Introduction
Coronary heart disease (CHD) is one of the primary
causes of mortality in humans in developed and developing
countries. Previous studies have demonstrated that the plasma
levels of intercellular adhesion molecule-1 (ICAM-1) may serve as a
molecular marker for atherosclerosis and the development of CHD
(1,2). ICAM-1, which belongs to the
immunoglobulin superfamily, is typically expressed in endothelial
cells and leukocytes where it serves as a receptor for the
leukocyte integrins lymphocyte function-associated antigen 1 and
Mac-1 (3–5). In the pathogenesis of
atherosclerosis, ICAM-1 plays an important role in recruiting
mononuclear cells into the basement membrane of the vasculature
(6,7). Therefore, ICAM-1 may be an important
factor in the development and progression of atherosclerosis.
Several polymorphisms for the ICAM-1 gene have been described.
rs5498, a single-base A–G transition polymorphism, is located in
exon 6 of the ICAM-1 gene. The missense mutation results in an
amino acid substitution from glutamine (E) to lysine (K), and it
has been found to be related to inflammatory diseases and
atherosclerosis. Thus, in the present case-control study, the
association between the rs5498 polymorphism of the ICAM-1
gene and CHD was investigated in patients with CHD and control
subjects in a Chinese Han population.
Subjects and methods
Ethics statement
This study was conducted according to the
Declaration of Helsinki, and was approved by the Ethics Committee
of the First Affiliated Hospital of Xinjiang Medical University
(Urumqi, China). Written informed consent was obtained from all
participants prior to the start of the study.
Subjects
All participants were genetically unrelated Chinese
Han individuals from Xinjiang, China. Participants with CHD were
recruited from the First Affiliated Hospital of Xinjiang Medical
University between 2007 and 2010. All patients with CHD were
diagnosed according to their medical history, clinical symptoms,
12-lead electrocardiogram and laboratory examinations, and the
diagnosis was confirmed by coronary arteriography (>50% stenosis
in at least one coronary artery). The control participants were
selected from the cardiovascular risk survey (CRS) (8). The CRS is a prospective,
observational cohort study designed to investigate the prevalence,
incidence and risk factors for cardiovascular disease in the Han,
Uygur and Kazakh populations in Xinjiang, China. The baseline data
for the control participants were collected between June 2007 and
March 2010. Inclusion criteria were as follows: No chest pain,
normal electrocardiograph, normal blood biochemistry values and
normal coronary arteriography.
Lifestyle data collection
Lifestyle data (age, smoking and drinking habits and
medical history) were collected using questionnaires, and height,
body weight and blood pressure were measured by doctors.
Participants who had smoked at least one cigarette per day in the
previous 12 months were considered as current smokers. Individuals
who had consumed ≤50 mg alcohol once a week in the previous 12
months were considered as current alcohol users. Essential
hypertension (EH) was defined as systolic blood pressure ≤140 mmHg
and/or diastolic blood pressure ≤90 mmHg on at least two separate
occasions, or antihypertensive treatment.
Laboratory examination and DNA
extraction
A total of 5 ml peripheral venous blood samples were
collected in EDTA-containing tubes from all participants following
fasting for 12 h. Serum and plasma collected for measurement were
immediately frozen at −80°C for further analysis. The
concentrations of triglyceride (TG), total cholesterol (TC),
high-density lipoprotein-cholesterol (HDL-C) and low-density
lipoprotein-cholesterol (LDL-C) were measured using standard
methods in the Central Laboratory of the First Affiliated Hospital
of Xinjiang Medical University. DNA was extracted from peripheral
vein blood leukocytes using a whole blood genome extraction kit
(BioTeke Corporation, Beijing, China).
Genotyping of ICAM-1
The primers were designed by Primer Premier 5.0
software (Premier Biosoft International, Vancouver, BC, Canada).
