Introduction
Vascular cognitive impairment (VCI) is the
phenotypic outcome of a cascade of events, involving vascular risk
factors that lead to vascular disease, which causes vascular brain
injury in networks important for cognitive functioning (1–4). VCI
encompasses numerous conditions from vascular dementia (VaD) to
mild cognitive impairment of vascular origin (5). Clinical studies have reported that
cerebral hypoperfusion associated with cognitive dysfunctions has
been identified in Alzheimer’s disease (AD) and in patients with
VaD (6,7).
The pathological mechanisms that underlie chronic
cerebral hypoperfusion-induced dementia are yet to be identified.
Reactive oxidative stress and inflammatory cytokines have important
roles in brain impairment and dementia progression (8). In addition, experimental evidence has
indicated that synapse loss occurs in AD and VaD patients and
studies revealed a strong correlation between synapse reduction and
the severity of dementia (9).
Although an increasing number of mechanistic pathological studies
focused on VaD and hypoperfusion, no effective pharmacological
intervention has been approved by the Food and Drug Administration
(Silver Spring, MD, USA) and used clinically.
Herbal medicines, including Traditional Chinese
Medicines, have been used for a long time as an alternative method
to treat cerebral vascular diseases and dementia-like symptoms
(10). A Chinese Medicine named
Bushen Huoxue decoction (BHD) was prepared, which has a good
clinical efficacy in treating patients with VaD. Two herbs are
included in BHD, namely Epimedium (Epimedium Davidii
Franch, Yin Yang Huo) and Salvia Miltrorrhiza (Salvia
Miltiorrhiza Bunge, Dan Shen). BHD has the efficacy of
nourishing kidney and activating blood (Bu Shen Huo Xue), which is
simplified from an impactful formula based on the Traditional
Chinese Medicine theory that has been previously confirmed to have
good efficacy in treating cognitive impairment, ischemic cerebral
stroke and VaD (11). Although the
formula has been proved to be effective in the clinical treatment
of VaD, whether the simplified decoction BHD may improve cognitive
deficits and the associated mechanism underlying its effects remain
to be elucidated.
In the present study, occlusion of bilateral common
carotid arteries (2VO) was used as a cerebral hypoperfusion model
to study the effect of BHD on cognitive improvement in
vivo.
Materials and methods
Plant materials and herbs water
extraction
BHD consists of Epimedium and Salvia
miltiorrhiza. The herbs were purchased from Fujian
Pharmaceutical Co. (Fuzhou, Fujian, China) and were identified as
eligible and pure medicinal material. These herbs were decocted at
boiling temperature and extracted with water. The decoction was
filtrated, concentrated and stored in a sterile bottle at 4°C until
use. An extract of Ginkgo biloba (GB) leaves was purchased
from Shenyang Green Pharmaceutical Co. (Shenyang, Liaoning,
China).
Animals and 2VO surgery
The current study was approved by the ethics
committee of Fujian University of Traditional Chinese Medicine,
Fuzhou, China. Wistar rats (Shanghai SLAC Laboratory Animal Co.,
Ltd., Shanghai, China), weighing 250±20 g, were allowed to
acclimatize for three days prior to experimentation and were housed
in 25±1°C, 65±5% humidity and a 12-h light/dark cycle. Food and
water were freely available. All experiments were conducted in
accordance with the Guidance Suggestions for the Care and Use of
Laboratory Animals, formulated by the Ministry of Science and
Technology of China. Efforts were made to minimize the suffering
and number of animals used.
Cerebral hypoperfusion in rats was performed
according to the previously described procedures (12). Each rat was anesthetized with
ketamine hydrochloride (0.3 ml/100 g, i.p.). Both of the common
carotid arteries were carefully separated from the cervical
sympathetic and vagal nerves through a ventral cervical incision.
The arteries were then ligated with 5.0 silk sutures. The
sham-operated animals were treated similarly to the 2VO rats except
for the carotid artery ligations.
Drug administration
The adult normal dosage of BHD was defined as a
moderate dose (M), 1/2 of the moderate dose as the low dose (L) and
two times the moderate dose as the high dose (H). On the day 18
following surgery, the 2VO rats were randomly divided into five
groups with n=12/group. The rats in each group were
intragastrically administrated twice daily with saline (vehicle
control, 2VO), 2.75 g/kg (BHD-L), 5.5 g/kg (BHD-M), 11 g/kg (BHD-H)
BHD and 5.85 mg/kg GB, respectively. GB, a well-known antioxidant
and a good anti-dementia drug, was used as a positive control
(13,14). The sham-operated and vehicle groups
were administered an equal volume of physiological saline solution.
The administration was performed for 14 consecutive days. The
animals were subjected to behavioral tests prior to the surgery,
post-surgery and on days 7 and 14 following administration,
respectively, to observe the learning and memory ability. The time
scale of the drug treatment regimen is illustrated in Fig. 1.
