Introduction
As the incidence of lung adenocarcinoma has rapidly
increased, it has become a major pathological type of lung cancer
(1). Revealing the molecular
pathogenesis of lung adenocarcinoma may enable improvement in the
diagnosis and treatment of this disease.
The gene solute carrier family 34 (sodium
phosphate), member 2 (SLC34A2) is a member of the SLC34
family located on chromosome 4p15–p16. The full-length cDNA of
SLC34A2 is 4,167 bp with an open reading frame that encodes
a 689-amino-acid protein. The SLC34A2 gene encodes the type
2b sodium-phosphate cotransporter NaPi-IIb (2,3),
which is responsible for the transcellular absorption of Pi in an
apical membrane (4–6). According to previous studies,
mutations in SLC34A2 led to the occurrence of pulmonary
alveolar microlithiasis, testicular microlithiasis and
hypophosphatemia (7–9). Previous studies have suggested that
the tumorigenesis of several types of cancer might be associated
with abnormal expression of SLC34A2, including papillary
thyroid, ovarian and breast cancer (10).
In 1999, Xu et al (6) revealed that SLC34A2 was
expressed in numerous human tissues, with adult and fetal lungs
demonstrating the highest levels of expression. Shibasaki et
al (11) confirmed that
targeted deletion of the SLC34A2 gene resulted in early
embryonic lethality, and suggested that SLC34A2 was a vital
gene in early embryonic development. Simultaneously, a study by
Kopantzev et al (12)
confirmed that the mRNA expression level of SLC34A2 was
increased during human lung embryogenesis; however, was decreased
in non-small cell lung carcinoma (NSCLC). These studies proposed
that SLC34A2 might be a novel candidate for a molecular
marker of NSCLC. It is widely accepted that the decreased
expression of a gene in lung cancer tends to exhibit a
monotonically increased expression during lung development. By
contrast, upregulated genes in various types of lung cancer tend to
exhibit a monotonically downregulated expression during lung
development (13–15). For example, a key gene of lung
embryogenesis (16,17), caveolin 1 (CAV1), which has
the opposite trend of expression between embryogenesis and
tumorigenesis (15), has been
implicated in oncogenic cell transformation, tumorigenesis and
metastasis (18). Furthermore,
downregulation of CAV1 has been confirmed to be a promoting factor
in the development of lung cancer (19,20).
These studies prompted us to hypothesize that SLC34A2 might
be linked to the onset of lung cancer, and further motivated the
investigation of the effects and molecular mechanisms of
SLC34A2 in the initiation and progression of lung
cancer.
In the lung, SLC34A2 is expressed primarily
in alveolar type II (AT II) cells (21). The AT II cells are not only
responsible for the production of surfactant fluids, but are also
the potential pulmonary alveolar epithelium stem cells, which are
able to differentiate into alveolar type I (AT I) cells and is
capable of self renewal (21–23).
Previous studies demonstrated that the AT II cells were a
progenitor cell of lung adenocarcinoma and bronchioloalveolar
carcinoma (24,25). In addition, Kitinya et al
(26) and Gazdar et al
(27) also found that AT II cells
might be the progenitor cells of several types of lung carcinoma,
including large cell carcinoma, adenocarcinoma and squamous cell
carcinoma, particularly lung adenocarcinoma. In addition, previous
studies verified that long-term exposure to carcinogenic factors
was able to cause AT II cells to transform into lung cancer cells
(28,29). In 2009, Xu et al (30) found that a diet low in Pi might
affect normal lung development by disturbing the Akt-FGF-2 signals
associated with tumor progression. Xu et al also indicated
that pulmonary NaPi-IIb was critical in Pi metabolism. These
studies highlighted that a lack of Pi might be associated with the
pathogenesis of lung cancer. Thus, it was hypothesized that a lower
expression of SLC34A2 in AT II cells might lead to the
deficiency in Pi, which might cause the hyperproliferation and lack
of differentiation of AT II cells, and then cause these abnormal AT
II cells to transform into lung adenocarcinoma. SLC34A2
might therefore be important in the development of lung
adenocarcinoma.
To examine this hypothesis, the expression of
SLC34A2 in A549 and H1299 lung adenocarcinoma cells compared
with normal human bronchial epithelial (HBE) cells was first
detected by quantitative polymerase chain reaction (qPCR). The AT
II cell-like A549 human lung adenocarcinoma cell line was then
selected for further identification of the biological functions of
SLC34A2 in lung cancer cells. The present study
preliminarily revealed the effects and mechanisms of SLC34A2
against A549 lung adenocarcinoma cells in vitro, and
provided insights into the effects and mechanisms of SLC34A2
in the generation and development of lung cancer.
Materials and methods
Cell culture
The HBE human bronchial epithelial cell line
obtained from the American Type Culture Collection (ATCC,
Arlington, VA, USA) was cultured in Dulbecco’s modified Eagle’s
medium supplemented with 10% fetal bovine serum (FBS; Gibco-BRL,
Carlsbad, CA, USA). The cells from the primary explants in their
first passage were infected with the recombinant retrovirus
LXSN16E6E7 containing the human papilloma virus E6E7 gene. The
cells were selected in the presence of 0.4 mg/ml G418 (31). The A549 human lung adenocarcinoma
cell line (32) and H1299 cell
line obtained from the ATCC were maintained in RPMI-1640
supplemented with 10% FBS. For all experiments described, the cells
were incubated in the aforementioned medium at 5% CO2
and at 37°C.
Plasmid construction
According to the SLCS4A2 full-length coding
region (Gene Bank serial no. NM_006424), the sense primer,
containing a BamHI site at its 5′-end (SLCS4A2 forward:
5′-GCGGATCCTAATGGCTCCCTGGCCTGAAT-3′) and an antisense primer,
containing an EcoRI site at its 5′-end (reverse:
5′-GCGAATTCCTACAAGGCCGTGCATTCG-3′) were used to clone the coding
DNA sequence of SLCS4A2 from the pcmv-sport6-SLCS4A2
(Open Biosystems, Inc., Huntsville, AL, USA) using a PrimeSTAR HS
PCR kit (Takara, Dalian, China). The cloned cDNA sequence was
connected to the pcDNA3.1 plasmid vector (InvivoGen, San Diego, CA,
USA) using a DNA Ligation kit Ver. 2.0 (Takara). Pure pcDNA3.1 and
pcDNA3.1-SLC34A2 plasmids were prepared using an
EndofreeTM Plasmid Giga kit (Qiagen, Chatsworth, CA,
USA) for the following experiments.
