Introduction
Among infants and young children under 5 years of
age, acute respiratory infection is the most common cause of
morbidity and mortality (1).
Globally, respiratory syncytial virus (RSV) is the most common
cause of childhood acute lower respiratory infection (ALRI) and a
major cause of hospital admissions when it results in severe ALRI.
Data suggest that RSV is a significant cause of mortality during
childhood due to its role in ALRI (2). Previous studies have demonstrated
that severe early RSV bronchiolitis is associated with an increased
prevalence of allergic asthma, which can persist into early
adulthood (3). RSV has also been
implicated in other respiratory illnesses, and can be serious in
elderly patients and patients with chronic lung disease or
immunological deficiency (4).
Cytokines and chemokines, which are secreted by
airway epithelial cells, have been demonstrated to be critical in
the regulation of local inflammatory processes in the lungs.
Production of proinflammatory cytokines, such as interleukin
(IL)-15, MICA and IL-6, increases in epithelial cells following RSV
infection (5). A number of the
underlying pathways or nuclear transcription factors involved have
been shown to be important for the replication and budding of RSV,
through regulation by protein kinase Cδ, hypoxia-inducible
factor-1α, or the nuclear factor (NF)-κB pathway (6). In a live RSV-infected human tracheal
epithelial cell line (9HTEo), the mRNA expression levels of
Toll-like receptors (TLRs) 1–10 were upregulated compared with
levels in cells infected with ultraviolet (UV)-inactivated RSV
(7). However, the mechanisms and
signaling pathways in RSV-induced reactive airway diseases remain
unclear.
Previous studies have indicated that IL-8 is
released in the upper respiratory tract in response to RSV
infection (8,9). Studies have suggested that IL-8
levels are correlated with clinical disease severity (10,11)
in full-term infants and that IL-8 may lead to a genetic
predisposition to asthma (12).
Another study demonstrated that exposure to IL-8 induced migration
and proliferation of airway smooth muscle cells (13). In the current study, the aim was to
observe the effects of RSV on secretion function in A549 cells
(human type II pulmonary epithelial cells) and to examine the
possible mechanisms involved.
Materials and methods
Viruses and cell lines
The RSV long strain was kindly donated by Professor
Hongwei Wang from the Medical School of Nanjing University,
Nanjing, China. The A549 cell line was obtained from the Cell
Resources Center of Shanghai Institutes for Biological Sciences,
Shanghai, China.
Cell culture and infection
The A549 cell line was cultured in Eagle’s minimal
essential medium (Gibco-BRL, Grand Island, NY, USA) containing 10%
fetal calf serum and 10 μg/ml ampicillin. A549 cells were seeded at
a density of 5×104 cells/well in compliance with the
manufacturer’s instructions in 6-well tissue culture plates
(Corning Inc., Corning, NY, USA). Cells were maintained until they
reached ~70% confluency. After 6 h of cell culture, half of the
A549 cells were infected with RSV at a multiplicity of infection of
1. The cells were infected with RSV for 24 h. Infection was
confirmed using a light microscope (Olympus, Tokyo, Japan).
Measurement of cytokine production
After 24 h of infection, the culture supernatants of
the RSV-infected and non-infected cells were collected. IL-8
protein levels in the culture supernatants were measured using an
enzyme-linked immunosorbent assay (ELISA) (EMD Millipore,
Darmstadt, Germany).
Microarray analysis
To identify possible signaling pathways involved in
the secretion of IL-8, infected and non-infected cells were
subjected to microarray analysis (KangChen Bio-tech, Inc.,
Shanghai, China). Total RNA from each sample was quantified with a
NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific,
Waltham, MA, USA) and RNA integrity was assessed using standard
denaturing agarose gel electrophoresis. Total RNA (~5 μg) of each
sample was used for labeling and array hybridization according to
the following steps: i) Reverse transcription with Superscript
ds-cDNA Synthesis kit (Invitrogen, Life Technologies, Carlsbad, CA,
USA); ii) ds-cDNA labeling with NimbleGen One-Color DNA Labeling
kit; iii) array hybridization using the NimbleGen Hybridization
system followed by washing with the NimbleGen Wash Buffer kit
(Roche Diagnostics, Basel, Switzerland); and iv) array scanning
using the Axon GenePix 4000B Microarray scanner (Molecular Devices,
Sunnyvale, CA, USA).