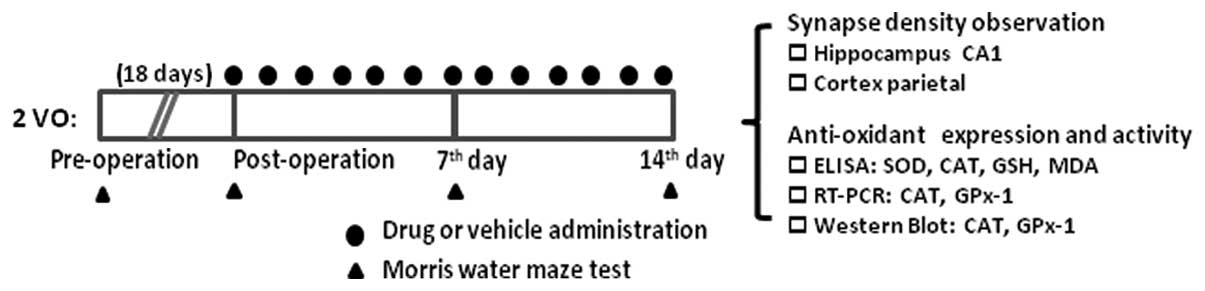 | Figure 1Outline of the experimental design
illustrating the time scale of drug administration and Morris water
maze test. The rats were subjected to permanent 2VO surgery.
Following 18 days, the rats in each group were intragastrically
administered twice daily with 2.75, 5.5 and 11 g/kg Bushen Huoxue
decoction, 5.85 mg/kg Ginkgo biloba or saline, respectively.
The administration was performed for 14 consecutive days. The
animals were subjected to behavioral testing prior to the surgery,
post-surgery without treatment, and on days 7 and 14 following
administration, respectively, followed by outcome parameters
detection, including synapses number and anti-oxidants levels. 2VO,
occlusion of bilateral common carotid arteries; GPx-1, glutathione
peroxidase-1; CAT, catalase; SOD, superoxide dismutase; GSH,
glutathione. |
Morris water maze tests (MWM)
Prior to the surgery, post-surgery and on days 7 and
14 following treatment, respectively, the rats were subjected to
spatial learning and memory tests as examined using the MWM as
previously described (15). The
MWM consisted of a black circular pool of water and a hidden
platform submerged 2 cm below the water surface, with the water
temperature maintained at 21±1°C. Four points around the edge of
the pool were designated as North (N), South (S), East (E) and West
(W). A video camera was mounted above the center of the pool and
all performance was recorded and then analyzed by a computerized
video imaging analysis system (Institute of Material Medical,
Chinese Academy of Medical Sciences, Beijing, China). At the
beginning of the trials, the rats were free to swim for 2 min and
the swimming distances were recorded. At first, the rats were
provided an escape latency trial once per day for four days. The
rats were placed from E, S, W and N one by one every day. If the
rat did not find the escape platform within the allotted time (2
min), the record was designated as 120 sec. On day 15, the
platforms were removed and the rats were placed into the maze as
the escape latency trial. A 120 sec probe trial was performed and
the number of crossings over the target quadrant was recorded. Each
test was repeated four times for each rat at every time-point. The
time scale of the MWM test is illustrated in Fig. 1.
Synapse density observed by transmission
electron microscopy
The synapse density in hippocampal CA1 and cortex
parietal was observed with a transmission electron microscope
(H7650, Hitachi, Ltd., Tokyo, Japan). Briefly, following regular
fixation, embedding and ultra-thin section, the brain sections of
each rat were placed onto three copper grids. Each grid was scanned
to capture 4–5 images, with a total of 10–15 pictures for each rat
brain. The numerical density of synapses per unit volume,
Nv, was calculated using the following formula:
Nv = Q−/Vdis, where Q−
is the mean number of synapses counted in each sector and
Vdis is the mean sector volume. The total number of
synapses, Nsyn, was calculated for each case using the
following formula: Nsyn =
Nv·Vref
ELISA
The hippocampus homogenates were isolated and stored
at −80°C until use. Superoxide dismutase (SOD) and catalase (CAT)
levels were determined with respected ELISA kits (Hufeng Tech,
Shanghai, China) according to manufacturer’s instructions.
Western blot analysis
The denatured protein samples were resolved by
SDS-PAGE and transferred to polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA). Following blocking, the membranes
were incubated overnight at 4°C with primary antibodies [β-actin
and CAT monoclonal rabbit anti-mouse antibodies, 1:1,000 dilution,
Cell Signaling Technology, Inc., Danvers, MA, USA; polyclonal
rabbit glutathione peroxidase-1 (GPx-1) antibody, 1:1,000 dilution,
Abcam, Cambridge, UK] followed by incubation with the goat
anti-rabbit horseradish peroxidase-conjugated secondary antibodies
(1:2,000 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, CA,
USA). Chemiluminescence detection was performed using enhanced
chemiluminescence advance western blotting detection reagents (BD
Biosciences, San Jose, CA, USA). Band intensities were measured
with the SX-300 image analysis system (Bio-Rad Laboratories,
Hercules, CA, USA).
