Introduction
Diabetes mellitus (DM) is a metabolic disorder
characterized by chronic hyperglycemia. While several causative
factors have been attributed to DM, pathogenesis of the disease is
complex and is not yet entirely understood. DM is becoming
increasingly prevalent, which may be due to changes in lifestyle,
an aging population and improved means of diagnostic detection
(1). Type 2 diabetes can lead to
complications of the liver (the organ commonly affected by
long-term hyperglycemia) in addition to the kidneys, heart, retina
and nervous system (2–5). It has been reported that 21–78% of
patients with DM have fatty livers, and certain serious cases
develop into liver fibrosis (6).
Therefore, the liver complications of type 2 diabetes have become a
key problem that requires a solution.
Bone morphogenetic protein-9 (BMP-9), also termed
growth differentiation factor 2, is a member of the transforming
growth factor-β (TGF-β) superfamily and is important in metabolism.
BMP-9 was first isolated in the developing mouse liver (7). It was also demonstrated to be
expressed in nonparenchymal adult neurons (8), liver cells (9) and bone cells (10). As the primary regulatory factors of
glucose and lipid metabolism, BMPs exert their biological effects
through a complex system of signal transduction pathways. BMPs bind
to their specific receptor and interact with Smad proteins. Smad-4
is the only established common-mediator-Smad (co-Smad) in a
plethora of mammalian cell types, and its role is unique within the
Smad family. Smad-4 is not only an effector of TGF-β signaling
(11), but also functions as a
co-Smad by heterodimerizing, entering the nucleus and helping to
activate transcription (12–15).
Therefore, its biological functions are regulated, in part, by its
interactions with other Smads or signaling proteins.
Platycodon grandiflorus is an edible root
that has been used for its medicinal properties. The root of this
plant contains high levels of pharmacologically active triterpenoid
saponins (16–20), which have been indicated to have
hepatoprotective properties. However, it is not clear whether the
BMP-9/Smad-4 pathway is involved in this hepatoprotective
mechanism. In the current study, it was determined whether the
BMP-9/Smad-4 pathway was involved in the effect of platycodins.
Clarification of the effective mechanism of platycodins in
glucolipid metabolic pathways may elucidate a potential therapeutic
agent for treating liver complications that arise from type 2
diabetes.
Materials and methods
Chemicals
Platycodins were obtained from Xi’an Guanyu Bio-Tech
Co., Ltd. (Xi’an, China). Streptozocin (STZ) was obtained from
Sigma-Aldrich (St. Louis, MO, USA). Molecular biology reagents and
kits for measuring fasting blood glucose (FBG), triglycerides (TG),
total cholesterol (TC), high-density lipoprotein (HDL), low-density
lipoprotein (LDL), glutamate pyruvate transaminase (GPT) and
glutamic oxalacetic transaminase (GOT) were obtained from Nanjing
KGI Biological Technology Development Co., Ltd. (Nanjing, China).
Antibodies against BMP-9, Smad-4 and β-actin were obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). A Fermentas protein
ladder was obtained from Thermo Fisher Scientific (Pittsburgh, PA,
USA).
Animals and treatment
Thirty pathogen-free Sprague-Dawley rats (180–220 g,
Animal Center of Jiamusi University, Jiamusi, China) were provided
standard rat chow and tap water ad libitum. The study was
approved by the ethics committee of Jiamusi University. All rats
were randomly divided into two groups: The control group (normal
diet, n=5) and the model group (diabetes and liver complications,
n=25). To produce animal models of diabetes with liver
complications, the rats in the model group were fed a high-fat and
-sugar diet for 8 weeks, with injection of 2% STZ (25 mg/kg body
weight) through the tail vein at week 4. The rats in the control
group were fed a normal diet containing corn, soybeans, wheat bran
and fish meal. The rats in the model group were fed the normal diet
supplemented with 10% sucrose, 15% lard, 2% cholesterol, and 0.25%
bile acid. After 8 weeks, the FBG and liver function of the models
were examined. The rats with FBG ≤16.8 mmol/l and abnormal liver
function were included in the model group (21,22).
Twenty successful model rats were produced, and were continued on
the high-fat and -sugar diet, then randomly divided into four
groups (n=5 in each group) that were treated with the following
doses of platycodins: The untreated controls, and the 50, 100 and
200 mg/kg body weight/day groups. The control group was maintained
with a normal diet. The control and untreated groups were treated
with distilled water, while the 50, 100 and 200 mg/kg body
weight/day groups were intragastrically administered with the
corresponding concentrations of platycodins dissolved in aqueous
solution. Platycodins treatment lasted for 12 weeks. After the
12-week treatment, the rats fasted for 12 h, then were anesthetized
by pentobarbital (0.1 mg/g body weight) and sacrificed by cervical
dislocation. The blood was collected and livers were resected to be
used for subsequent analysis.