The primers were as follows: forward, 5′-GGCCAGCTTATACACAAGAACC-3′
and reverse, 5′-TGTCATCATACTGTGGTAGCA-3′. Synthesis was performed
by Shanghai Hi-Tech Bioengineering Co., Ltd. (Shanghai, China). The
reaction was performed in a 25-μl volume, containing 12.5 μl Power
Mix (BioTeke Corporation), 10.5 μl distilled water, 0.5 mM forward
and reverse primer, respectively, and 1 μl genomic DNA. The
reaction conditions were as follows: Initial denaturation at 95°C
for 5 min, 30 cycles of denaturation at 95°C for 30 sec, annealing
at 53°C for 30 sec, extension at 72°C for 40 sec and extension at
72°C for 7 min. The reaction products were then stored at 4°C. The
polymerase chain reaction (PCR) products (10 μl) were incubated
with 3 units Bsh1236I (Fermentas, Burlington, ON, Canada) in a
total volume of 20 μl at 37°C overnight. The fragments were
separated on a 2% agarose gel with ethidium bromide. Under the
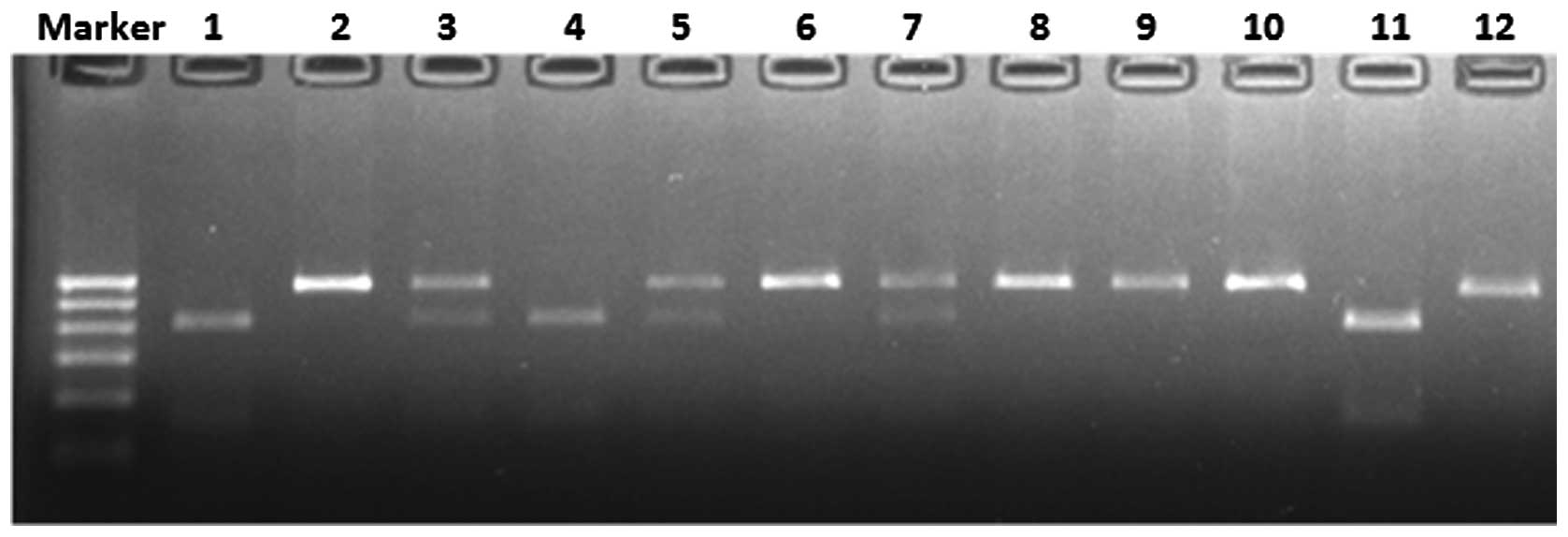
ultraviolet ray, three genotypes were observed: The AA genotype
produced one 541-base pair (bp) band; the AG genotype produced
three bands of 541, 417 and 124 bp; and the GG genotype produced
two bands of 417 and 124 bp (Fig.