Scanned images were then imported into NimbleScan
software (version 2.6; Roche Diagnostics) for grid alignment and
expression data analysis. Expression data were normalized through
quantile normalization and the Robust Multi-chip Average algorithm
in the NimbleScan software. The probe level and gene level were
imported into GeneSpring GX software (version 11.5.1; Agilent
Technologies, Inc., Santa Clara, CA, USA) for further analysis.
Genes with values ≥50.0 in 2/2 samples were selected for data
analysis. Differentially expressed genes were identified through
fold change filtering. Pathway analysis was applied to determine
the roles of these differentially expressed genes in these
biological pathways. Hierarchical clustering was performed to
clarify distinguishable gene expression profiling among
samples.
Quantitative polymerase chain reaction
(qPCR)
For microarray data validation, qPCR was performed
as previously described (7).
Briefly, first-strand synthesis was performed using an RNA
First-Strand Synthesis kit (Roche Diagnostics) with 40 ng of total
RNA (Roche Diagnostics). SYBR Green (Roche Diagnostics) PCR was
performed for MYD88, TRAM and TRIF according to the manufacturer’s
instructions. GAPDH was used as an endogenous control. The primer
sequences were as follows: MYD88 F, 5′-GATGGTGGTGGTTGTCTCTGAT-3′
and R, 5′-GCTGGGGAACTCTTTCTTCATT-3′; TRAM F, 5′-TCA
AACCCGGAATAATCTTTGCT-3′ and R, 5′-GGGCCGCAT GGGTATAACAG-3′; TRIF F,
5′-GCCAGCAACTTGGAA ATCAGC-3′ and R, 5′-GGGGTCGTCACAGAGCTTG-3′;
GAPDH F, 5′-AGAAGGCTGGGGCTCATTTG-3′ and R,
5′-AGGGGCCATCCACAGTCTTC-3′. Data obtained by qPCR were evaluated
using the 2−ΔΔCt method.
Electrophoretic mobility shift assay
(EMSA)
EMSAs were performed. AP-1 protein was extracted
from the nuclei of the two groups of cells (Vazyme, Piscataway, NJ,
USA) according to manufacturer’s instructions. The EMSA kit was
procured from Pierce (Rockford, IL, USA).
Briefly, an AP-1 consensus oligonucleotide was
prepared with the following sequence: F, 5′-CGCTTGATGACTCAG
CCGGAA-3′ and R, 3′-GCGAACTACTGAGTCGGCCTT-5′ (Beyotime, Shanghai,
China). The nuclear extracts (10 μg) were incubated for 20 min with
the gel shift-binding buffer (Beyotime), prior to the separation of
the labeled probe and protein-DNA complexes by electrophoresis on a
6% polyacrylamide gel. Following the electrophoretic transfer of
the bound complexes to a nylon membrane (Amersham, Uppsala,
Sweden), the transferred DNA was cross-linked to the membrane.
Biotin-labeled DNA was then detected through chemiluminescence
using ChemiDoc XRS+ System with Image Lab Software (Bio-Rad,
Berkeley, CA, USA).
Statistical analysis
Data were analyzed using SPSS software, version 13.0
(SPSS, Inc., Chicago, IL, USA). Homogeneity of variance F-tests
were used to compare the samples, followed by t- or t′-test.
P<0.05 was considered to indicate a statistically significant
difference. Data are presented as the mean ± standard deviation
(SD).