Detection contents
Liver index
The weights of the bodies and livers of the rats
were recorded and then used to calculate the liver index according
to the following formula: Liver index = liver weight/body
weight.
FBG, blood lipids and liver function
An automatic biochemical analyzer (Olympus, Osaka,
Japan) was used to detect FBG, the levels of blood lipids (TG, TC,
LDL and HDL) and the liver function (GPT and GOT).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was purified from liver tissue using the
Protein & RNA Extraction kit for Mammalian Cells (Takara
Biotechnology, Dalian, China). RNA was quantified using
spectrophotometry (Beckman Coulter, CA, USA) and
reverse-transcribed to cDNA. cDNA was amplified using a master mix
containing LA Taq DNA Polymerase (Takara Biotechnology). The
following primer sequences were used (Augct DNA-Syn Biotechnology
Co., Ltd., Beijing, China): Sense: 5′-GGGACCAAGGAGACCAGACTG-3′ and
antisense: 5′-CGCCCATGTCATCCTTGTAGA-3′ for BMP-9; sense:
5′-TAAAGGTGAAGGGGACGTGTG-3′ and antisense:
5′-CATGGTGTGCAGGACTTCATC-3′ for Smad-4; and sense:
5′-TCCTCCCTGGAGAAGAGCTA-3′ and antisense:
5′-TCAGGAGGAGCAATGATCTTG-3′ for β-actin. The final
concentration of each primer was 20 μM. The PCR products were
separated on a 1.5% agarose gel and stained with ethidium bromide
to identify fragments of BMP-9, Smad-4 and β-actin.
The reaction conditions were as follows: 94°C for 5 min,
denaturation at 94°C for 30 sec, annealing at 56°C for 45 sec,
extension 25 cycles at 72°C for 1 min, followed by a final step at
72°C for 10 min for β-actin, fragment length 357 bp; 94°C
for 5 min, denaturation at 94°C for 30 sec, annealing at 64°C for
45 sec, extension 31 cycles at 72°C for 1 min, followed by a final
step at 72°C for 10 min for BMP-9, fragment length 427 bp;
and 94°C for 5 min, denaturation at 94°C for 30 sec, annealing at
60°C for 45 sec, extension 33 cycles at 72°C for 1 min, followed by
a final step at 72°C for 10 min for Smad-4, fragment length
357 bp.
Western blot analysis
Protein samples from liver tissues were separated
using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred to nitrocellulose membranes (Pall Life Sciences,
Port Washington, NY, USA). Membranes were blocked with 5% non-fat
dry milk in 0.1% Tris-buffered saline with Tween-20 (TBST,
Sigma-Aldrich), and then washed with 0.1% TBST. The membranes were
incubated with the primary antibodies overnight at 4°C. After four
10-min washes with 0.1% TBST, the membranes were incubated with
horseradish peroxidase-conjugated AffiniPure goat anti-rabbit
secondary antibody (Zsgb-Bio, Beijing, China) for 1 h. SuperSignal
West Pico Chemiluminescent Substrate (Pierce Biotechnology, Inc.,
Rockford, IL, USA) was used to detect the protein bands. Protein
band intensities were quantified by densitometry using Quantity One
software (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Student’s t-tests were utilized to analyze differences between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
FBG levels in rat models of type 2
diabetes with liver complications
Rats in the 200 mg/kg body weight/day group
exhibited reduced FBG levels compared with the untreated group
(P<0.05); the FBG levels were reduced to the level of the
healthy control mice. No changes were observed in the 50 and 100
mg/kg body weight/day groups compared with the untreated group
(Table I).
 | Table ILevels of FBG, GPT, GOT and liver
index in each group. |
Table I
Levels of FBG, GPT, GOT and liver
index in each group.
| Group | FBG (mmo1/l) | GPT (mmo1/l) | GOT (mmo1/l) | Liver index |
|---|
| Control | 7.784±0.591 | 68.088±1.942 | 98.360±1.467 | 0.022±0.001 |
| Untreated |
21.704±0.423a |
129.638±3.240a |
282.966±4.039a | 0.044±0.006a |
| 50 mg/kg/day |
21.082±0.492a |
126.154±1.558a |
279.798±1.494a | 0.041±0.004a |
| 100 mg/kg/day |
20.302±1.122a |
121.312±5.774a |
272.086±7.743a | 0.039±0.005a |
| 200 mg/kg/day | 8.488±0.369b |
71.944±2.245b |
191.448±2.566a,b | 0.027±0.003a,b |
GPT and GOT levels
As markers of liver health, elevated levels of GPT
and GOT in the serum signify a disease state. Compared with the
untreated group, GPT and GOT levels in the 200 mg/kg body
weight/day group were significantly reduced (P<0.05), and GPT
levels were reduced to the level of the controls. No changes in GPT
and GOT levels were detected in the 50 and 100 mg/kg body
weight/day groups compared with the untreated model group (Table I).