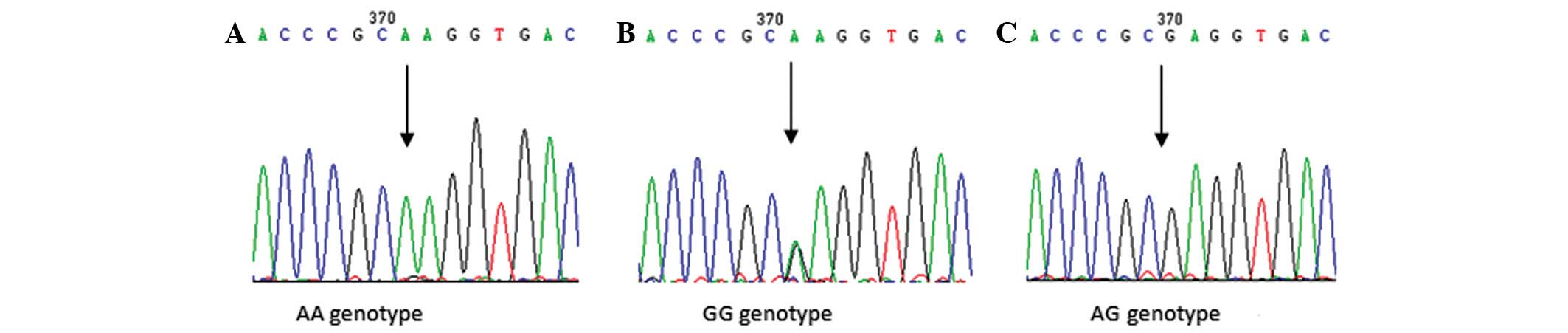
1). To confirm the results, sequenced genomic DNAs were used as
positive controls (Fig. 2).
Sequencing reactions were undertaken by Biomed-Beijing (Beijing,
China).
Statistical analysis
All analyses were performed using SPSS version 17.0
(SPSS, Inc., Chicago, IL, USA). Continuous data are shown as the
mean ± standard deviation and categorical data are shown as
percentages (%). The differences between patients with CHD and
control subjects were assessed using an independent-sample t-test.
The Hardy-Weinberg equilibrium and differences in categorical data
between the two groups were analyzed using a χ2 test.
Differences in the distribution of genotypes and alleles between
the two groups were also analyzed using a χ2 test.
Logistic regression analysis was used to assess the contribution of
the major risk factors to CHD. P<0.05 was considered to indicate
a statistically significant difference.
Results
Characteristics of participants
A total of 1,453 individuals (674 patients with CHD
and 779 healthy controls) participated in this study. The clinical
characteristics of the individuals are shown in Table I. For the three groups, i.e. total
study population, males and females, there were no differences in
age between patients with CHD and healthy controls, suggesting that
the study was an age-matched case-control study. For the total
study population, males and females, there were significantly
higher concentrations of TG, TC and LDL-C and a greater percentage
of EH in patients with CHD than those in healthy controls. However,
the concentration of HDL-C was significantly lower in the CHD group
than that in the control group. For the total study population and
males, the body mass index (BMI) and percentage of individuals with
a smoking habit were significantly different between the two
groups; however, for females, no significant differences in BMI or
smoking were observed between the two groups.
 | Table IDemographic and risk profile of the
study population. |
Table I
Demographic and risk profile of the
study population.
| Total | Males | Females |
|---|
|
|
|
|
|---|
| Controls | CHD | P-value | Controls | CHD | P-value | Controls | CHD | P-value |
|---|
| Total n | 779 | 674 | | 543 | 490 | | 236 | 184 | |
| Age (years)b | 58.71±14.17 | 58.47±9.52 | 0.71 | 59.39±16.00 | 58.75±10.43 | 0.454 | 57.14±8.43 | 57.72±6.465 | 0.445 |
| BMI
(kg/m2)b | 24.46±3.43 | 25.83±3.28 | <0.001a | 24.16±3.27 | 26.19±3.32 | <0.001a | 25.18±3.69 | 24.84±2.96 | 0.322 |
| TG (mmol/l)b | 1.49±1.56 | 7.15±17.47 | <0.001a | 1.49±1.23 | 7.05±19.41 | <0.001a | 1.51±0.98 | 7.38±10.93 | <0.001a |
| TC (mmol/l)b | 4.59±0.98 | 5.56±3.95 | <0.001a | 4.5±0.93 | 5.55±4.11 | <0.001a | 4.79±1.05 | 5.83±3.5 | <0.001a |
| LDL-C
(mmol/l)b | 2.27±1.45 | 2.87±0.92 | <0.001a | 2.3±1.53 | 2.87±0.92 | <0.001a | 2.17±1.2 | 2.84±0.93 | <0.001a |
| HDL-C
(mmol/l)b | 1.26±0.42 | 0.89±0.4 | <0.001a | 1.27±0.41 | 5.55±4.11 | <0.001a | 1.23±0.43 | 1.00±0.62 | <0.001a |
| Smokers (%) | 28.3 | 35.8 | 0.002a | 40.0 | 48.8 | 0.004a | 1.3 | 1.1 | 0.859 |
| EH (%) | 24.8 | 55.2 | <0.001a | 22.8 | 54.5 | <0.001a | 29.2 | 57.1 | <0.001a |
Allele and genotype distribution of
rs5498 (A>G)
The distribution of genotypes between patients with
CHD and healthy controls in the three groups (total study
population, males and females) was consistent with the
Hardy-Weinberg equilibrium. Table
II shows the distribution of alleles and genotypes of rs5498
for the ICAM-1 gene. For the total study population and
males, the distribution of the three genotypes was significantly
different between patients with CHD and control subjects (total
study population, AA vs. AG, P=0.003; AA vs. GG, P=0.01; males, AA
vs. AG, P=0.007; AA vs. GG, P=0.003). The frequency of the G allele
was significantly higher in the CHD group than that in the control
group (P<0.001 in the total study population and males). The
distributions of the three models of rs5498 were significantly
different between the two groups (all P<0.05). There was no
significant difference in the distribution between the two groups
in the females.