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from the hippocampi with
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. Reverse transcription
was performed using the AMV reverse transcription system
(Fermentas, Burlington, Canada). Amplification of cDNA fragments of
CAT, GPx-1 and β-actin was performed using the Green PCR Master Mix
kit (Fermentas). DNA was amplified immediately with a single cycle
at 94°C for 5 min, 30 cycles at 94°C for 30 sec, annealing
temperature of each gene for 30 sec and 72°C for 30 sec followed by
a final extension step 72°C for 10 min. Ethidium bromide-stained
gels were scanned and qualified using Tanon Image software (Science
& Technology Co. Ltd., Shanghai, China). The intensity of each
band was normalized against the intensity of β-actin. The PCR
primers, fragment length and annealing time of GPX-1, CAT and
β-actin are summarized in Table
I.
 | Table IPolymerase chain reaction primers,
fragment length and annealing time of GPx-1, CAT and β-actin. |
Table I
Polymerase chain reaction primers,
fragment length and annealing time of GPx-1, CAT and β-actin.
| Gene | Primer sequence | Product (bp) | Annealing temperature
(°C) |
|---|
| CAT | F:
5′-CTATCCTGACACTCACCGCCAT-3′
R: 5′-TTCTTGACCGCTTTCTTCTGGA-3′ | 372 | 58 |
| GPx-1 | F:
5′-CTCGGTTTCCCGTGCAATCAG-3′
R: 5′-GTGCAGCCAGTAATCACCAAG-3′ | 431 | 65 |
| β-actin | F:
5′-CTGACCGAGCGTGGCTAC-3′
R: 5′-CCTGCTTGCTGATCCACA-3′ | 505 | 58 |
Statistical analysis
Statistical analysis was performed with SPSS 12.0
(SPSS, Inc, Chicago, IL, USA). Data are expressed as the mean ±
standard deviation. Analysis of variance was used to determine
statistical significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
BHD improves spatial learning and memory
of rats with 2VO in MWM
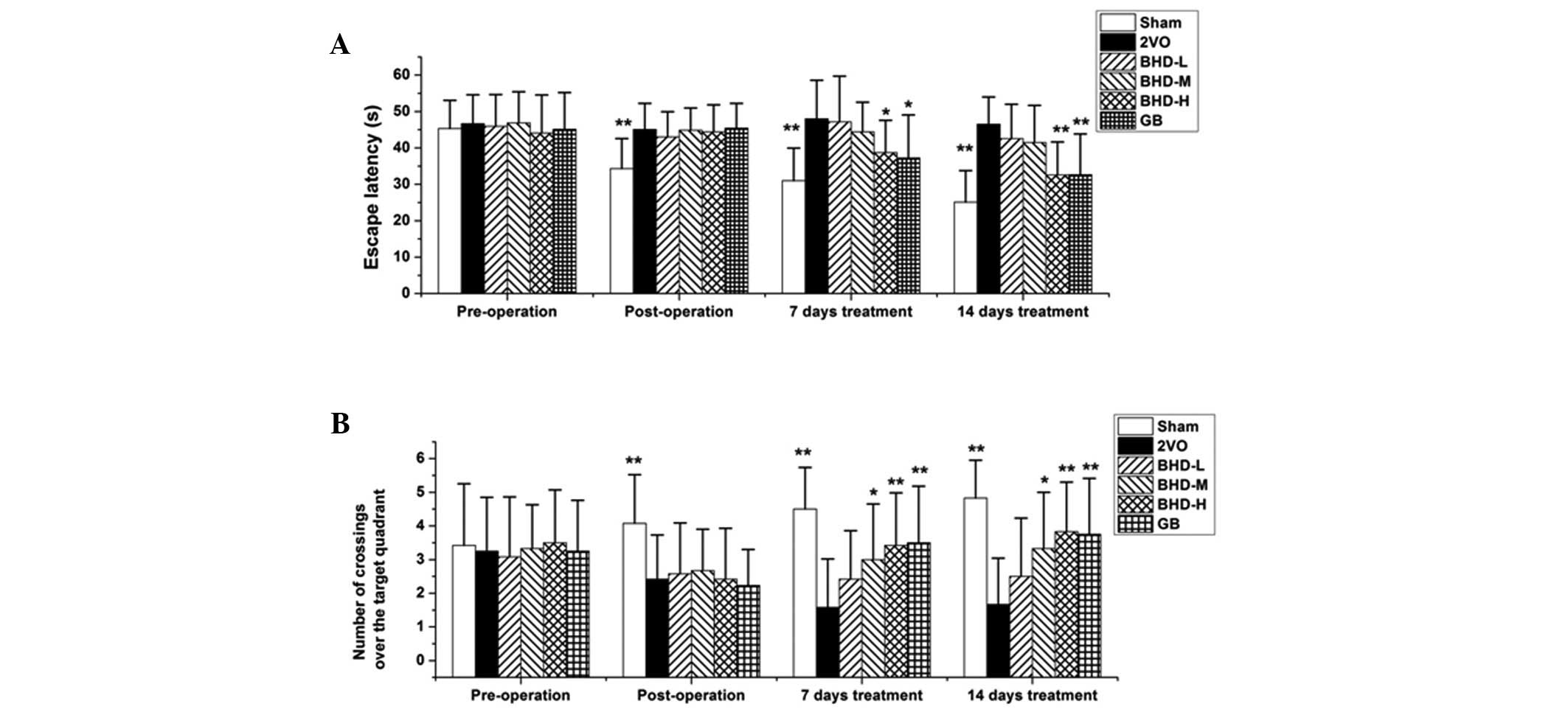
To observe whether BHD improves cognitive deficits,
the classical MWM test was used to examine spatial learning and
memory, as described in materials and methods above. As