Liver index following treatment with
platycodins
Compared with the untreated group, the liver index
of the 200 mg/kg body weight/day group was reduced, but remained
significantly higher than the healthy control group (P<0.05),
indicating that platycodins treatment significantly reduces the
liver size of rat models of diabetes with liver complications. No
changes were observed in the 50 and 100 mg/kg body weight/day
groups compared with the untreated group (Table I).
Effects of platycodins on lipid
regulation
Compared with the untreated group, TG, TC and LDL
levels in the 200 mg/kg body weight/day group were significantly
reduced (P<0.05), while the HDL levels significantly increased
(P<0.05). The levels of TC and LDL detected in the 200 mg/kg
body weight/day group were close to levels in the control group,
suggesting that 200 mg/kg body weight platycodins successfully
returned the levels of TC and LDL to healthy levels. Consistent
with the data regarding FBG and liver health, no changes were
detected when rats were treated with <200 mg/kg body weight
platycodins (Table II).
 | Table IIBlood lipid levels in each group. |
Table II
Blood lipid levels in each group.
| Group | TG (mmo1/l) | TC (mmo1/l) | LDL (mmo1/l) | HDL (mmo1/l) |
|---|
| Control | 0.798±0.037 | 1.784±0.043 | 0.216±0.015 | 2.956±0.031 |
| Untreated | 2.898±0.065a | 4.480±0.045a | 0.856±0.028a | 1.414±0.032a |
| 50 mg/kg/day | 2.792±0.105a | 4.440±0.039a | 0.824±0.036a | 1.448±0.026a |
| 100 mg/kg/day | 2.730±0.132a | 4.406±0.059a | 0.810±0.032a | 1.490±0.067a |
| 200 mg/kg/day | 0.898±0.070a,b | 1.988±0.208b | 0.234±0.019b | 2.868±0.040a,b |
Changes in BMP-9 and Smad-4 expression
following treatment with platycodins
The aforementioned results suggest that treatment
with 200 mg/kg body weight platycodins rectifies liver
complications and glucolipid regulation in rats with type 2
diabetes. Since the BMP-9 and Smad-4 signaling pathway is crucial
to lipid and glucose metabolism, the effects of platycodins on
these proteins was investigated. Total RNA was isolated from liver
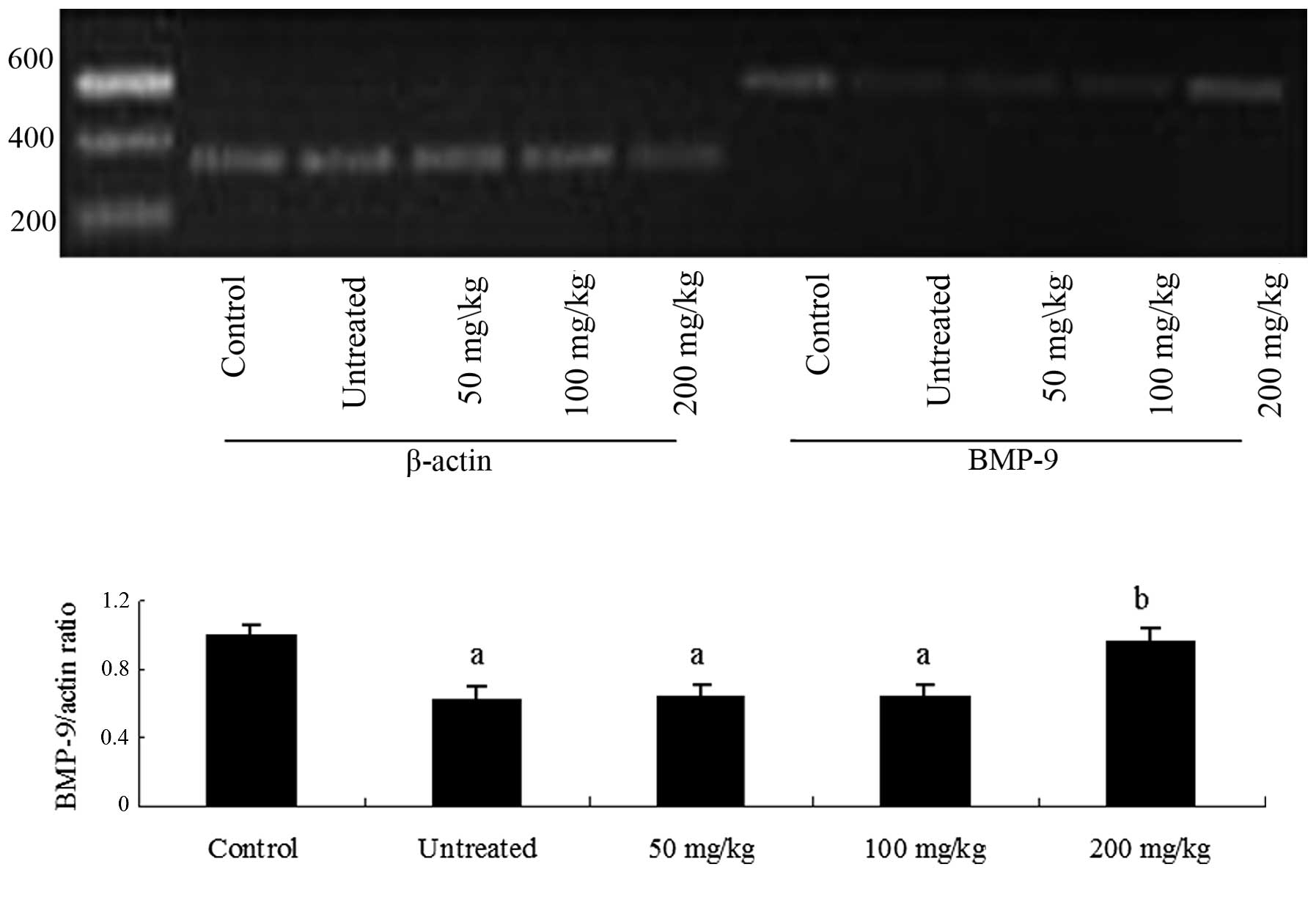
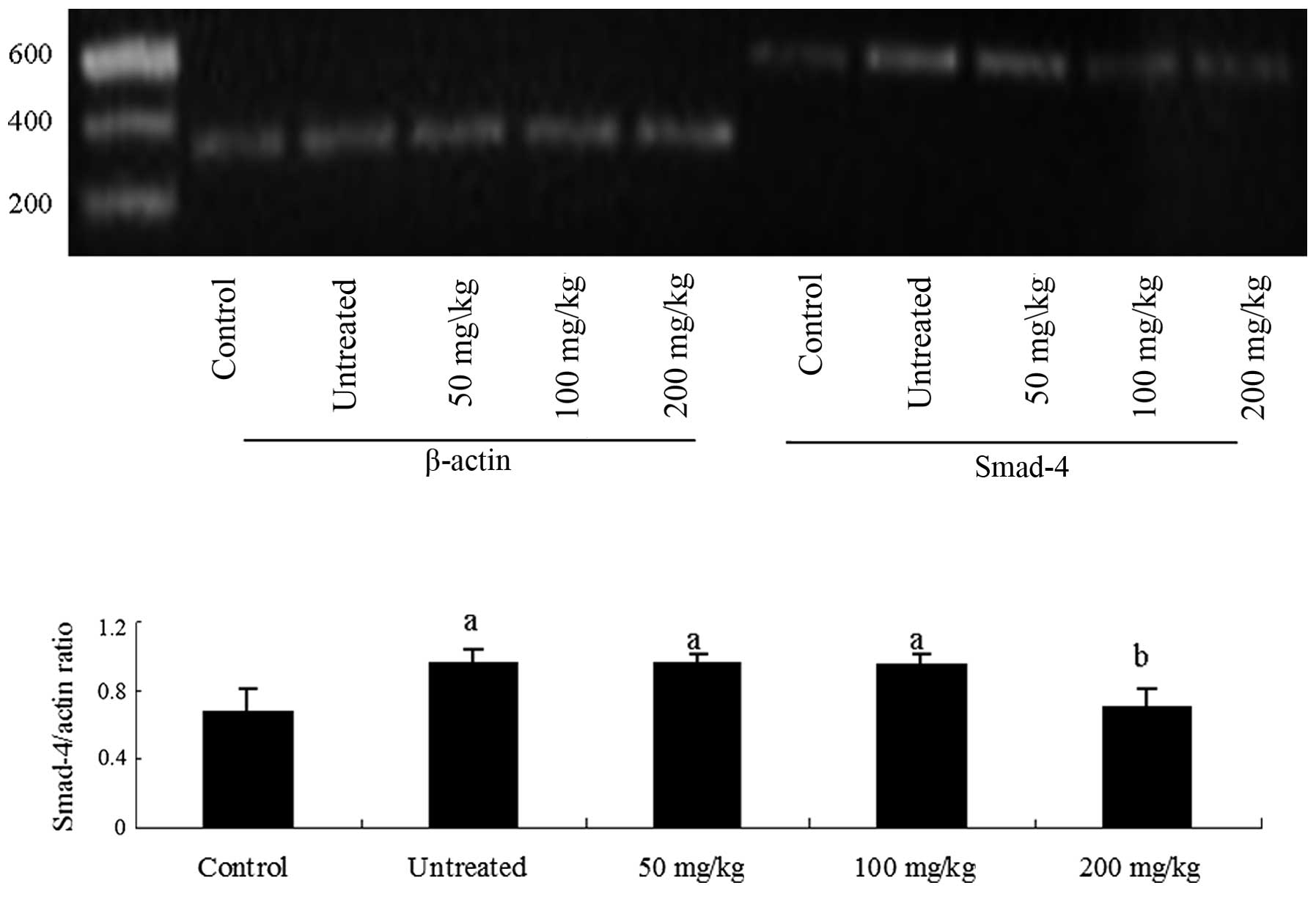
tissue and mRNA levels were detected by RT-PCR. BMP-9 mRNA
expression levels were reduced (Fig.