 | Table IIGenotype and allele distributions in
patients with CHD and controls subjects. |
Table II
Genotype and allele distributions in
patients with CHD and controls subjects.
| Total | Males | Females |
|---|
|
|
|
|
|---|
| Controls, n (%) | CHD, n (%) | OR | P-value | Controls, n (%) | CHD, n (%) | OR | P-value | Controls, n (%) | CHD, n (%) | OR | P-value |
|---|
| Total n | 779 | 674 | | | 543 | 490 | | | 236 | 184 | | |
| Genotype |
| AA | 461 (0.592) | 339 (0.503) | 1.000 | - | 324 (0.597) | 242 (0.494) | 1.000 | - | 137 (0.581) | 97 (0.527) | 1.000 | - |
| AG | 273 (0.350) | 278 (0.412) | 1.385 | 0.003a | 191 (0.352) | 203 (0.414) | 1.423 | 0.007a | 82 (0.347) | 75 (0.408) | 1.292 | 0.218 |
| GG | 45 (0.058) | 57 (0.085) | 1.723 | 0.01a | 28 (0.051) | 45 (0.092) | 2.152 | 0.003a | 17 (0.072) | 12 (0.065) | 0.997 | 0.994 |
| Dominant model |
| AA | 461 (0.592) | 339 (0.503) | | | 324 (0.597) | 242 (0.494) | | | 137 (0.581) | 97 (0.527) | | |
| AG+GG | 318 (0.408) | 335 (0.497) | 1.433 | 0.001a | 219 (0.403) | 248 (0.506) | 1.516 | 0.001a | 99 (0.419) | 87 (0.592) | 1.241 | 0.275 |
| Recessive
model |
| AG+AA | 734 (0.942) | 617 (0.915) | | | 515 (0.948) | 445 (0.908) | | | 219 (0.928) | 172 (0.935) | | |
| GG | 45 (0.058) | 57 (0.085) | 1.507 | 0.047a | 28 (0.052) | 45 (0.092) | 1.860 | 0.013a | 17 (0.072) | 12 (0.065) | 1.113 | 0.785 |
| Additive model |
| AA | 461 (0.911) | 339 (0.856) | | | 324 (0.921) | 242 (0.843) | | | 137 (0.890) | 97 (0.890) | | |
| GG | 45 (0.089) | 57 (0.144) | 1.723 | 0.01a | 28 (0.079) | 45 (0.157) | 2.152 | 0.003a | 17 (0.110) | 12 (0.110) | 1.003 | 0.994 |
| Allele |
| A | 1195 (0.767) | 956 (0.709) | | | 839 (0.773) | 687 (0.701) | | | 356 (0.754) | 269 (0.731) | | |
| G | 363 (0.233) | 392 (0.291) | 1.350 | <0.001a | 247 (0.227) | 293 (0.299) | 1.449 | <0.001a | 116 (0.246) | 99 (0.269) | 1.129 | 0.444 |
Logistic regression analysis of CHD risk
factors
Multifactor logistic regression analysis revealed
six independent risk factors for CHD: AG+GG dominant model,
smoking, TC, TG, LDL-C and EH. It also showed that HDL-C was a
protective factor for CHD. Following adjustments for smoking, TC,
TG, LDL-C, HDL-C and EH, the subjects with the G allele in rs5498
of the ICAM-1 gene had a significantly higher risk of CHD
[total study population, odds ratio (OR), 1.919; 95% confidence
intervals (CI), 1.471–2.503; P<0.001; males, OR, 2.028; 95% CI,
1.456–2.825; P<0.001; females, OR, 1.661; 95% CI, 1.046–2.637;
P=0.031] (Table III).