Results
Airway epithelial responses to RSV
infection
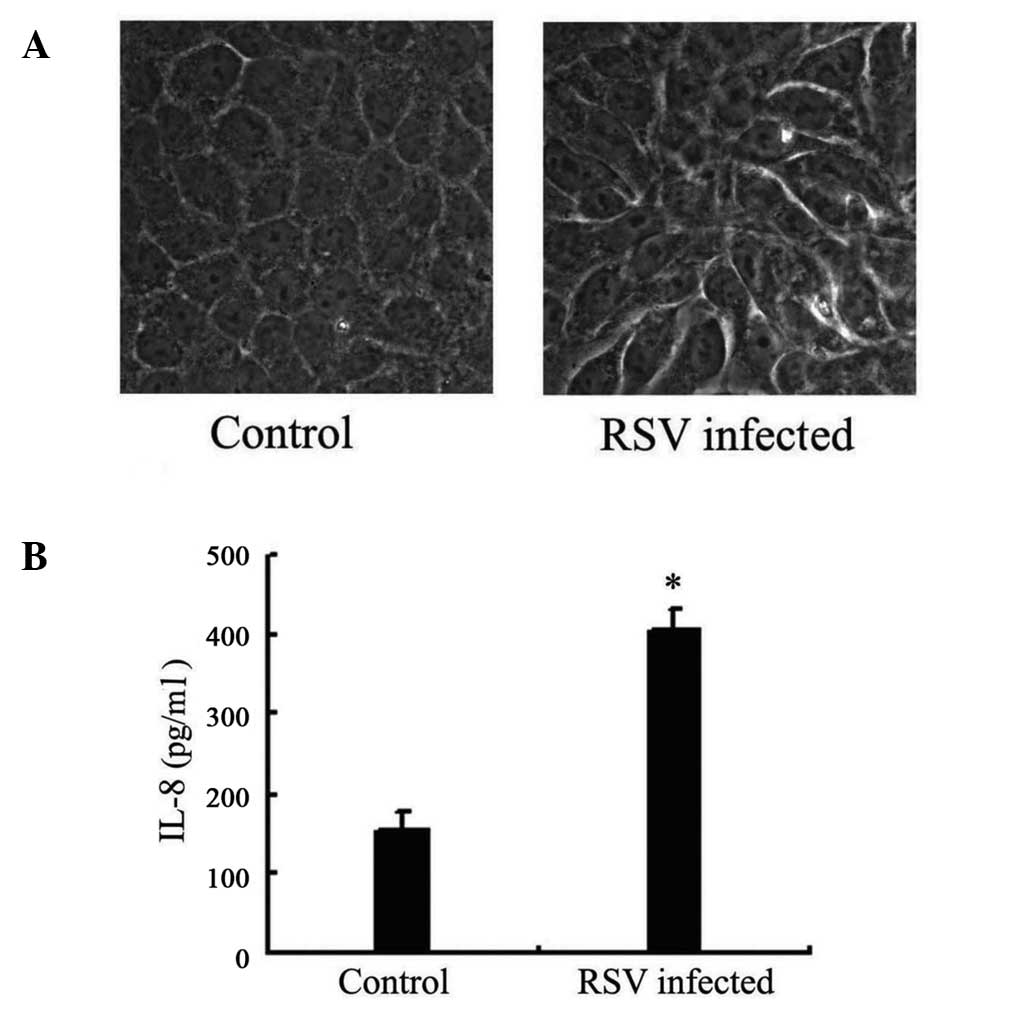
Following RSV infection for 24 h, morphological
changes in the A549 cells were detected. Cell fusion was observed
with a light microscope, which was considered to indicate RSV
infection (Fig. 1A).
Release of IL-8 increases in A549 cells
following RSV infection
Following RSV infection for 24 h, the IL-8
concentration in the supernatant of A549 cells was measured. ELISA
results demonstrated that the IL-8 concentration in the supernatant
of infected cells was 405 ng/ml, while the concentration in
non-infected cells was 155 ng/ml, indicating that RSV causes a
significant increase (P=0.01; Fig.
1B).