SLC34A2 gene expression analysis by
qPCR
Total RNA from cultured cells was isolated using
TRIzol reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. Synthesis of cDNA with reverse
transcriptase was performed using a PrimeScript RT reagent kit
(Perfect Real Time) (Takara). For SLC34A2 gene expression
analysis, qPCR analysis was performed in the iCycler iQ5 real-time
PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA) with
SYBR-Green reagents (Bio-Rad Laboratories), SLC34A2-specific
primers and a probe (Invitrogen Life Technologies). The human GAPDH
primer and probe reagents were used as the normalization control in
subsequent quantitative analysis.
Stable transfection of A549 cells with
SLC34A2
A549 cells were seeded on 6-well plates at
2×105/well. When the cell density reached 60–70%, they
were transfected with the pcDNA3.1-SLC34A2 and pcDNA3.1
plasmids using Lipofectamine 2000 (InvivoGen). Following 48 h, the
transfected cells were replaced with a medium containing G418 (800
μg/ml; Sigma, St. Louis, MO, USA) to eliminate nontransfected
cells. Individual colonies appeared following 2 weeks. Then,
G418-resistant colonies were isolated into a 96-well plate to be
expanded. Following 2–3 weeks, when the quantity of positive
transfected cells was enough, they were cultured in 6-well plates
with 500 μg/ml G418. Following this, the positive transfected cells
were identified by determining whether SLC34A2 was expressed
stably by qPCR and western blot analysis. Stable transfectants were
maintained in the medium containing G418 (500 μg/ml). A549 cells
stably expressing pcDNA3.1-SLC34A2 and pcDNA3.1 were
designated A549-pcDNA3.1-SLC34A2 (A549-P-S) and
A549-pcDNA3.1 (A549-P), respectively, for further analysis.
Growth curve
The cells were plated into 96-well plates at
2.0×104 cells/well. The effect of SLC34A2 on the
cell viability of A549 cells was determined using an
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. At the indicated time points (1, 2, 3, 4, 5, 6 and 7 days),
the culture medium was replaced with 180 μl of fresh medium, and 20
μl MTT solution (5 mg/ml; Sigma) was added to the culture medium
and incubated for another 4 h at 37°C. Following 4 h, the unreacted
dye was removed by aspiration. Blue formazan crystals were observed
in the well when examined under a microscope (Olympus IX51;
Olympus, Tokyo, Japan). Following this, 150 ml dimethylsulfoxide
was added to each well and then incubated on a shaker for 10 min at
room temperature to dissolve the blue crystal. The absorbance was
measured using a microplate reader (Bio-Rad Laboratories) at a
wavelength of 490 nm. The percentage cell viability was then
calculated using the following formula: [optical density
(OD)490 (treated cells) / OD490 (control)] ×
100.
Colony formation assay
The cells were seeded into 6-well plates in
triplicates at a density of 300 cells/well in 2 ml of medium
containing 10% FBS. The cultures were regularly replaced with fresh
medium in a 37°C humidified atmosphere containing 5% CO2
and grown for 3 weeks. The cell clones were fixed with pure
methanol and stained for 15 min with a solution containing 0.05%
crystal violet, then followed by three rinses with double distilled
water to remove excess dye. The colony numbers were counted using a
gel documentation system (ImagePro Plus software; Media
Cybernetics, Rockville, MD, USA). Additionally, the colony
formation rates were calculated in terms of the number of A549-P-S
cells and A549-P cells relative to A549 cells.
Cell invasion analysis
The cell invasion assay was performed using a Boyden
chamber (Millipore, Billerica, MA, USA) with BD Matrigel™ (BD
Biosciences, Franklin Lakes, NJ, USA). The filters in the upper
compartment were loaded with 400 μl serum-free RPMI-1640 containing
5×104 cells, and filters in the lower compartment were
filled with 600 μl RPMI-1640 containing 10% FBS. The chamber was
then cultured in 5% CO2 at 37°C for 24 h. Then, the
Matrigel and cells in the upper chamber were removed, and the
attached cells in the lower section were fixed with pure methanol
and stained with 0.05% crystal violet. The number of migrated cells
was counted in five randomly selected power fields (magnification,
×200) under a light microscope. The invasion rates were calculated
in terms of the number of A549-P-S cells and A549-P cells relative
to A549 cells.
Microarray analysis
Total RNA extraction from A549-P and A549-P-S using
TRIzol reagent (Invitrogen Life Technologies, Gaithersburg, MD,
USA) and purification of the RNA using a NucleoSpin® RNA
clean-up kit (Macherey-Nagel, Düren, Germany) were completed and
prepared for microarray analysis. The commercially available 35K
Human Genome Array, including 25,000 human genes, was obtained from
CapitalBio Corporation (Beijing, China). Double-stranded cDNAs were
synthesized from 1 μg total RNA using the CbcScript reverse
transcriptase with the T7 Oligo (dT). The dsDNA was transcribed
into cRNA in vitro transcription reactions at 37°C for 4–14
h using a T7 enzyme mix. Next, 2 μg of cRNA was reverse transcribed
to generate cDNA using CbcScript II reverse transcriptase. The cDNA
was fluorescently labeled by Cy5 or Cy3 CPT with the Klenow enzyme
following reverse transcription. The cDNA of A549-P-S cells was
labeled with Cy3-CPT and the cDNA of A549-P cells was labeled with
Cy5-CPT as a control. Array hybridization was performed in a
CapitalBio BioMixerTM II Hybridization station overnight
at a rotation speed of 8 rpm and a temperature of 42°C, and
subsequently washed with two consecutive solutions (0.2% sodium
dodecyl sulfate, 2X saline-sodium citrate at 42°C for 5 min and
0.2X saline-sodium citrate for 5 min at room temperature). These
arrays were scanned with a confocal LuxScan™ scanner and the images
obtained were then analyzed using LuxScan 3.0 software (CapitalBio
Corporation). The obtained images were analyzed using LuxScan 3.0
(CapitalBio Corporation), which employed the LOWESS normalization
method (33). The differentially
expressed genes that exhibited an average ratio in triplicate tests
>2.0-fold upregulated or <0.5-fold downregulated were
obtained. Significance Analysis of Microarrays (LightCycler
software version 3.02) was performed.