 | Table IIIMutiple logistic regression analysis
for patients with CHD and control subjects. |
Table III
Mutiple logistic regression analysis
for patients with CHD and control subjects.
| Total | Males | Females |
|---|
|
|
|
|
|---|
| Risk factors | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| AG+GG vs. AA | 1.919 | 1.471–2.503 | <0.001a | 2.028 | 1.456–2.825 | <0.001a | 1.661 | 1.046–2.637 | 0.031a |
| Smoking | 1.231 | 0.993–1.625 | 0.141 | 1.223 | 1.456–2.825 | <0.001a | 0.445 | 0.043–4.734 | 0.506 |
| TC | 2.590 | 1.586–4.230 | <0.001a | 4.831 | 2.227–10.479 | <0.001a | 1.557 | 0.801–3.106 | 0.187 |
| TG | 2.001 | 1.443–2.775 | <0.001a | 2.085 | 1.383–3.143 | <0.001a | 1.933 | 1.098–3.403 | 0.022a |
| LDL-C | 2.142 | 1.223–3.750 | 0.008a | 3.099 | 1.428–6.725 | 0.004a | 1.307 | 0.565–3.025 | 0.531 |
| HDL-C | 0.244 | 0.188–0.316 | <0.001a | 1.995 | 1.769–2.249 | <0.001a | 0.549 | 0.348–0.865 | 0.01a |
| EH | 4.251 | 3.236–5.585 | <0.001a | 4.473 | 3.429–5.835 | <0.001a | 3.114 | 1.956–4.958 | <0.001a |
Discussion
The human ICAM-1 gene is located on
chromosome 19p13.3-p13.2, and it consists of seven exons separated
by six introns. The rs5498 polymorphism of the ICAM-1 gene,
which is located in exon 6, is a functional mutation resulting in
the substitution of lysine to glutamate (K469E). This mutation may
have a possible functional value in the etiology of atherosclerosis
(9). Using human peripheral
mononuclear cells and Epstein-Barr virus-transformed peripheral
mononuclear cells, Iwao et al (10) demonstrated that individuals
carrying the G-allele may have increased difficulty splicing the
ICAM-1 short isoform (ICAM-1-S) compared with individuals carrying
the A-allele. Therefore, cells with the GG genotype may have
decreased levels of ICAM-1-S mRNA and, thus, higher sensitivity to
apoptosis than cells with the AA genotype. Kitagawa et al
(11) demonstrated that the level
of soluble ICAM-1 (sICAM-1) was closely associated with the
severity of atherosclerosis and cardiovascular events. It was also
suggested that the inhibition of ICAM-1 expression could delay the
progression of atherosclerosis in apolipoprotein E-knockout
mice.
The effect of rs5498 on plasma ICAM-1 levels has
also been investigated. Hulthe et al (12) reported that the level of sICAM-1
was associated with subclinical atherosclerosis and inflammatory
variables in clinically healthy middle-aged males. Puthothu et
al (13) found a significant
association between rs5498 and the serum levels of sICAM-1 in
German children with asthma. It was also found that rs5498 was
associated with sICAM-1 levels in Chinese and European Americans
(14). Therefore, in this study,
it was hypothesized that variability in the rs5498 polymorphism of
the ICAM-1 gene had the potential to affect the risk of CHD.
To investigate this, the polymorphism of the ICAM-1 gene was
genotyped in a Chinese population and the association between the
ICAM-1 gene and CHD was determined using case-control
analysis.
In the present study it was found that there was a
significant difference in the genotype distribution of rs5498
between patients with CHD and control subjects in the total
population. When analyzing males and females separately, the G
allele frequency was higher in males with CHD than that in male
control subjects. This indicates that the risk of CHD was increased
in males carrying the G allele. For males, the distribution of the
dominant model (AG+GG vs. AA) was significantly higher in patients
with CHD than that in control subjects, and this difference
remained significant following adjustment for risk factors. This
indicates that the risk of CHD may increase in males with the AG or
GG genotype of rs5498. In the present analysis, however, no
differences in allele frequency or genotype distribution were
observed between the two groups in females. The results of the
present study were in accordance with the results from Zhou et
al (15). Using nested PCR,
Zhou et al demonstrated that the G allele of rs5498 was a
genetic risk factor for CHD in the Han population in Hubei, China.