RSV induces changes in mRNA expression
levels as detected by microarray analysis
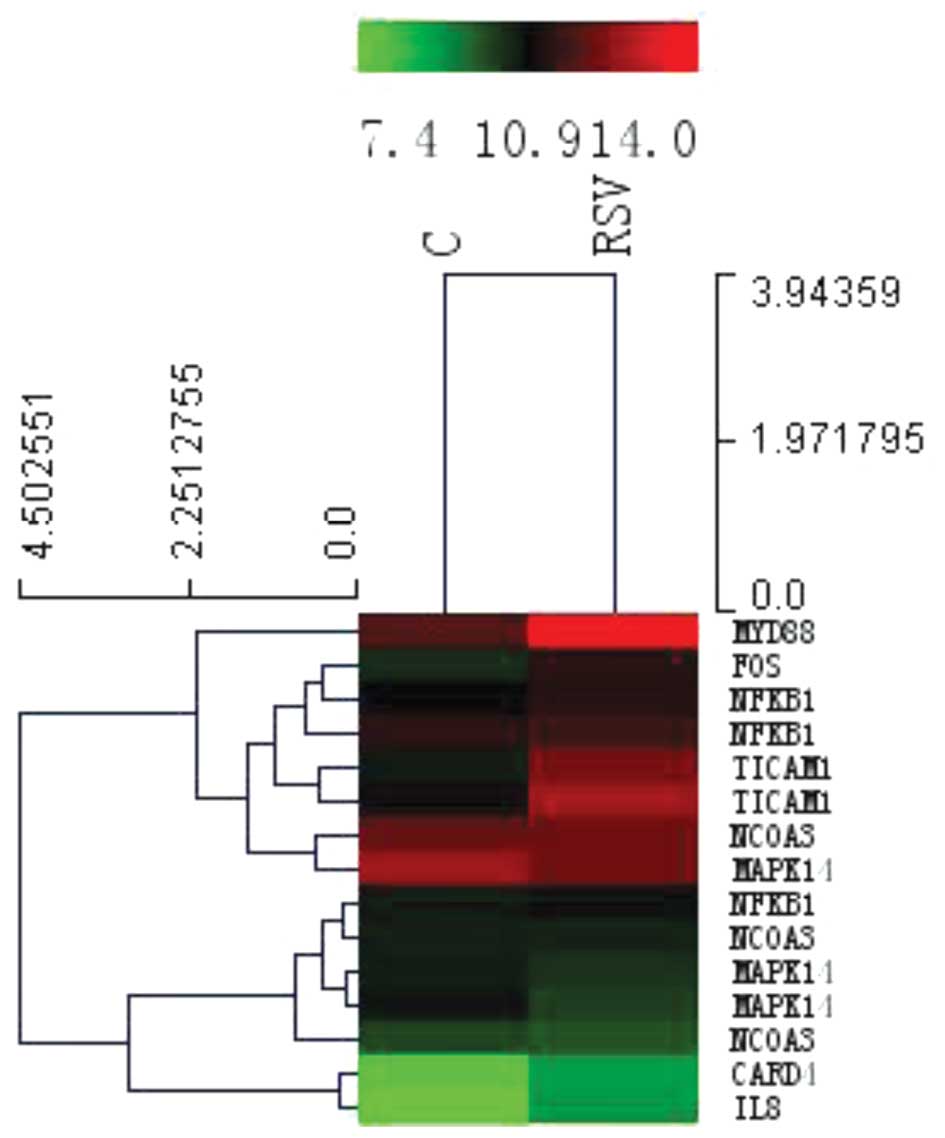
Microarray analysis of human bronchial epithelial
cells displayed significant changes in the global profile of mRNA
expression following infection with RSV. The heat map with
hierarchical cluster analysis (Fig.
2) and the bar plots displaying the top ten enrichment score
[−log10(P-value)] values of the significantly enriched pathways for
each group (Fig. 3A and B) are
presented. These imply that the TLR signaling pathways, in
particular those of TLR4, are important in the mechanism of IL-8
secretion occurring in RSV-infected A549 cells compared with that
in non-infected control cells, in which MYD88, TRAM, TRIF and AP-1
are also involved.
mRNA levels of MYD88, TRAM and TRIF
In order to confirm the effect of the TLR4 signaling
pathway on A549 cells following RSV infection, qPCR was performed.
mRNA expression levels of MYD88, TRAM and TRIF were significantly
increased (P=0.035, SD=0.0116; P=0.026, SD=0.00095; and P=0.001,
SD=0.00051, respectively) in RSV-infected cells compared with those
in non-infected cells (Fig.
4).