Identification of differentially
expressed genes by qPCR
To further identify the results of the cDNA
microarray data, qPCR was performed in the iCycler iQ5 real-time
PCR detection system (Bio-Rad Laboratories) using EQ SYBR-Green dye
(Bio-Rad Laboratories) according to the manufacturer’s
instructions. The comparative threshold cycle (CT) method was used
to calculate the amplification fold. β-actin (forward:
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and reverse:
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′) was used as a reference control
gene to normalize the expression value of each gene. The primer
sequences are listed in Table
I.
 | Table IPrimer sequences and detection
results of differentially expressed genes identified by qPCR. |
Table I
Primer sequences and detection
results of differentially expressed genes identified by qPCR.
| Gene name | Primer
direction | Primer sequence
(5′-3′) | Product (bp) | Upregulation
fold |
|---|
| C3 | F-1 |
AAAGATAAGAACCGCTGGGAGG | 117 | 20.88 |
| R-1 |
CACGACGGGAGGCACAAA | | |
| C5 | F-1 |
CCCACTACAGAGGCTACG | 305 | 3.18 |
| R-1 |
AGGACTGAGAAACCCAAC | | |
| C4b | F-1 |
CTGTCTGCCTACTGGATTGC | 205 | 12.15 |
| R-1 |
CCTTCAGGGTTCCTTTGC | | |
| FGA | F-1 |
AGGCAACACTTACCACTG | 176 | 26.15 |
| R-1 |
GTAATCTCATTTCCACCAG | | |
| FGG | F-1 |
ACATTGCCAATAAGGGAG | 209 | 10.15 |
| R-1 |
TGTTGTGCCAGTAGGAGA | | |
| FGB | F-1 |
TTGCCCATAGAAACGAGG | 153 | 14.82 |
| R-1 |
GTGGCGACTTGGAGTGAA | | |
| SELENBP1 | F-1 |
CAAAGTATGGCTACAGGG | 84 | 10.16 |
| R-1 |
AGTGGCTCTAAGACGATT | | |
| TXNIP | F-1 |
CCACCGTCATTTCTAACT | 147 | 20.90 |
| R-1 |
ACACCTCCACTATCACCC | | |
| PDZK1IP1 | F-1 |
TTTCAGGCGGACACCAAT | 217 | 8.41 |
| R-1 |
ACGAGGACCAGGAACACG | | |
| DUSP6 | F-1 |
AGCGACTGGAACGAGAAT | 297 | 7.01 |
| R-1 |
GTTGGACAGCGGACTACC | | |
Measurement of C3 and C4b concentrations
by ELISA
The cells were plated onto 96-well plates at
4.0×104 cells/well with three duplicate wells. Then, the
supernatants of each group of cells at 24, 48, 72 and 96 h were
respectively collected to measure human complement factor C3 using
the C3 ELISA kit (Cusabio, Wuhan, China) and C4b concentration
using the C4b ELISA kit (Cusabio) according to the manufacturer’s
instructions (34). The protein
concentrations of C3 and C4b were quantified in the media
supernatants.
Determination of extracellular phosphate
ion concentrations using the phosphomolybdic acid method
The same supernatants of each group of cells at 24,
48, 72 and 96 h were used to detect extracellular phosphate ion
concentration by the phosphomolybdic acid method (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer’s instructions (35).
The quantities of Pi were quantified in the media supernatants.
Statistical analysis
Each experiment was performed at least three times.
Statistical differences between the A549-P-S groups and A549-P
groups (or A549 groups) were assessed using one-way analysis of
variance and an unpaired Student’s t-test. All analyses were
performed using SPSS software, version 19.0 (IBM, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically
significant difference.
Results
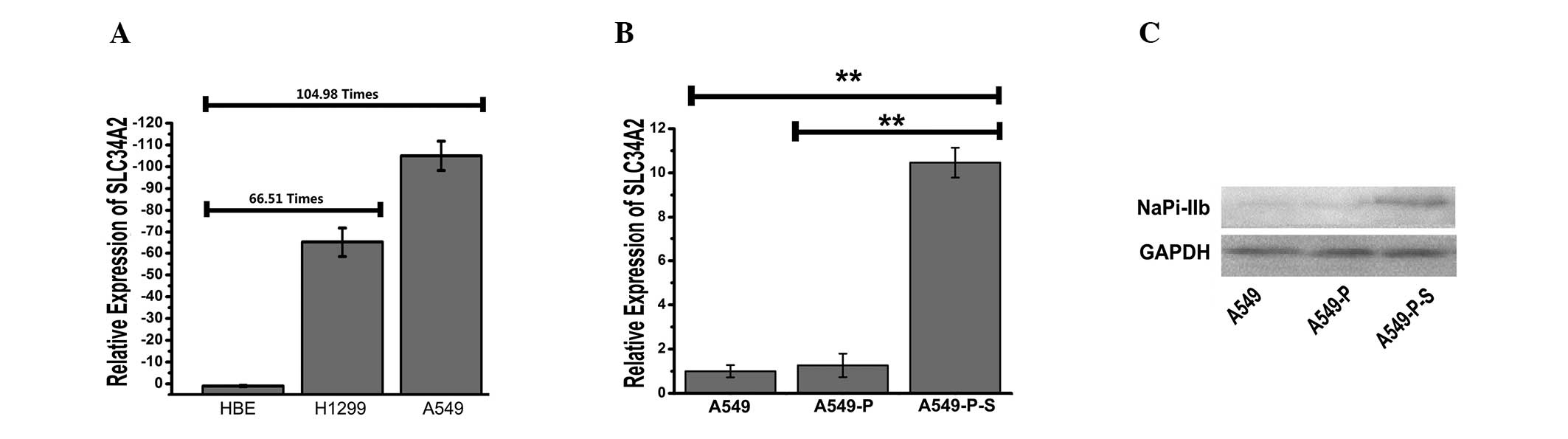
Downregulation of SLC34A2 in A549
and H1299 lung adenocarcinoma cell lines
qPCR was performed to investigate whether the
expression of SLC34A2 was different in A549 and H1299 lung
adenocarcinoma cells compared with normal human bronchial HBE cells
(Fig. 1A). The results indicated
that the expression levels of SLC34A2 were downregulated
66.51-fold in the H1299 cell line and 104.98-fold in the A549 cell
line compared with the HBE cell line (P<0.01). This suggested
that SLC34A2 might be associated with the initiation and
progression of lung adenocarcinoma.