1), while those of Smad-4 were increased (Fig. 2) in the model rats compared with
healthy control rats. Notably, treatment with 200 mg/kg body weight
platycodins significantly upregulated the expression of
BMP-9 and downregulated the expression of Smad-4
(P<0.05 vs. untreated models) to levels that were not
significantly higher than those of healthy control rats (Figs. 1 and 2).
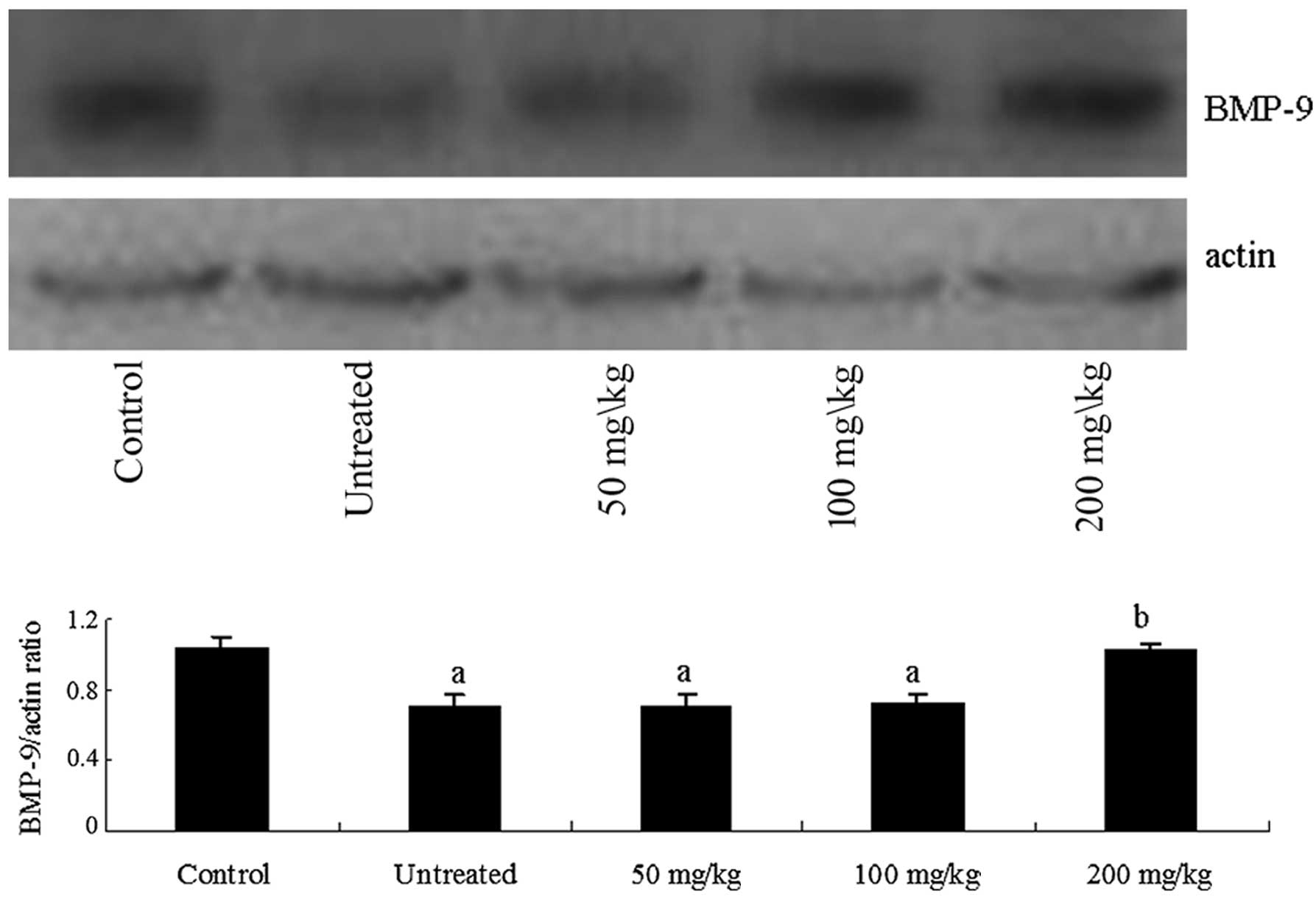
To confirm that protein levels of BMP-9 and Smad-4
followed the same pattern as the mRNA levels, proteins were
extracted from liver tissues and western blot analysis was
performed. Consistent with mRNA expression patterns, BMP-9
expression increased following treatment with 200 mg/kg body weight
platycodins (Fig. 3). Similarly,
Smad-4 expression was reduced to the level of the healthy controls
(Fig. 4).