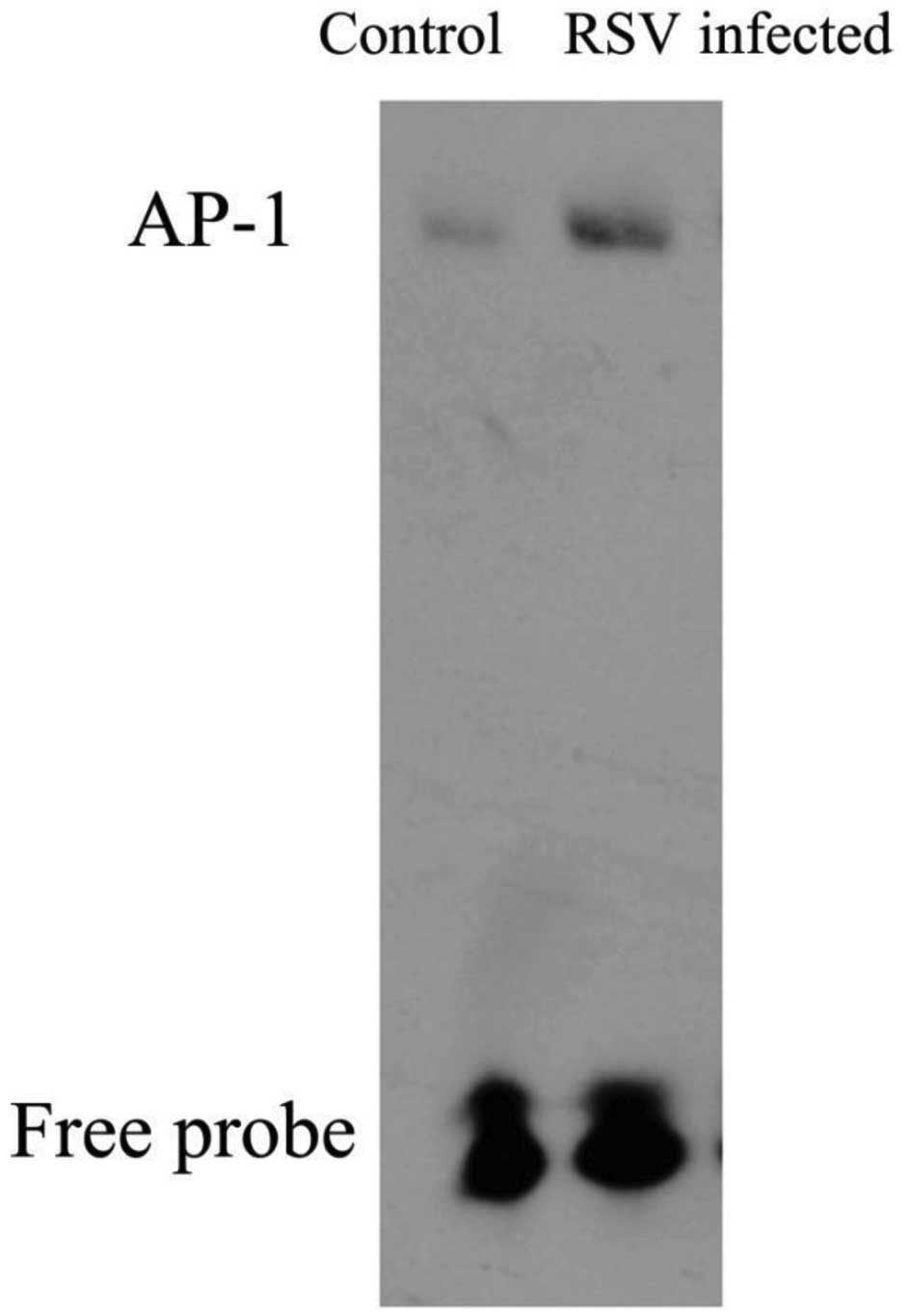
AP-1 protein expression differences in
EMSA
Nuclear factor AP-1 protein expression was
investigated by EMSA using nuclear extracts from control and
RSV-infected cells at 24 h post-infection. As shown in Fig. 5, the levels of AP-1 DNA binding in
the nuclear fractions were clearly higher in the RSV-infected cells
compared with those in the control group. This suggests that RSV
infection may induce the activation of AP-1.
Discussion
Since the primary site for RSV entry and replication
is the respiratory tract, the respiratory epithelium has emerged as
the major origin of airway inflammation (7). It actively participates in the innate
immune response to foreign antigens, which involves the release of
chemokines and cytokines, and the initiation of an inflammatory
reaction. This is followed by the recruitment of phagocytes,
including dendritic cells and lymphocytes, which participate in the
clearance of invading pathogens to facilitate the adaptive immune
response (9).