Several previous studies have shown that the A allele of rs5498 is
associated with CHD in different ethnicities. In the Chinese Han
population, Zhang et al (16) genotyped 173 patients with CHD and
141 control subjects using PCR-restriction fragment length
polymorphism, and they demonstrated that the rs5498 polymorphism
was associated with CHD and that the A allele may serve as a
genetic risk factor for CHD. Lu et al (17) used nested PCR with allele-specific
oligonucleotide primers in 160 patients with CHD and 64 control
subjects and found that the A allele frequency and plasma levels of
ICAM-1 were higher in patients with CHD than those in control
subjects. It was also found that the frequency of the A genotype
(AG and GG) was significantly higher in patients with CHD than that
in controls in the Egyptian population (18). However, McGlinchey et al
(19) found that rs5498 was not
associated with ischemic heart disease in Irish populations.
Another study also demonstrated that there was no strong
association between rs5498 polymorphisms and the occurrence of CHD
and myocardial infarction in the studied population in Iran
(20). In addition, Milutinović
and Petrovic (21) found that the
rs5498 polymorphism was not associated with myocardial infarction
in subjects with type 2 diabetes in the Slovenian population. These
contradictory results may be due to differences in the ethnic
background, geographical factors and the relatively small sample
size in each study.
There were certain limitations in this study.
Firstly, in the present study, only one variant, rs5498, was
investigated rather than other variants in the ICAM-1 gene.
Although the main aim of the present study was to investigate the
role of rs5498, this may underestimate the association between the
ICAM-1 gene and CHD. The rs5498 and rs5491 polymorphisms of
the ICAM-1 gene may also affect the plasma sICAM-1
expression levels (22). Secondly,
the sICAM-1 levels were not measured in either patients with CHD or
in control subjects. Therefore, the association between the rs5498
polymorphism and sICAM-1 levels was not investigated. There were
two advantages in the present study. One was that the sample size
in the study was relatively large. The other was that it was the
first study, to the best of our knowledge, to demonstrate the
different distribution of the rs5498 polymorphism of the
ICAM-1 gene in males and females.
In conclusion, the present study demonstrated that
the rs5498 polymorphism of the ICAM-1 gene was associated
with CHD in males in the Chinese Han population. Males with the G
allele (AG and GG genotype) may have a higher risk of CHD than
those with the AA genotype. However, an age-matched study with a
larger sample size is required to investigate the association
between the ICAM-1 gene and CHD in females.
Acknowledgements
This study was supported by a grant from the
Technology Support Program of Xinjiang Uygur Autonomous Region (no.
201233138).
References
|
1
|
Hwang SJ, Ballantyne CM, Sharrett AR,
Smith LC, Davis CE, et al: Circulating adhesion molecules VCAM-1,
ICAM-1, and E-selectin in carotid atherosclerosis and incident
coronary heart disease cases: the Atherosclerosis Risk In
Communities (ARIC) study. Circulation. 96:4219–4225. 1997.
View Article : Google Scholar
|
|
2
|
Mizia-Stec K, Zahorska-Markiewicz B and
Goliszek L: Adhesion molecules: atherosclerosis and coronary artery
disease. Przegl Lek. 60:147–150. 2003.(In Polish).
|
|
3
|
Greve JM, Davis G, Meyer AM, Forte CP,
Yost SC, et al: The major human rhinovirus receptor is ICAM-1.
Cell. 56:839–847. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dietrich JB: The adhesion molecule ICAM-1
and its regulation in relation with the blood-brain barrier. J
Neuroimmunol. 128:58–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van de Stolpe A and van der Saag PT:
Intercellular adhesion molecule-1. J Mol Med (Berl). 74:13–33.
1996.
|
|
6
|
Watanabe T and Fan J: Atherosclerosis and
inflammation mononuclear cell recruitment and adhesion molecules
with reference to the implication of ICAM-1/LFA-1 pathway in
atherogenesis. Int J Cardiol. 66(Suppl 1): S45–S53; discussion S55.