Generation of A549 cells with stably
expressing SLC34A2
In order to investigate the effects of
SLC34A2 upregulation in human lung adenocarcinoma, A549
cells stably expressing SLC34A2 (A549-P-S) or PcDNA3.1
vector (A549-P) were initially selected by G418. qPCR and western
blot analysis were performed to detect the expression of
SLC34A2 in A549-P-S compared with A549-P and A549 cells. As
shown in Fig. 1B, the expression
level of SLC34A2 in the A549-P-S group was upregulated
10.46-fold compared with the A549 cell line (P<0.01). As shown
in Fig. 1C, the SLC34A2
protein (NaPi-IIb) was identified in the A549-P-S group and was
also functionally expressed compared with the A549-P group. The
results confirmed that the A549 cell line with stable transfection
of SLC34A2 was constructed successfully.
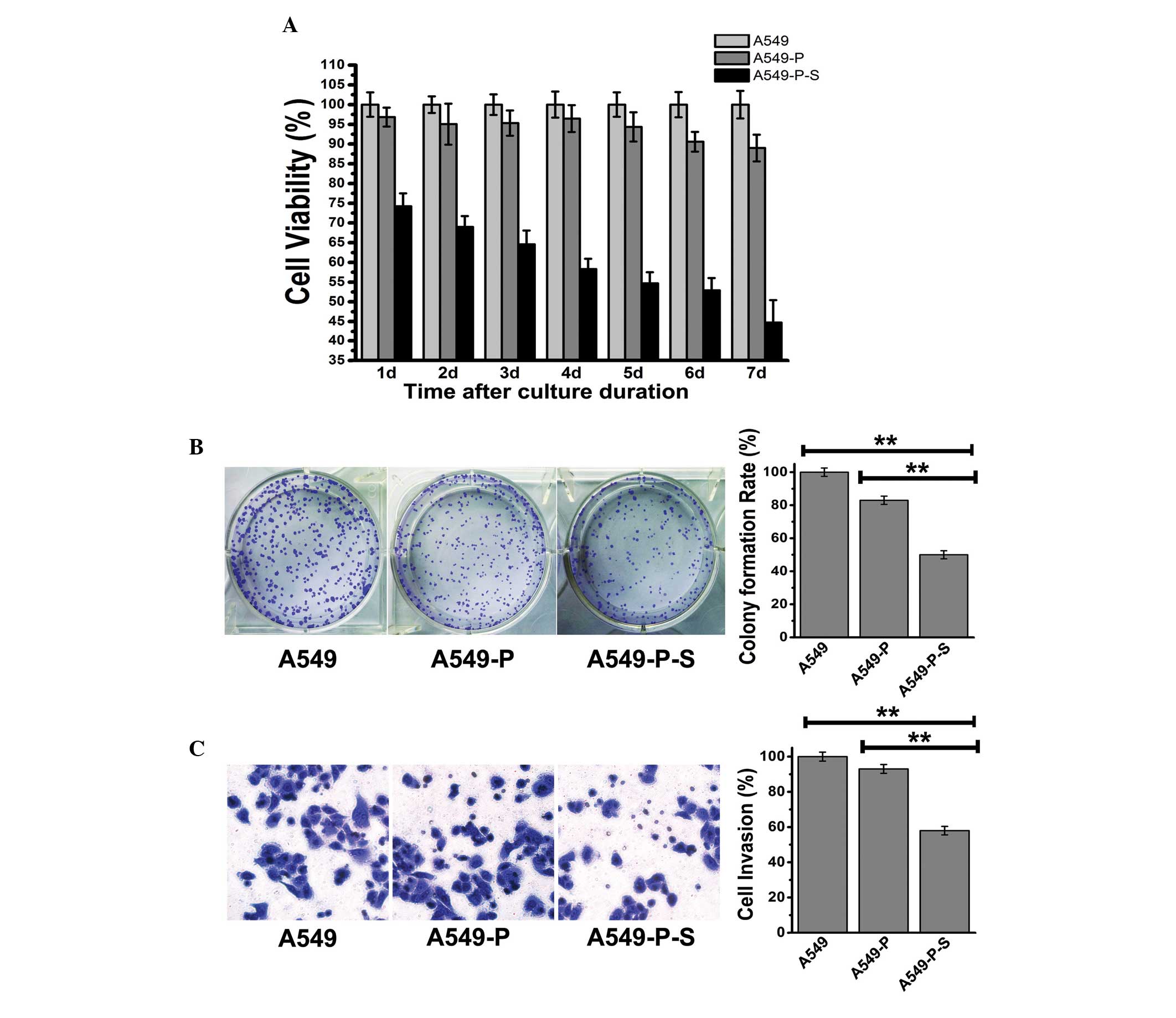
Effects of SLC34A2 on the
viability and invasion of A549 cells
The cell viability of A549, A549-P and A549-P-S
cells was examined at consecutive time points (1, 2, 3, 4, 5, 6 and
7 days) using the MTT assay and colony forming assay. As shown in
Fig. 2A, the cell viability of the
A549-P-S group was the lowest at each time point. As shown in
Fig. 2B, the colony formation rate
of the A549-P group was 83%; however, it was only 53% in the
A549-P-S group (P<0.01). The data were consistent with the
results of the growth curve of SLC34A2. In addition, a
transwell assay was performed to detect the cell invasion ability
of SLC34A2 (Fig. 2C). It
was revealed that the quantity of the cells in the chamber
decreased by 7% in the A549-P group, however, decreased by 42% in
the A549-P-S group (P<0.01). These results indicated that
enhancing the expression of SLC34A2 significantly inhibited
the viability and invasion of A549 cells.