Discussion
The findings of the present study demonstrate that
treatment with 200 mg/kg body weight platycodins can rectify
disorders of blood glucose and lipid metabolism in rat models of
type 2 diabetes with liver complications, by improving liver index
and protecting liver function. Furthermore, it was demonstrated
that the therapeutic effect of 200 mg/kg body weight platycodins
was mediated through the BMP-9/Smad-4 pathway. This was supported
by the observation that platycodins increased BMP-9 and reduced
Smad-4 expression.
Chronic high blood glucose is a serious factor
contributing to complications in type 2 diabetes. Persistent
hyperglycemia can result in liver damage, aggravate lipid
metabolism disorder, alter the structure of liver and compromise
liver function. In addition, patients with diabetes usually have
microvascular and microcirculation complications that can result in
ischemia and hypoxia in other organs. Ischemia can cause carbon
dioxide accumulation, acidosis, oxygen deficiency and bilirubin
metabolism disorders. Ischemia can also reduce the phosphorylation
capacity and increase the activity of GPT and GOT in liver cells.
Platycodins have been indicated to improve liver function and
alleviate the hepatotoxicity caused by tert-butyl hydroperoxide
(23), acetaminophen activation
(24), carbon tetrachloride
(25) and cholestasis (26). They can reduce the serum levels of
alanine aminotransferase and aspartate aminotransferase, scavenge
oxygen free radicals and protect cells from oxidative stress
(23). Furthermore, it can inhibit
P450-mediated acetaminophen bioactivation (24). In addition to the hepatoprotection,
platycodon root is involved in the modulation of glucose and lipid
metabolisms. Previous studies demonstrated that platycodin D
regulates adipogenesis via the Wnt/β-catenin (27) and AMPK-dependent signaling pathways
(28). In another study, it
ameliorated obesity and insulin resistance in obese mice via the
activation of the AMPK/ACC pathways, and also reduced adipocyte
differentiation (29). It has been
reported that Platycodon grandiflorum extract ameliorated
obesity in mice fed a high fiber diet and increased glucose uptake
in L6 muscle cells by modifying adipokines, which indicates
clinical benefits of platycodon as a supplement to treat obesity
and diabetes (30). It has also
been indicated that platycodins promote pancreatic exocrine
activity and improve the function of the pancreas by promoting the
release of cholecystokinin (31).
Platycodins significantly reduced cholesterol (32) and postprandial glucose
concentration by increasing insulin and glucose transporter
protein-4 expression levels in the plasma of obese and lean Zucker
rats (33). Other studies have
also confirmed that platycodins can inhibit pancreatic lipase
activity and reduce lipid content of the plasma and liver in a
dose-dependent manner (26,30,34).
As a potential autocrine/paracrine mediator in the
hepatic reticuloendothelial system (9), BMP-9 has been implicated in recent
studies in the regulation of glucose and lipid metabolism (35). BMP-9 has insulin-like regulatory
functions in glucose metabolism. It promotes insulin secretion of
pancreatic β cells and glycogen synthesis in muscle cells, while it
suppresses gluconeogenesis in the liver (36). These processes act synergistically
to reduce blood glucose levels. Additionally, BMP-9 is important in
liver regeneration and has an antifibrotic function in liver
(37,38). In addition to its roles in glucose
metabolism and liver health, BMP-9 is also involved in lipid
metabolism. It stimulates the synthesis of malic enzyme and fatty
acid synthase, which are key enzymes in the hepatic fatty acid
metabolism pathway (36,39). BMP-9 functions similarly to leptin
(39) and opposite to resistin and
resistin-like proteins (40). As a
glucose and lipid metabolism regulatory factor, BMP-9 may have
far-reaching roles in the development and treatment of diabetes,
and it has previously been proposed as a candidate for the hepatic
insulin-sensitizing substance (HISS) (41). BMPs function by binding to specific
receptors and interacting with Smad proteins. Smad-4 is the only
established co-Smad in mammals, and its role is unique within the
Smad family. Smad-4 dysfunction has been implicated in liver
fibrosis. Experiments using a cirrhotic mouse model indicated that
the downregulation of Smad-4 expression by RNA interference leads
to a reduction in the mRNA levels of TGF-β1, type II collagen and
matrix metalloproteinase inhibitor I. Furthermore, the protein
levels of type I collagen were reduced. Together, these changes
lead to improvements in liver function (42).
Overall, the data from the present study have
indicated that the mechanism underlying the effects of platycodins
in liver complications associated with type 2 diabetes involves the
BMP-9/Smad-4 pathway. Notably, the present study is limited, since
other regulatory pathways may be affected by platycodins and
contribute to their therapeutic effects. Therefore, additional
studies are required in order to examine its therapeutic potential
in a clinical setting.