In the present study, IL-8 production in A549 human
type II pulmonary epithelial cells increased following RSV
infection. IL-8 is one of the most abundant chemokines produced by
airway epithelial cells. As an important member of the CXC branch
of the chemokine family, these proteins may primarily mediate the
activation and migration of neutrophils from the peripheral blood
into tissue and be involved in the initiation and amplification of
inflammatory processes. These processes occur in the human immune
system in response to a wide variety of pathogens.
In the current study, the underlying mechanism of
the upstream signaling pathway was investigated using microarray
experiments and the results indicated that the TLR4 signaling
pathway may be partly responsible for increasing the levels of IL-8
secretion. To confirm these results, qPCR and EMSA were conducted
for MYD88, TRAM and TRIF, and AP-1, respectively. The results
suggested that the transcription factor AP-1 may be important in
IL-8 secretion in RSV-stimulated A549 cells.
TLRs are important in the innate immune response,
and although they are capable of detecting various molecules
derived from viruses, fungi, bacteria and protozoa, intracellular
TLRs primarily function in virus detection. All TLRs, with the
exception of TLR3, depend to a certain extent on the MyD88 adaptor
protein for full signaling activity. TLR4, which is located on the
cell surface (14), induces MyD88
signaling at the plasma membrane prior to being endocytosed and
subsequently activates TRAM-TRIF signaling from early endosomes
(15). When TLR4 migrates to late
endosomes, it encounters TAG, a splice variant of TRAM that
negatively modulates TLR4-TRAM signaling from endosomes, ultimately
promoting the degradation of the signaling complex (16). Activation of interferon regulatory
factors, NF-κB and AP-1 transcription factors induces gene
transcription of proinflammatory cytokines such as tumor necrosis
factor and IL-12 (17). The AP-1
transcription factor is a dimeric complex that comprises members of
the JUN, FOS, activating transcription factor and
musculoaponeurotic fibrosarcoma protein families (18). Fos and Jun family proteins function
as dimeric transcription factors that bind to AP-1 regulatory
elements in the promoter and enhancer regions of numerous mammalian
genes. The DNA-binding and dimerization domains of different family
members are highly conserved and different members of the Fos and
Jun families have similar DNA-binding and dimerization
specificities (19). In the
process of inflammation, attraction and activity of immune cells
are regulated by a plethora of different cytokines that are
predominantly activated by the transcription factors, including
AP-1. Specifically in the innate immune system, TLRs are an
important initiation point of specific sensing for environmental
changes. The signaling of TLRs leading to cytokine production may
consequently activate AP-1 (20).
In conclusion, the present study demonstrates one
critical signaling pathway in IL-8 secretion of RSV-infected airway
epithelial cells, which may be the MyD88/TRAM/TRIF/AP-1 signaling
pathway. However, further studies concerning the signaling pathways
involved in the increased cytokine production of RSV-infected A549
cells are required.
Acknowledgements
The current research was supported by the National
Natural Science Foundation of China (81200012; Feng Liu), by the
Nanjing Medical Science and Technique Development Foundation (Feng
Liu), and by a grant from Key Project supported by Medical Science
and Technology Development Foundation, Nanjing Department of Health
(201108012; Deyu Zhao). The authors also thank the Medical School
of Nanjing University for kindly donating RSV, long strain, and
Professor Hongwei Wang and Qiuqin Feng for technical support.
References
|
1
|
Liu L, Johnson HL, Cousens S, et al; Child
Health Epidemiology Reference Group of WHO and UNICEF. Global,
regional, and national causes of child mortality: an updated
systematic analysis for 2010 with time trends since 2000. Lancet.