Microarray data analysis and
identification of differentially expressed genes
In order to investigate the molecular mechanisms
underlying the effects of SLC34A2 on the viability and
invasion of A549 cells, an oligonucleotide microarray was used to
screen differentially expressed genes between A549-P-S and A549-P
cells (Fig. 3A). As shown in
Table II, the expression levels
of 10 genes were higher in the A549-P-S group compared with the
A549-P group. The upregulated genes included complement genes (C3,
C4b and C5), complement-associated genes [fibrinogen α chain
precursor (FGA), fibrinogen β chain precursor (FGB) and fibrinogen
γ chain precursor (FGG)] and tumor suppressor genes [selenium
binding protein 1 (SELENBP1), thioredoxin-interacting protein
(TXNIP), PDZK1-interacting protein 1 (PDZK1IP) and dual specificity
protein phosphatase 6 (DUSP6)]. To further validate the precision
of microarray data, qPCR was performed using the same RNA sample as
that used in the microarray. As shown in Fig. 3B, the results of qPCR were
marginally different to the microarray; however, the upregulation
observed was consistent with the microarray. Therefore, the results
suggest that the effects of SLC34A2 on A549 cells might be
associated with alterations in these genes.
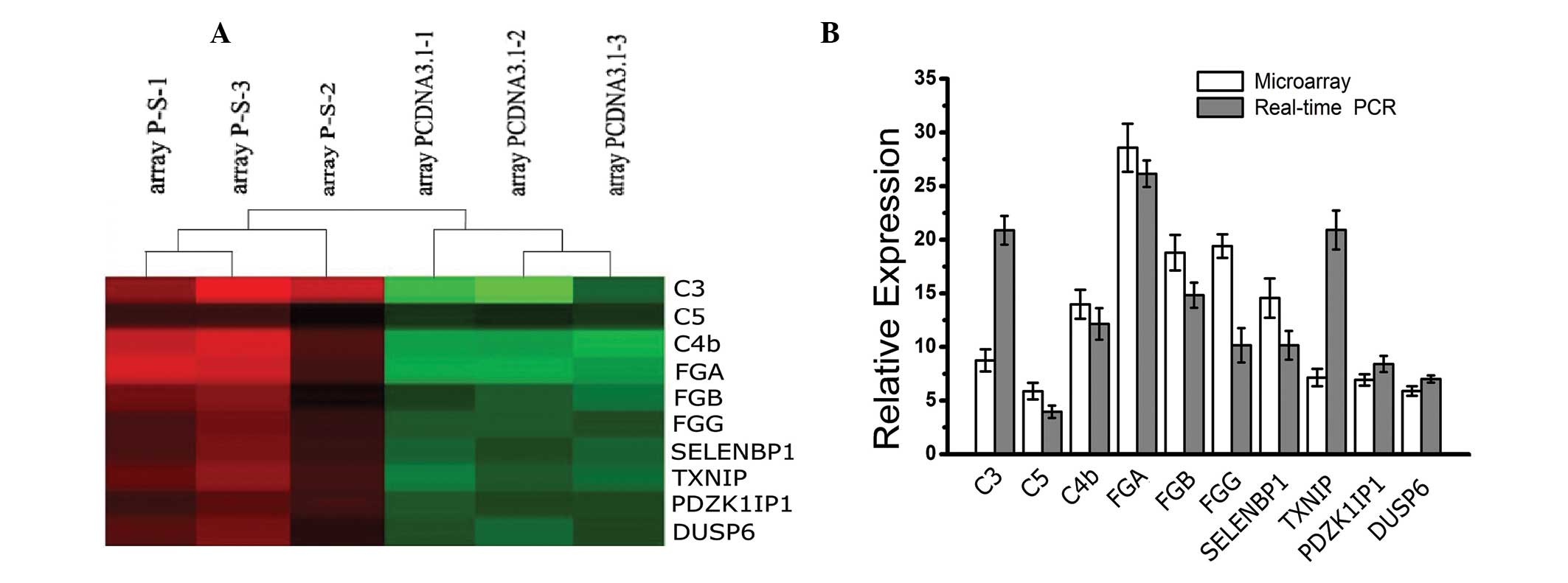 | Figure 3Differentially expressed genes
between A549-P-S and A549-P cells by microarray assay and qPCR
validation. (A) Each group in triplicate was shown on the same
chip. Color intensity was assigned to ratios of gene expression;
shades of red indicate genes that were upregulated; shades of green
indicate genes that were downregulated. (B) 10 upregulated genes
were identified by qPCR, which was consistent with cDNA microarray
data. qPCR, quantitative polymerase chain reaction; C3, complement
C3 precursor; C4b, complement 4B preproprotein; FGA, fibrinogen α
chain precursor; FGB, fibrinogen β chain precursor; FGG, fibrinogen
γ chain precursor; SELENBP1, selenium binding protein 1; TXNIP,
thioredoxin-interacting protein; PDZK1IP1, PDZK-interacting protein
1; DUSP6, dual specificity protein phosphatase 6. |
 | Table IIMicroarray analysis to determing
overexpressed genes in an A549 cell line stably expressing
SLC34A2. |
Table II
Microarray analysis to determing
overexpressed genes in an A549 cell line stably expressing
SLC34A2.
| GenBank acc.