demonstrated in Fig. 2A, the
escape latency to find the hidden platform in the sham group
decreased gradually, while the 2VO rats spent more time in the
training trials (P<0.01, compared with the model group). These
observations are consistent with the results of behavioral tests
reported elsewhere (16). The rats
in the BHD-M, BHD-H dosage of BHD and positive control (GB) groups
demonstrated improved cognitive abilities compared with the 2VO
rats from days 7 and 14 of the tests (P<0.05 or P<0.01,
compared with the model group), whereas low-dose BHD did not induce
any significant improvements.
The number of crossings over the target quadrant in
the probe trials was also examined in each group. A large number of
times of crossing over the target quadrant where the hidden
platform was placed demonstrated good spatial learning and memory.
Fig. 2B demonstrated that the rats
in the 2VO group required less time than the sham group
(P<0.01), and the number of crossings over the target quadrant
increased following treatment with BHD as well as GB. The results
suggested that rats with 2VO treated with BHD-M or BHD-H exhibited
a significant increase in the number crossings over the target
quadrant (P<0.05, compared with the model group), particularly
at a high dose (P<0.01, compared with the model group). Overall,
these results demonstrated that BHD dose-dependently improved
cognitive deficits.
BHD increases the number of synapses in
hippocampal CA1 regions and the parietal cortex in rats with
2VO
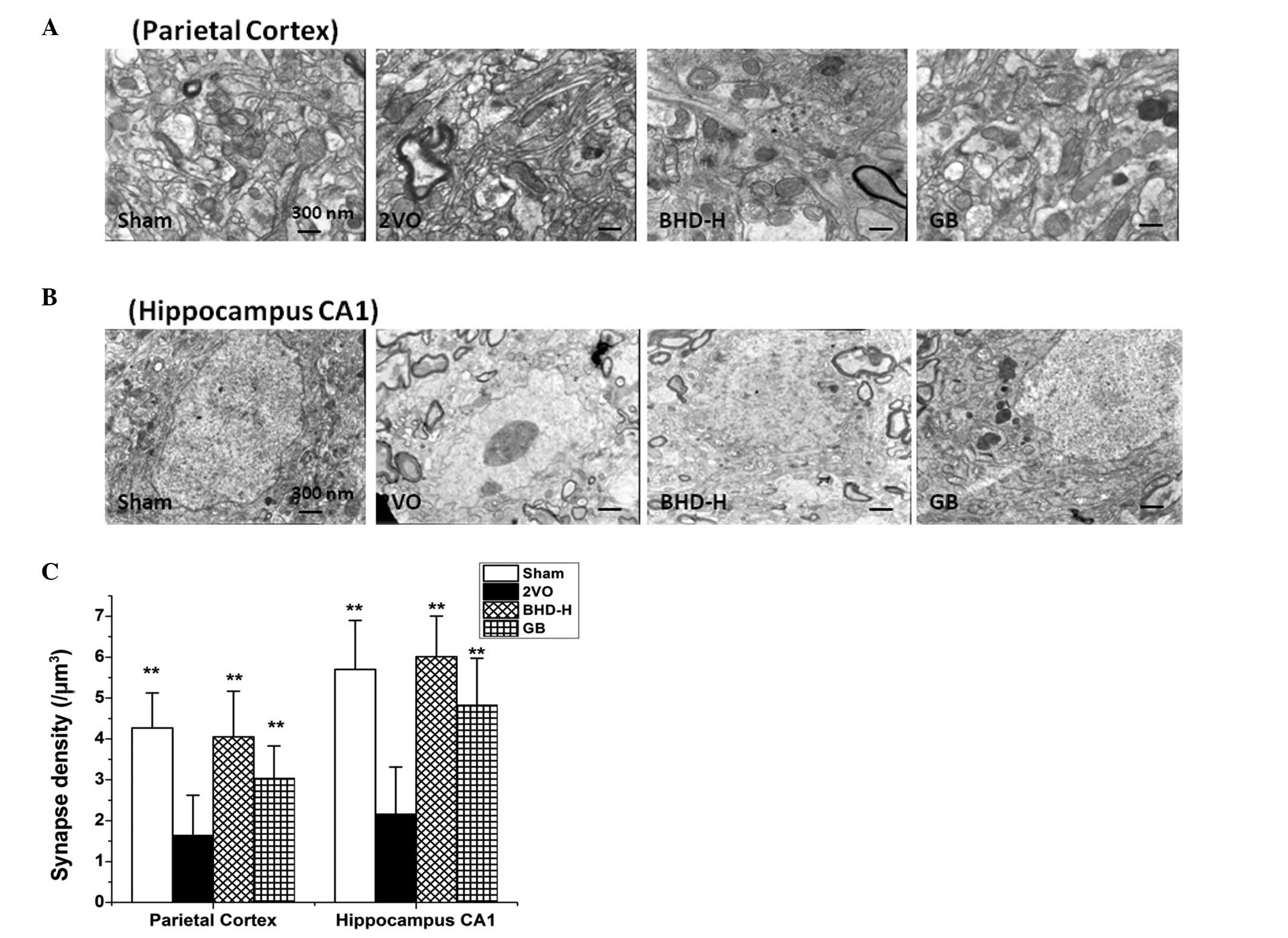
Cognitive impairment is strongly associated with a
reduction in the number of synapses. (17) To detect whether the resultant loss
in the number of synapses due to cerebral hypoperfusion could be
alleviated by BHD administration, the synapses in the hippocampal
CA1 region and parietal cortex in rats with 2VO were observed using
transmission electron microscopy, counting the number of synapses