379:2151–2161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nair H, Nokes DJ, Gessner BD, Dherani M,
Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, et
al: Global burden of acute lower respiratory infections due to
respiratory syncytial virus in young children: a systematic review
and meta-analysis. Lancet. 375:1545–1555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sigurs N, Aljassim F, Kjellman B, Robinson
PD, Sigurbergsson F, Bjarnason R and Gustafsson PM: Asthma and
allergy patterns over 18 years after severe RSV bronchiolitis in
the first year of life. Thorax. 65:1045–1052. 2010.PubMed/NCBI
|
|
4
|
Walsh EE: Respiratory syncytial virus
infection in adults. Semin Respir Crit Care Med. 32:423–432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zdrenghea MT, Telcian AG, Laza-Stanca V,
Bellettato CM, Edwards MR, Nikonova A, Khaitov MR, Azimi N, Groh V,
Mallia P, et al: RSV infection modulates IL-15 production and MICA
levels in respiratory epithelial cells. Eur Respir J. 39:712–720.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masaki T, Kojima T, Okabayashi T, et al: A
nuclear factor-κB signaling pathway via protein kinase C δ
regulates replication of respiratory syncytial virus in polarized
normal human nasal epithelial cells. Mol Biol Cell. 22:2144–2156.
2011.
|
|
7
|
Xie XH, Law HK, Wang LJ, Li X, Yang XQ and
Liu EM: Lipopolysaccharide induces IL-6 production in respiratory
syncytial virus-infected airway epithelial cells through the
toll-like receptor 4 signaling pathway. Pediatr Res. 65:156–162.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abu-Harb M, Bell F, Finn A, Rao WH, Nixon
L, Shale D and Everard ML: IL-8 and neutrophil elastase levels in
the respiratory tract of infants with RSV bronchiolitis. Eur Respir
J. 14:139–143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gern JE, Martin MS, Anklam KA, Shen K,
Roberg KA, Carlson-Dakes KT, Adler K, Gilbertson-White S, Hamilton
R, Shult PA, et al: Relationships among specific viral pathogens,
virus-induced interleukin-8, and respiratory symptoms in infancy.
Pediatr Allergy Immunol. 13:386–393. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hornsleth A, Loland L and Larsen LB:
Cytokines and chemokines in respiratory secretion and severity of
disease in infants with respiratory syncytial virus (RSV)
infection. J Clin Virol. 21:163–170. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Assefa D, Amin N, Dozor AJ and Parton LA:
Attenuated interleukin-8/leukocyte immunoresponse in preterm
infants compared with term infants hospitalized with respiratory
syncytial virus bronchiolitis: a pilot study. Hum Immunol.
72:708–711. 2011. View Article : Google Scholar
|
|
12
|
Puthothu B, Krueger M, Heinze J, Forster J
and Heinzmann A: Impact of IL8 and IL8-receptor alpha polymorphisms
on the genetics of bronchial asthma and severe RSV infections. Clin
Mol Allergy. 4:22006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Govindaraju V, Michoud MC, Ferraro P,
Arkinson J, Safka K, Valderrama-Carvajal H and Martin JG: The
effects of interleukin-8 on airway smooth muscle contraction in
cystic fibrosis. Respir Res. 9:762008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toshchakov V, Jones BW, Perera PY, Thomas
K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ
and Vogel SN: TLR4, but not TLR2, mediates IFN-beta-induced
STAT1alpha/beta-dependent gene expression in macrophages. Nat
Immunol. 3:392–398. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kagan JC, Su T, Horng T, Chow A, Akira S
and Medzhitov R: TRAM couples endocytosis of Toll-like receptor 4
to the induction of interferon-beta. Nat Immunol. 9:361–368. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palsson-McDermott EM, Doyle SL, McGettrick
AF, Hardy M, Husebye H, Banahan K, Gong M, Golenbock D, Espevik T
and O’Neill LA: TAG, a splice variant of the adaptor TRAM,
negatively regulates the adaptor MyD88-independent TLR4 pathway.
Nat Immunol. 10:579–586. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blasius AL and Beutler B: Intracellular
toll-like receptors. Immunity. 32:305–315. 2010. View Article : Google Scholar
|
|
18
|
Eferl R and Wagner EF: AP-1: a
double-edged sword in tumorigenesis. Nat Rev Cancer. 3:859–868.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Curran T and Franza BR Jr: Fos and Jun:
the AP-1 connection. Cell. 55:395–397. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawai T, Sato S, Ishii KJ, Coban C, Hemmi
H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uematsu S, et al:
Interferon-alpha induction through Toll-like receptors involves a
direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol.
5:1061–1068. 2004. View
Article : Google Scholar : PubMed/NCBI
|