no. | Average ratio
(Cy5/Cy3) | GenBank
identity | Gene ontology
molecular function |
|---|
| NM_001002029 | 13.98 | Homo sapiens
complement component 4B preproprotein (C4B) | Complement and
coagulation cascades |
| NM_000064 | 8.75 | Homo sapiens
complement C3 precursor (C3) | Complement and
coagulation cascades |
| NM_001735 | 5.88 | Homo sapiens
complement C5 precursor (C5) | Complement and
coagulation cascades |
| NM_021871 | 28.58 | Homo sapiens
fibrinogen α chain precursor (FGA) | Complement and
coagulation cascades |
| NM_000509 | 19.41 | Homo sapiens
fibrinogen γ chain precursor (FGG) | Complement and
coagulation cascades |
| NM_005141 | 18.80 | Homo sapiens
fibrinogen β chain precursor (FGB) | Complement and
coagulation cascades |
| NM_005025 | 14.56 | Homo sapiens
selenium binding protein 1 (SELENBP1) | Contributed -
metabolic_process--Hs_Selenoproteins |
| NM_006472 | 7.15 | Homo sapiens
Thioredoxin-interacting protein (TXNIP) | Tumor suppressor
and thioredoxin pathway |
| NM_005764 | 6.93 | Homo sapiens
PDZK1-interacting protein 1 (PDZK1IP1) | Tumor
suppressor |
| NM_001946 | 5.90 | Homo sapiens
dual specificity protein phosphatase 6 (DUSP6) | Activates
extracellular signal-regulated kinase 2 (ERK2) |
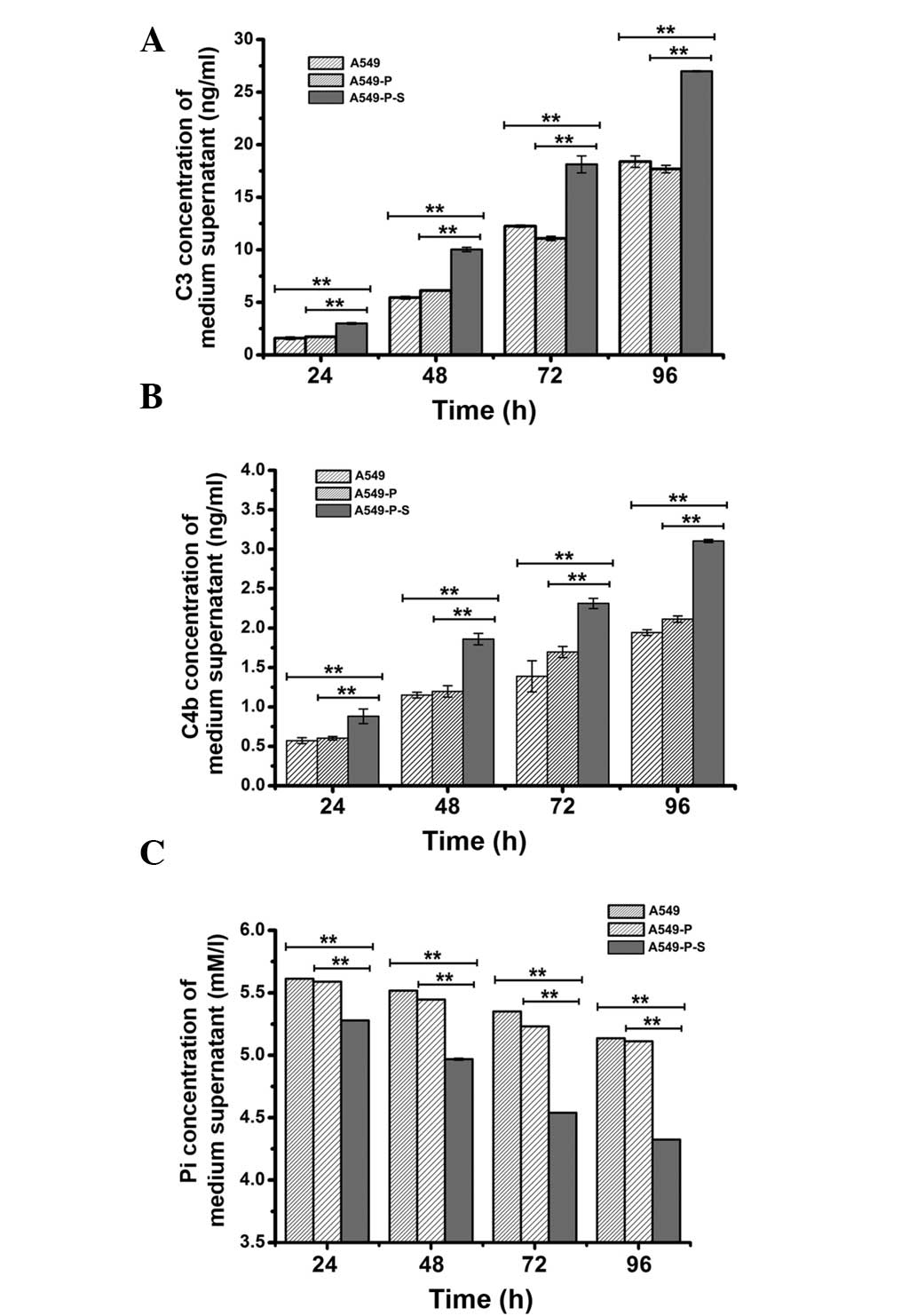
Effect of SLC34A2 on C3 and C4b
production in A549 cells
To further evaluate whether enhancing the expression
of SLC34A2 was able to increase the secretion of C3 and C4b
in A549 cells, cell culture supernatants of A549, A549-P and
A549-P-S cells were used to detect the concentration of C3 and C4b
by ELISA at consecutive time points (24, 48, 72 and 96 h). As shown
in Fig. 4A, the C3 concentration
in cell culture supernatants of the A549-P-S group respectively
increased to 1.40 ng/ml (1.26 ng/ml), 4.58 ng/ml (3.90 ng/ml), 5.87
ng/ml (7.24 ng/ml) and 8.59 ng/ml (9.30 ng/ml) at the continuous
time points compared with the A549 group (A549-P group; P<0.01).
Simultaneously, the other results, shown in Fig. 4B, demonstrated that C4b
concentration in the cell culture supernatants of the A549-P-S
group respectively increased to 0.30 ng/ml (0.27 ng/ml), 0.71 ng/ml
(0.66 ng/ml), 0.92 ng/ml (0.61 ng/ml), 1.16 ng/ml (0.99 ng/ml) at
the continuous time points compared with the A549 group (A549-P
group; P<0.01). These data further indicated that enhancing the
expression of SLC34A2 was able to significantly increase the
secretion of the complement factor C3 and C4b in A549 cells.