1998.PubMed/NCBI
|
|
7
|
Cybulsky MI, Iiyama K, Li H, Zhu S, Chen
M, et al: A major role for VCAM-1, but not ICAM-1, in early
atherosclerosis. J Clin Invest. 107:1255–1262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie X, Ma YT, Yang YN, Fu ZY, Li XM, et
al: Polymorphisms in the SAA1/2 gene are associated with carotid
intima media thickness in healthy Han Chinese subjects: the
Cardiovascular Risk Survey. PLoS One. 5:e139972010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gaetani E, Flex A, Pola R, Papaleo P, De
Martini D, et al: The K469E polymorphism of the ICAM-1 gene is a
risk factor for peripheral arterial occlusive disease. Blood Coagul
Fibrinolysis. 13:483–488. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwao M, Morisaki H and Morisaki T:
Single-nucleotide polymorphism g. 1548G > A (E469K) in human
ICAM-1 gene affects mRNA splicing pattern and TPA-induced
apoptosis. Biochem Biophys Res Commun. 317:729–735. 2004.PubMed/NCBI
|
|
11
|
Kitagawa K, Matsumoto M, Sasaki T,
Hashimoto H, Kuwabara K, et al: Involvement of ICAM-1 in the
progression of atherosclerosis in APOE-knockout mice.
Atherosclerosis. 160:305–310. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hulthe J, Wikstrand J, Mattsson-Hultén L
and Fagerberg B: Circulating ICAM-1 (intercellular cell-adhesion
molecule 1) is associated with early stages of atherosclerosis
development and with inflammatory cytokines in healthy 58-year-old
men: the Atherosclerosis and Insulin Resistance (AIR) study. Clin
Sci (Lond). 103:123–129. 2002.PubMed/NCBI
|
|
13
|
Puthothu B, Krueger M, Bernhardt M and
Heinzmann A: ICAM1 amino-acid variant K469E is associated with
paediatric bronchial asthma and elevated sICAM1 levels. Genes
Immun. 7:322–326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bielinski SJ, Reiner AP, Nickerson D,
Carlson C, Bailey KR, et al: Polymorphisms in the ICAM1 gene
predict circulating soluble intercellular adhesion
molecule-1(sICAM-1). Atherosclerosis. 216:390–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou YL, Zhu MA, Ding Y, et al:
Association between intercellular adhension molecule-1 gene
polymorphism and coronary heart disease. J Clin Cardiol. 9:519–522.
2006.(In Chinese).
|
|
16
|
Zhang SR, Xu LX, Gao QQ, Zhang HQ, Xu BS,
et al: The correlation between ICAM-1 gene K469E polymorphism and
coronary heart disease. Zhonghua Yi Xue Yi Chuan Xue Za Zhi.
23:205–207. 2006.(In Chinese).
|
|
17
|
Lu FH, Shang Q, Wen PE, Su GH, Wu JM, et
al: A study on K469E polymorphism of ICAM1 gene and ICAM1 plasma
level in patients with coronary heart disease. Zhonghua Yi Xue Yi
Chuan Xue Za Zhi. 23:195–197. 2006.(In Chinese).
|
|
18
|
Mohamed AA, Rashed L, Amin H, Abu-Farha M,
El Fadl SA and Pakhoum S: K469E polymorphism of the intercellular
adhesion molecule-1 gene in Egyptians with coronary heart disease.
Ann Saudi Med. 30:432–436. 2010.PubMed/NCBI
|
|
19
|
McGlinchey PG, Spence MS, Patterson CC,
Allen AR, Murphy G, et al: The intercellular adhesion molecule-1
(ICAM-1) gene K469E polymorphism is not associated with ischaemic
heart disease: an investigation using family-based tests of
association. Eur J Immunogenet. 31:201–206. 2004. View Article : Google Scholar
|
|
20
|
Aminian B, Abdi Ardekani AR and Arandi N:
ICAM-1 polymorphisms (G241R, K469E), in coronary artery disease and
myocardial infarction. Iran J Immunol. 4:227–235. 2007.PubMed/NCBI
|
|
21
|
Milutinović A and Petrovic D: The K469E
polymorphism of the intracellular adhesion molecule 1 (ICAM-1) gene
is not associated with myocardial infarction in Caucasians with
type 2 diabetes. Folia Biol (Praha). 52:79–80. 2006.PubMed/NCBI
|
|
22
|
Wang MY, Bai DC, Zhu P, Fu Y, Bu DF and
Zhang Y: Effects of ICAM-1 gene K469E, K56M polymorphisms on plasma
sICAM-1 expression levels in Chinese Yugur, Tibetan and Han
nationalities. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 20:1205–1211.
2012.(In Chinese).
|