in each specified area. The results in Fig. 3 revealed that the synapse density
in both areas was reduced in the 2VO model, which is consistent
with a previous study (18).
Notably, both the high dosage of BHD and GB treatment reversed the
synaptic impairments observed in the parietal cortex and
hippocampal CA1 regions.
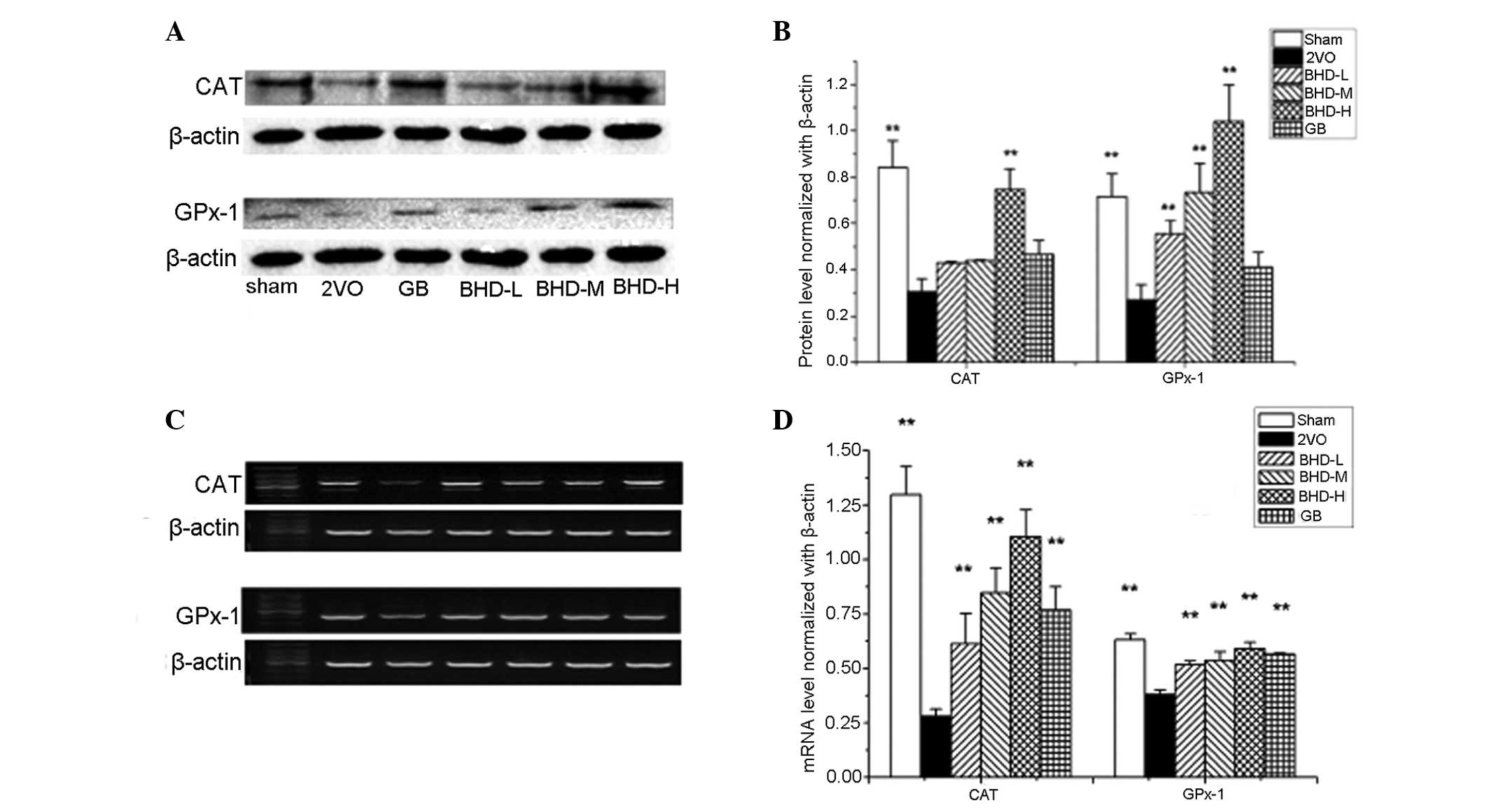
Expression of antioxidants increases
following BHD treatment in the hippocampi of rats with 2VO
To further study the molecular mechanism underlying
the BHD-induced cognitive improvement, antioxidant levels and
activities were examined. Increased levels of malondialdehyde (MDA)
and a decrease in the activity of SOD, CAT and glutathione (GSH) in
cerebral hypoperfusion induced by 2VO was observed (P<0.01,
compared with the sham group; Table
II). Both the GB and BHD groups demonstrated a significant
reduction in the MDA levels in the hippocampus, with the GB, BHD-M
and BHD-H groups exhibiting significantly decreased MDA levels
(P<0.01, compared with the model group). In addition, compared
with the model group, both GB and BHD increased the activities of
SOD, CAT and GSH (P<0.01). 2VO markedly downregulated the mRNA
and protein expression levels of CAT and GPx-1 in the hippocampus
(P<0.01, compared with the sham group; Fig. 4). Both GPx-1 and CAT expression
increased at the mRNA and protein levels in a dose-dependent manner
following BHD treatment, which is consistent with the ELISA
data.
 | Table IIEffects of BHD on the content of SOD,
CAT, GSH and MDA in the 2VO rats. |
Table II
Effects of BHD on the content of SOD,
CAT, GSH and MDA in the 2VO rats.
| Groups | MDA (nmol/mg) | SOD (U/mg) | CAT (U/mg) | GSH (μg/mg) |
|---|
| Sham | 8.32±0.52b | 115.03±9.67b | 6.94±0.69b |
104.90±13.33b |
| 2VO | 11.49±0.54 | 100.19±8.53 | 5.20±0.35 | 79.54±9.85 |
| BHD-L | 10.79±1.45 | 109.16±2.83 | 6.01±0.63a | 88.97±7.99a |
| BHD-M | 9.81±0.67b |
110.68±12.43a | 6.51±0.79b | 94.41±16.66a |
| BHD-H | 8.49±0.49b | 130.60±9.13b | 6.65±0.43b | 98.47±10.16b |