Effect of SLC34A2 on Pi absorption in
A549 cells
To determine whether enhancing the expression of
SLC34A2 in A549 cells was able to increase the absorption of
Pi in A549 cells and whether increasing Pi absorption was
associated with the effect of SLC34A2 on the secretion of C3
and C4b, cell culture supernatants used in the detection of C3 and
C4b concentration were also used to detect Pi concentration using
the phosphomolybdic acid method. As shown in Fig. 4C, the Pi concentration in the cell
culture supernatants of the A549-P-S group decreased to 0.33 mmol/l
(0.31 mmol/l), 0.54 mmol/l (0.47 mmol/l), 0.811 mmol/l (0.69
mmol/l) and 0.812 mmol/l (0.78 mmol/l), respectively, at the
continuous time points compared with the A549 group (A549-P group;
P<0.01). The Pi concentration and the secretion of C3 and C4b in
the A549-P-S group was greater than in the other two groups. The
results suggested that not only was enhancing the expression of
SLC34A2 in A549 cells able to increase Pi absorption of A549
cells, but also enhancing Pi absorption capacity may have a certain
connection with the effect of SLC34A2 on the secretion of C3
and C4b in A549 cells.
Discussion
The role of SLC34A2 in carcinogenesis has not
been fully elucidated, however, previous studies have indicated
that this gene might be a potential molecular marker in
carcinogenesis. Gaiłza et al (36) found that the expression of
SLC34A2 was increased in papillary thyroid carcinoma, and
suggested that this gene might be used as a potential biomarker in
the diagnosis of papillary thyroid carcinoma. Blanchard et
al (37) revealed that the
expression of SLC34A2 was decreased in breast cancer,
suggesting that this gene had a certain association with breast
cancer. Yin et al (38)
found that SLC34A2 was able to be used as a target for MX35
to treat ovarian cancer. Previous studies have demonstrated that
the expression of SLC34A2 between normal lung tissue and
lung cancer tissue was significantly different (12). To the best of our knowledge, the
present study provided the first evidence, that the expression
levels of the SLC34A2 gene in A549 and H1299 cells are
clearly different compared with HBE cells by qPCR. Furthermore, the
results demonstrate that enhancing the expression of SLC34A2
in A549 cells was able to significantly suppress the viability and
invasion of A549 cells in vitro. These results imply that
downregulation of SLC34A2 may be associated with the
initiation and progression of lung adenocarcinoma.
Microarray analysis is able to simultaneously
analyze almost 10,000 genes in a chip with added benefits,
including high-flux and high-sensitivity (39). For further examining the potential
mechanisms of SLC34A2 in lung adenocarcinoma, differentially
expressed genes between A549-P and A549-P-S cells were screened
using microarray analysis. A total of 10 upregulated genes,
including complement genes (C3, C4b and C5), complement associated
genes (FGA, FGB and FGG) and tumor suppressor genes (SELENBP1,
TXNIP, PDZK1IP1 and DUSP6) were identified by microarray and qPCR.
The results suggested that the effects of SLC34A2 on A549 cells
might be associated with the expression changes of these genes.
AT II cells possess immune functions. They can
directly synthesize factors of immune regulation, including the
complement C2, C3, C4 and C5 factors and interleukin (IL)-3
(40). It is well known that the
complement system (C1–C9) is important in immunosurveillance in the
initial stage of tumorigenesis (41). Usually, the antigen-antibody
complex formed through an antibody combining with a tumor cell
antigen is able to activate C1 and C4, thereby further activating
C3, and then activating a complement cascade to kill tumor cells
in vivo (42). Another
study indicated that the tumor cells themselves could also directly
activate complement C3, resulting in activation of the complement
alternative pathway to kill tumor cells (43). In addition, the complement
alternative pathway was also activated when complement C3 bound to
the tumor cell receptor in vitro, resulting in the formation
of the membrane attack complex (C5b-9) to destroy the tumor cell
membrane lipid bilayer and cause the tumor cell to be lysed
(44). As the key to activating
the complement alternative pathway, C3 is the most important
complement factor in the complement alternative pathway. In
addition, C4b is an active fragment of C4, which is involved in
activating C3 (43,44). C5 is a key complement factor
involved in the formation of the membrane attack complex. The
present study revealed that enhancing the expression of
SLC34A2 was able to increase mRNA expression levels of C3,
C4b and C5 in A549 cells by microarray analysis. Furthermore, the
present study confirmed that enhancing the expression of
SLC34A2 was also able to stimulate the secretion of C3 and
C4b in A549 cells by an ELISA assay. Rothman et al (45,46)
found that cytokines IL-1, IL-2 as well as lipopolysaccharide
increased C3 production in A549 cells. In addition, dexamethasone
and interferon-γ had the same effect on A549 cells (34). Duerst et al (47) found that monoclonal antibodies
(mAbs) were able to activate C3 to induce complement-dependent
cytotoxicity, which led to tumor cells lysis. With the exception of
mAbs, corresponding inhibitors of membrane complement regulatory
proteins were also able to activate the complement alternative
pathway through activating C3 (48). However, at present, there is no
report of a relevant gene that is capable of activating C3
production in vitro. To the best of our knowledge, the
present study was the first to reveal that SLC34A2 was able
to increase the secretion of C3 and C4b in A549 cells.
In AT II cells, SLC34A2 is responsible for
the synthesis of phosphate, which is the main component of
surfactant in AT II cells. The surfactant is able to increase the
activity of membrane proteins and the mobility of phospholipids
(49). Previous studies found that
improving the activity of cell surface proteins was useful for
activating C3 to trigger the complement alternative pathway
(50,51). The present study confirmed that
enhancing the expression of SLC34A2 was able to
significantly strengthen the ability to absorb Pi in the A549 cell
line. The results of the present study also demonstrated that the
effect of SLC34A2 on promoting the secretion of C3 and C4b
may be associated with a time-dependent increase of Pi absorption.
Based on the these results, it was hypothesized that the effect of
the inhibition of SLC34A2 on the viability and invasion of
A549 cells in vitro might be attributed to the activation of
the complement alternative pathway by C3 activation and increased
Pi absorption. It was also suggested that the lack of Pi might aid
the escape of abnormal AT II cells from complement-associated
immunosurveillance in the initial stages of lung adenocarcinoma
development, when downregulation of SLC34A2 induces aberrant
Pi transport. Therefore, downregulation of SLC34A2 might
cause abnormal AT II cells to develop into lung adenocarcinoma
cells. However, in the future, investigation of the effects of
inhibiting C3 and C4b on the proliferation of lung cancer cells is
required.