Acknowledgements
The current study was partially supported by the
grant from the Chinese Heilongjiang Provincial Health Department
Science Foundation (grant no. 2011-429) and Chinese Heilongjiang
Province Pharmaceutical Administration Science Foundation (grant
no. ZHY12-W043).
References
|
1
|
Gujral UP, Pradeepa R, Weber MB, Narayan
KM and Mohan V: Type 2 diabetes in South Asians: similarities and
differences with white Caucasian and other populations. Ann NY Acad
Sci. 1281:51–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Börgeson E and Godson C: Resolution of
inflammation: therapeutic potential of pro-resolving lipids in type
2 diabetes mellitus and associated renal complications. Front
Immunol. 3:3182012.PubMed/NCBI
|
|
3
|
Uslu S, Kebapçi N, Kara M and Bal C:
Relationship between adipocytokines and cardiovascular risk factors
in patients with type 2 diabetes mellitus. Exp Ther Med. 4:113–120.
2012.PubMed/NCBI
|
|
4
|
Sen S, Chen S, Feng B, Iglarz M and
Chakrabarti S: Renal, retinal and cardiac changes in type 2
diabetes are attenuated by macitentan, a dual endothelin receptor
antagonist. Life Sci. 91:658–668. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tovilla-Zárate C, Juárez-Rojop I, Peralta
Jimenez Y, et al: Prevalence of anxiety and depression among
outpatients with type 2 diabetes in the Mexican population. PLoS
One. 7:e368872012.PubMed/NCBI
|
|
6
|
Salas-Flores R, González-Pérez B and
Echegollen-Guzmán A: Hepatic steatosis and type 2 diabetes mellitus
in health workers. Rev Med Inst Mex Seguro Soc. 50:13–18.
2012.PubMed/NCBI
|
|
7
|
Song JJ, Celeste AJ, Kong FM, et al: Bone
morphogenetic protein-9 binds to liver cells and stimulates
proliferation. Endocrinology. 136:4293–4297. 1995.PubMed/NCBI
|
|
8
|
López-Coviella I, Berse B, Krauss R, Thies
RS and Blusztajn JK: Induction and maintenance of the neuronal
cholinergic phenotype in the central nervous system by BMP-9.
Science. 289:313–316. 2000.
|
|
9
|
Miller AF, Harvey SA, Thies RS and Olson
MS: Bone morphogenetic protein-9. An autocrine/paracrine cytokine
in the liver. J Biol Chem. 275:17937–17945. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suttapreyasri S, Koontongkaew S, Phongdara
A and Leggat U: Expression of bone morphogenetic proteins in normal
human intramembranous and endochondral bones. Int J Oral Maxillofac
Surg. 35:444–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyazono K: TGF-beta/SMAD signaling and
its involvement in tumor progression. Biol Pharm Bull.
23:1125–1130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ten Dijke P, Goumans MJ, Itoh F and Itoh
S: Regulation of cell proliferation by Smad proteins. J Cell
Physiol. 191:1–16. 2002.PubMed/NCBI
|
|
13
|
Feng XH and Derynck R: A kinase subdomain
of transforming growth factor-beta (TGF-beta) type I receptor
determines the TGF-beta intracellular signaling specificity. EMBO
J. 16:3912–3923. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bedossa P and Paradis V: Transforming
growth factor-beta (TGF-beta): a key-role in liver fibrogenesis. J
Hepatol. 22(Suppl): 37–42. 1995.PubMed/NCBI
|
|
15
|
Attisano L and Wrana JL: Signal
transduction by the TGF-beta superfamily. Science. 296:1646–1647.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma G, Guo W, Zhao L, et al: Two new
triterpenoid saponins from the root of Platycodon
grandiflorum. Chem Pharm Bull (Tokyo). 61:101–104. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu WW, Fu JN, Zhang WM, et al: Platycoside
O, a new triterpenoid saponin from the roots of Platycodon
grandiflorum. Molecules. 16:4371–4378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Zhang W, Xiang L, et al: Platycoside
N, a new oleanane-type triterpenoid saponin from the roots of
Platycodon grandiflorum. Molecules. 15:8702–8708. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi YH, Yoo DS, Choi CW, et al:
Platyconic acid A, a genuine triterpenoid saponin from the roots of
Platycodon grandiflorum. Molecules. 13:2871–2879. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu WW, Shimizu N, Dou DQ, et al: Five new
triterpenoid saponins from the roots of Platycodon
grandiflorum. Chem Pharm Bull (Tokyo). 54:557–560. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu A-M, Xiao F-Y and Zheng Y: Establishing
and valuating animal model resembling to type two diabetes mellitus
with intercurrent fatty liver. Chin J Int Trad Western Med
Digestion. 14:156–159. 2006.(In Chinese).
|
|
22
|
Reed MJ, Meszaros K, Entes LJ, et al: A
new rat model of type 2 diabetes: the fat-fed,
streptozotocin-treated rat. Metabolism. 49:1390–1394. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee KJ, Choi CY, Chung YC, et al:
Protective effect of saponins derived from roots of Platycodon
grandiflorum on tert-butyl hydroperoxide-induced oxidative
hepatotoxicity. Toxicol Lett. 147:271–282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee KJ, You HJ, Park SJ, et al:
Hepatoprotective effects of Platycodon grandiflorum on
acetaminophen-induced liver damage in mice. Cancer Lett. 174:73–81.