| GB | 8.90±0.53b | 110.84±7.11a | 6.05±0.63a | 92.63±7.36a |
Discussion
Cognitive impairment of all types, from mild to
severe associated with cerebrovascular damage, is defined as
vascular cognitive impairment, as first proposed by O’Brien et
al (19) which is
characterized as the prodromal stages of ischemic VaD (20). Accumulating evidence has implicated
VCI as a prominent cause of cognitive decline in the elderly
(21,22).
The MWM test is a classic method to examine spatial
learning and memory function (23). In the present study, to exclude the
possibility that the 2VO model and drug administration affect
motoric disability rather than cognitive decline, the swimming
velocity was measured prior to the formal MWM test. There was no
significant difference in the time to swim the distance (120 sec)
between the groups (P>0.05, data not shown). This quality
control ensured the equal basic physical and motoric ability in the
water for each group. Next, escape latency and probe trials were
conducted to determine the acquisition and retention ability of the
experimental rats. The results demonstrated that in rats with
cerebral hypoperfusion, treatment with BHD significantly increased
the number of crossings over the target quadrant and decreased the
escape latency to reach the platform, particularly at the mid and
high dosages, indicating that BHD improved cognitive functioning in
the rats with 2VO.
Synapse formation and plasticity are necessary for
memory recall and consolidation. Previous studies have revealed
that synapse loss is associated with cognitive impairment observed
in various cognitive dysfunction models, including AD (24,25),
middle cerebral artery occlusion (26) and 2VO (27). In the present study, BHD treatment
evidently alleviated the synaptic loss induced by cerebral
hypoperfusion. Reversing this pathological change may have largely
contributed to the memory recall and improvement of cognitive
impairment.
