemeno-Guanzon JG, Lee S, Berg JR, Jo YH,
Yeo JE, Nam BM, Koh YG and Lee JI: Trends in tissue engineering for
blood vessels. J Biomed Biotechnol. 2012:9563452012.PubMed/NCBI
|
|
2
|
Schimidt CE and Baier JM: Acellular
vascular tissues: natural biomaterials for tissue repair and tissue
engineering. Biomaterials. 21:2215–2231. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hung HS, Chen HC, Tsai CH and Lin SZ:
Novel approach by nanobiomaterials in vascular tissue engineering.
Cell Transplant. 20:63–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baker MI, Walsh SP, Schwartz Z and Boyan
BD: A review of polyvinyl alcohol and its uses in cartilage and
orthopedic applications. J Biomed Mater Res B Appl Biomater.
100:1451–1457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cascone MG, Barbani N, Cristallini C,
Giusti P, Ciardelli G and Lazzeri L: Bioartificial polymeric
materials based on polysaccharides. J Biomater Sci Polym.
12:267–281. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu KC and Rutt BK: Polyvinyl alcohol
cryogel: an ideal phantom material for MR studies of arterial flow
and elasticity. Magn Reson Med. 37:314–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O’Flynn PM, Roche ET and Pandit AS:
Generating an ex vivo vascular model. ASAIO J. 51:426–433.
2005.PubMed/NCBI
|
|
8
|
King DM, Moran CM, McNamara JD, Fagan AJ
and Browne JE: Development of a vessel mimicking material for use
in anatomically realistic Doppler flow phantoms. Ultrasound Med
Biol. 37:813–826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Vrana NE, Cahill PA and McGuinness
GB: Physically crosslinked composite hydrogels of PVA with natural
macromolecules: structure, mechanical properties, and endothelial
cell compatibility. J Biomed Mater Res B Appl Biomater. 90:492–502.
2009. View Article : Google Scholar
|
|
10
|
Vrana NE, Cahill PA and McGuinnes GB:
Endothelization of PVA/gelatin cryogels for vascular tissue
engineering: effect of disturbed shear stress conditions. J Biomed
Mater Res A. 94:1080–1090. 2010.PubMed/NCBI
|
|
11
|
Mathews DT, Birney YA, Cahill PA and
McGuinness GB: Vascular cell viability on polyvinyl alcohol
hydrogels modified with water-soluble and -insoluble chitosan. J
Biomed Mater Res B Appl Biomater. 84:531–540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas LV, Arun U, Remya S and Nair PD: A
biodegradable and biocompatible PVA-citric acid polyester with
potential applications as matrix for vascular tissue engineering. J
Mater Sci Mater Med. 20:S259–S269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tamura K, Mizuno H, Okada K, Katoh H,
Hitomi S, Teramatsu T, Shimizu Y and Hino T: Experimental
application of polyvinyl alcohol-silica for small artificial
vessels. Biomater Med Devices Artif Organs. 13:133–152.
1986.PubMed/NCBI
|
|
14
|
Miyake H, Handa H, Yonekawa Y, Taki W,
Naruo Y, Yamagata S, Ikada Y, Iwata H and Suzuki M: New
small-caliber antithrombotic vascular prosthesis: experimental
study. Microsurgery. 5:144–150. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grandi C, Martorina F, Lora S, Dalzoppo D,
Amistà P, Sartore L, Di Liddo R, Conconi MT and Parnigotto PP:
ECM-based triple layered scaffolds for vascular tissue engineering.
Int J Mol Med. 28:947–952. 2011.PubMed/NCBI
|
|
16
|
Meezan E, Hjelle JT, Brendel K and Carlson
EC: A simple, versatile, non disruptive method for the isolation of
morphologically and chemically pure basement membranes from several
tissues. Life Sci. 17:1721–1732. 1975. View Article : Google Scholar
|
|
17
|
Grandi C, Baiguera S, Martorina F, Lora S,
Amistà P, Dalzoppo D, Del Gaudio C, Bianco A, Di Liddo R, Conconi
MT and Parnigotto PP: Decellularized bovine reinforced vessels for
small-diameter tissue-engineered vascular grafts. Int J Mol Med.
28:315–325. 2011.
|
|
18
|
Weinberg CB and Bell E: A blood vessel
model constructed from collagen and cultured vascular cells.
Science. 231:397–400. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Udelsman BV, Maxfield MW and Breuer CK:
Tissue engineering of blood vessels in cardiovascular disease:
moving towards clinical translation. Heart. 99:454–460. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Coppi P, Bellini S, Conconi MT, Sabatti
M, Simonato E, Gamba PG, Nussdorfer GG and Parnigotto PP:
Myoblast-acellular skeletal muscle matrix constructs guarantee a
long-term repair of experimental full-thickness abdominal wall
defects. Tissue Eng. 12:1929–1936. 2006.PubMed/NCBI
|
|
21
|
Conconi MT, Bellini S, Teoli D, de Coppi
P, Ribatti D, Nico B, Simonato E, Gamba PG, Nussdorfer GG, Morpurgo
M and Parnigotto PP: In vitro and in vivo evaluation of acellular
diaphragmatic matrices seeded with muscle precursors cells and
coated with VEGF silica gels to repair muscle defect of the
diaphragm. J Biomed Mater Res A. 89:304–316. 2009. View Article : Google Scholar
|
|
22
|
Ju YM, Choi JS, Atala A, Yoo JJ and Lee
SJ: Bilayered scaffold for engineering cellularized blood vessels.
Biomaterials. 31:4313–4321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stephan S, Ball SG, Williamson M, Bax DV,
Lomas A, Shuttleworth CA and Kielty CM: Cell-matrix biology in
vascular tissue engineering. J Anat. 209:495–502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomas LV, Lekshmi V and Nair PD: Tissue
engineered vascular grafts - preclinical aspects. Int J Cardiol.
167:1091–1100. 2013. View Article : Google Scholar : PubMed/NCBI
|