2001.
|
|
25
|
Lee KJ and Jeong HG: Protective effect of
Platycodi radix on carbon tetrachloride-induced hepatotoxicity.
Food Chem Toxicol. 40:517–525. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim TW, Lee HK, Song IB, et al: Platycodin
D attenuates bile duct ligation-induced hepatic injury and fibrosis
in mice. Food Chem Toxicol. 51:364–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee H, Bae S, Kim YS and Yoon Y:
WNT/β-catenin pathway mediates the anti-adipogenic effect of
platycodin D, a natural compound found in Platycodon
grandiflorum. Life Sci. 89:388–394. 2011.
|
|
28
|
Hwang YP, Choi JH, Kim HG, et al:
Saponins, especially platycodin D, from Platycodon
grandiflorum modulate hepatic lipogenesis in high-fat diet-fed
rats and high glucose-exposed HepG2 cells. Toxicol Appl Pharmacol.
267:174–183. 2013.PubMed/NCBI
|
|
29
|
Lee CE, Hur HJ, Hwang JT, et al: Long-term
consumption of Platycodi radix ameliorates obesity and insulin
resistance via the activation of AMPK pathways. Evid Based
Complement Alternat Med. 2012:7591432012.PubMed/NCBI
|
|
30
|
Ahn YM, Kim SK, Kang JS and Lee BC:
Platycodon grandiflorum modifies adipokines and the glucose
uptake in high-fat diet in mice and L6 muscle cells. J Pharm
Pharmacol. 64:697–704. 2012. View Article : Google Scholar
|
|
31
|
Arai I, Komatsu Y, Hirai Y, et al:
Stimulative effects of saponin from kikyo-to, a Japanese herbal
medicine, on pancreatic exocrine secretion of conscious rats.
Planta Med. 63:419–424. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao HL, Harding SV, Marinangeli CP, Kim
YS and Jones PJ: Hypocholesterolemic and anti-obesity effects of
saponins from Platycodon grandiflorum in hamsters fed
atherogenic diets. J Food Sci. 73:H195–H200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim KS, Seo EK, Lee YC, et al: Effect of
dietary Platycodon grandiflorum on the improvement of
insulin resistance in obese Zucker rats. J Nutr Biochem.
11:420–424. 2000.
|
|
34
|
Zhao HL and Kim YS: Determination of the
kinetic properties of platycodin D for the inhibition of pancreatic
lipase using a 1,2-diglyceride-based colorimetric assay. Arch Pharm
Res. 27:968–972. 2004. View Article : Google Scholar
|
|
35
|
Groop L: Bringing diabetes therapeutics to
the big screen. Nat Biotechnol. 21:240–241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen C, Grzegorzewski KJ, Barash S, et al:
An integrated functional genomics screening program reveals a role
for BMP-9 in glucose homeostasis. Nat Biotechnol. 21:294–301. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kan NG, Junghans D and Izpisua Belmonte
JC: Compensatory growth mechanisms regulated by BMP and FGF
signaling mediate liver regeneration in zebrafish after partial
hepatectomy. FASEB J. 23:3516–3525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sosa I, Cvijanovic O, Celic T, et al:
Hepatoregenerative role of bone morphogenetic protein-9. Med Sci
Monit. 17:HY33–HY35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Machinal-Quélin F, Dieudonné MN, Leneveu
MC, Pecquery R and Giudicelli Y: Proadipogenic effect of leptin on
rat preadipocytes in vitro: activation of MAPK and STAT3 signaling
pathways. Am J Physiol Cell Physiol. 282:C853–C863. 2002.PubMed/NCBI
|
|
40
|
Stadler K, Jenei V, von Bölcsházy G,
Somogyi A and Jakus J: Role of free radicals and reactive nitrogen
species in the late complications of diabetes mellitus in rats. Orv
Hetil. 145:1135–1140. 2004.(In Hungarian).
|
|
41
|
Caperuto LC, Anhê GF, Cambiaghi TD, et al:
Modulation of bone morphogenetic protein-9 expression and
processing by insulin, glucose, and glucocorticoids: possible
candidate for hepatic insulin-sensitizing substance. Endocrinology.
149:6326–6335. 2008. View Article : Google Scholar
|
|
42
|
Xu XB, Leng XS, He ZP, et al: Inhibitory
effect of retroviral vector containing anti-sense Smad4 gene on Ito
cell line, LI90. Chin Med J (Engl). 117:1170–1177. 2004.PubMed/NCBI
|