Increased levels of oxidative stress and antioxidant
deficiencies may pose as risk factors for cognitive decline
(28). Chronic vascular
hypoperfusion induces oxidative stress and brain energy failure and
leads to neuronal death. This is because the brain is particularly
susceptible to free radical attack, which generates more toxins per
gram of tissue than any other organ in the body (29). Therefore, anti-oxidative therapy is
considered as a good way to improve cognitive deficits (30). MDA is a marker of lipid
peroxidation and the levels of MDA indicate the degree of oxidative
stress (31). In the antioxidant
enzyme defense system, SOD catalyzes the formation of hydrogen
peroxide from superoxide radicals, while CAT and GPx prevent or
remove toxic hydroxyl radicals generated by hydrogen peroxide
(32).
The most significant ingredient of BHD,
Epimedium, an important Chinese Herb which nourishes the
kidney and enhances brain functioning, has been proved to possess
antioxidant activities (33,34).
The ministerial drug Salvia Miltrorrhiza, which has an
activating effect on the blood and is often used for
cerebrovascular diseases, was also demonstrated to have
anti-oxidant activities (35). The
extract of GB leaves, the positive control used in this study, was
found to possess cognitive enhancing effects associated with its
antioxidant properties (36). In
the present study, BHD reduced MDA levels and enhanced the activity
of SOD, CAT and GSH in the hippocampi of rats with 2VO. BHD
treatment also increased the expression of SOD and GPx at both the
protein and mRNA levels. These results indicated that the role of
BHD improving learning and memory is associated with the contents
or activity of antioxidants and antioxidative enzymes.
In conclusion, the present study evidently
demonstrated that BHD improved learning and memory deficits induced
by cerebral hypoperfusion, and the restoration of elevated
antioxidants and synapse density may have contributed to this
improvement. The present study provided evidence that will
facilitate developing BHD as a preventive or therapeutic for
VaD.
Acknowledgements
This study was supported by the Developmental Fund
of Chen Keji Integrative Medicine, no. CKJ2010025, and the Key
Foundation of Society Development in Fujian province, no.
2013Y0059.
References
|
1
|
Gorelick PB and Pantoni L: Advances in
vascular cognitive impairment. Stroke. 44:307–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chui HC: Vascular cognitive impairment:
today and tomorrow. Alzheimers Dement. 2:185–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moorhouse P and Rockwood K: Vascular
cognitive impairment: current concepts and clinical developments.
Lancet Neurol. 7:246–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Consoli A, Pasi M and Pantoni L: Vascular
mild cognitive impairment: concept, definition, and directions for
future studies. Aging Clin Exp Res. 24:113–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dichgans M and Zietemann V: Prevention of
vascular cognitive impairment. Stroke. 43:3137–3146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalaria RN: Cerebrovascular disease and
mechanisms of cognitive impairment: evidence from
clinicopathological studies in humans. Stroke. 43:2526–2534. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luckhaus C, Flüb MO, Wittsack HJ, et al:
Detection of changed regional cerebral blood flow in mild cognitive
impairment and early Alzheimer’s dementia by perfusion-weighted
magnetic resonance imaging. Neuroimage. 40:495–503. 2008.PubMed/NCBI
|
|
8
|
Panza F, Frisardi V, Capurso C, et al:
Metabolic syndrome and cognitive impairment: current epidemiology
and possible underlying mechanisms. J Alzheimers Dis. 21:691–724.
2010.PubMed/NCBI
|
|
9
|
Clare R, King VG, Wirenfeldt M and Vinters
HV: Synapse loss in dementias. J Neurosci Res. 88:2083–2090. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jesky R and Hailong C: Are herbal
compounds the next frontier for alleviating learning and memory
impairments? An integrative look at memory, dementia and the
promising therapeutics of traditional chinese medicines. Phytother
Res. 25:1105–1118. 2011. View
Article : Google Scholar
|
|
11
|
Liu X, Du J, Cai J, Xu G, Lin A and Teng
Q: Clinical systematic observation of Kangxin capsule curing
vascular dementia of senile kidney deficiency and blood stagnation
type. J Ethnopharmacol. 112:350–355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moss G: The adequacy of the cerebral
collateral circulation: tolerance of awake experimental animals to
acute bilateral common carotid artery occlusion. J Surg Res.
16:337–338. 1974. View Article : Google Scholar
|
|
13
|
Brondino N, De Silvestri A, Re S, et al: A
Systematic review and meta-analysis of ginkgo biloba in
neuropsychiatric disorders: from ancient tradition to modern-day
medicine. Evid Based Complement Alternat Med. 2013:9156912013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ihl R: Effects of Ginkgo biloba extract
EGb 761 in dementia with neuropsychiatric features: review of
recently completed randomised, controlled trials. Int J Psychiatry
Clin Pract. 1(Suppl 1): 8–14. 2013. View Article : Google Scholar
|
|
15
|
Morris R: Developments of a water-maze
procedure for studying spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984. View Article : Google Scholar
|
|
16
|
Feng Z, Lu Y, Wu X, et al: Ligustilide
alleviates brain damage and improves cognitive function in rats of
chronic cerebral hypoperfusion. J Ethnopharmacol. 144:313–321.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scheff SW, Price DA, Schmitt FA, et al:
Synaptic alterations in CA1 in mild Alzheimer disease and mild
cognitive impairment. Neurology. 68:1501–1508. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Xing A, Xu C, Cai Q, Liu H and Li
L: Cerebrovascular hypoperfusion induces spatial memory impairment,
synaptic changes, and amyloid-β oligomerization in rats. J
Alzheimers Dis. 21:813–822. 2010.PubMed/NCBI
|
|
19
|
O’Brien JT, Erkinjuntti T, Reisberg B, et
al: Vascular cognitive impairment. Lancet Neurol. 2:89–98.
2003.
|
|
20
|
Ramos-Estébanez C, Moral-Arce I,
Muñoz-Arrondo R, et al: Vascular cognitive impairment: prodromal
stages of ischemic vascular dementia. Dement Geriatr Cogn Disord.
25:451–460. 2008.PubMed/NCBI
|
|
21
|
Hachinski V, Iadecola C, Petersen RC, et
al: National Institute of Neurological Disorders and
Stroke-Canadian Stroke Network vascular cognitive impairment
harmonization standards. Stroke. 37:2220–2241. 2006. View Article : Google Scholar
|
|
22
|
Bowler JV: Modern concept of vascular
cognitive impairment. Br Med Bull. 83:291–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D’Hooge R and De Deyn PP: Applications of
the Morris water maze in the study of learning and memory. Brain
Res Brain Res Rev. 36:60–90. 2001.PubMed/NCBI
|
|
24
|
Dong H, Martin MV, Chambers S and
Csernansky JG: Spatial relationship between synapse loss and
beta-amyloid deposition in Tg2576 mice. J Comp Neurol. 500:311–321.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marcello E, Epis R, Saraceno C and Di Luca
M: Synaptic dysfunction in Alzheimer’s disease. Adv Exp Med Biol.
970:573–601. 2012.
|
|
26
|
Yao ZB, Li X and Xu ZC: GABAergic and
asymmetrical synapses on somata of GABAergic neurons in CA1 and CA3
regions of rat hippocampus. A quantitative electron microscopic
analysis. Stroke. 27:1411–1415. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hai J, Yu F, Lin Q and Su SH: The changes
of signal transduction pathways in hippocampal regions and
postsynaptic densities after chronic cerebral hypoperfusion in
rats. Brain Res. 1429:9–17. 2012. View Article : Google Scholar
|
|
28
|
Berr C, Balansard B, Arnaud J, Roussel AM
and Alperovitch A: Cognitive decline is associated with systemic
oxidative stress: the EVA study. Etude du Vieillissement Artériel.
J Am Geriatr Soc. 48:1285–1291. 2000.PubMed/NCBI
|
|
29
|
Reiter RJ: Oxidative processes and
antioxidative defense mechanisms in the aging brain. FASEB J.
9:526–533. 1995.PubMed/NCBI
|
|
30
|
Mecocci P, Mariani E, Cornacchiola V and
Polidori MC: Antioxidants for the treatment of mild cognitive
impairment. Neurol Res. 26:598–602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bennett S, Grant MM and Aldred S:
Oxidative stress in vascular dementia and Alzheimer’s disease: a
common pathology. J Alzheimers Dis. 17:245–257. 2009.
|
|
32
|
López N, Tormo C, De Blas I, Llinares I
and Alom J: Oxidative stress in Alzheimer’s disease and mild
cognitive impairment with high sensitivity and specificity. J
Alzheimers Dis. 33:823–829. 2013.
|
|
33
|
Yuan D, Wang H, He H, et al: Protective
effects of total flavonoids from Epimedium on the male mouse
reproductive system against cyclophosphamide-induced oxidative
injury by up-regulating the expressions of SOD3 and GPX1. Phytother
Res. 28:88–97. 2014. View
Article : Google Scholar
|
|
34
|
Cheng H, Feng S, Jia X, Li Q, Zhou Y and
Ding C: Structural characterization and antioxidant activities of
polysaccharides extracted from Epimedium acuminatum. Carbohydr
Polym. 92:63–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu YC and Hsieh CL: Pharmacological
effects of Radix Angelica Sinensis (Danggui) on cerebral
infarction. Chin Med. 6:322011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mansour SM, Bahgat AK, El-Khatib AS and
Khayyal MT: Ginkgo biloba extract (EGb 761) normalizes hypertension
in 2K, 1C hypertensive rats: role of antioxidant mechanisms, ACE
inhibiting activity and improvement of endothelial dysfunction.
Phytomedicine. 18:641–647. 2011. View Article : Google Scholar
|