Adequate phosphate absorption is important for the
maintenance of cellular metabolism (52). The study by Xu et al
(30) demonstrated that the low Pi
environment was able to activate certain cell signaling pathways
associated with tumorigenesis in the normal lung cells, including
AKT signaling pathways. AKT signaling pathways were generally
active in lung cancer cells (53).
A total of four tumor suppressor genes were identified including,
SELENBP1, TXNIP, PDZK1IP1 and DUSP, which were upregulated in
A549-P-S cells compared with A549-P cells. DUSP6 is an
upstream inhibitor of ERK2, which is an important signaling protein
in the AKT signaling pathway. Overexpression of DUSP6 in
NSCLC may inactivate ERK2 and further act as a natural terminator
of AKT/MAPK signal transduction (54). The present study found that
increasing the expression of SLC34A2 in A549 cells was able
to increase DUSP6 expression. Furthermore, Pi absorption in A549
cells increased. Therefore, it was hypothesized that the effects of
SLC34A2 on the viability and invasion of A549 cells might be
associated with the upregulation of DUSP6 in the AKT/ERK2 signaling
pathway. However, further investigation is in progress. As a member
of the selenium-containing protein family, the expression of
SELENBP1 is commonly decreased in numerous types of human
epithelial cancer, and this decrease is correlated with a poor
prognosis. Previous studies have suggested that SELENBP1 exerts a
tumor suppressor function by inhibition of proliferation and was
identified as a lung cancer suppressor HIF-1 target gene (55–58).
TXNIP is a member of the thioredoxin pathway and a tumor metastasis
suppressor gene (59). PDZK1IP1
exhibited a tumor-suppressor phenotype in cultured colon cancer
cells by negatively affecting proliferation and tumor growth
(60). Therefore, our data
suggested that a low Pi environment induced by downregulation of
SLC34A2 in A549 cells may cause expression changes of these
genes associated with tumorigenesis signaling pathways, resulting
in AT II cells that develop into lung adenocarcinoma cells.
The present study first determined that the
expression levels of SLC34A2 were downregulated in A549 and
H1299 lung adenocarcinoma cells, then further revealed that the
elevated expression of SLC34A2 was able to significantly
inhibit the viability and invasion of A549 cells in vitro.
These results indicated that SLC34A2 may be important in the
initiation and progression of lung adenocarcinoma. The present
study also indicated that the relative mechanisms of SLC34A2
in A549 lung cancer may be associated with the activation of the
complement alternative pathway (C3 and C4b) and upregulation of the
expression of SELENBP1, TXNIP, PDZK1IP1 and DUSP6. These
incidents might be attributed to enhancing Pi transport in A549
cells by elevating the expression of SLC34A2. From this it
was hypothesized that the downregulation of SLC34A2,
expressed primarily in AT II cells, might cause abnormal AT II
cells to escape from complement-associated immunosurveillance and
abnormally express certain tumor-suppressor genes, inducing
development of these cells into lung adenocarcinoma. The present
study has provided further insights into the effects and mechanisms
of SLC34A2 in lung cancer.
Acknowledgements
This study was partly supported by the National
Science and Technology Major Projects of New Drugs (grant no.
2012ZX09103301-009).
Abbreviations:
|
SLC34A2
|
solute carrier family 34 (sodium
phosphate), member 2
|
|
NSCLC
|
non-small cell lung cancer
|
|
CAV1
|
caveolin 1
|
|
C3
|
complement C3 precursor
|
|
C4b
|
complement 4B preproprotein
|
|
C5
|
complement C5 precursor
|
|
FGA
|
fibrinogen α chain precursor
|
|
FGB
|
fibrinogen β chain precursor
|
|
FGG
|
fibrinogen γ chain precursor
|
|
SELENBP1
|
selenium binding protein 1
|
|
TXNIP
|
thioredoxin-interacting protein
|
|
PDZK1IP1
|
PDZK1-interacting protein 1
|
|
DUSP6
|
dual specificity protein phosphatase
6
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
AT II
|
alveolar type II
|
|
mAbs
|
monoclonal antibodies
|
|
OD
|
optical density
|
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Murer H, Forster I and Biber J: The sodium
phosphate cotransporter family SLC34. Pfugers Arch. 447:763–767.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng X, Kammerer CM, Cox LA, Morrison A,
Turner ST and Ferrell RE: Association of SLC34A2 variation and
sodium-lithium countertransport activity in humans and baboons. Am
J Hypertens. 22:288–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lituiev DS and Kiyamova RG: Mutations in
the gene of human type IIb sodium-phosphate cotransporter SLC34A2.
Biopolym Cell. 26:13–22. 2010. View Article : Google Scholar
|
|
5
|
Tenenhouse HS: Phosphate transport:
Molecular basis, regulation and pathophysiology. J Steroid Biochem
Mol Biol. 103:572–577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu H, Bai L, Collins JF and Ghishan FK:
Molecular cloning, functional characterization, tissue
distribution, and chromosomal localization of a human, small
intestinal sodium-phosphate (Na+-Pi) transporter (SLC34A2).
Genomics. 62:281–284. 1999. View Article : Google Scholar
|
|
7
|
Corut A, Senyigit A, Ugur S, Altin S,
Ozcelik U, Calisir H, Yildirim Z, Gocmen A and Tolun A: Mutations
in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly
associated with testicular microlithiasis. Am J Hum Genet.
79:650–656. 2006. View
Article : Google Scholar
|
|
8
|
Vachon L, Fareau GE, Wilson MG and Chan
LS: Testicular microlithiasis in patients with Down syndrome. J
Pediatr. 149:233–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hernando N, Sheikh S, Karim-Jimenez Z,
Galliker H, Forgo J and Biber J: Asymmetrical targeting of type II
Na-P(i) cotransporters in renal and intestinal epithelial cell
lines. Am J Physiol Renal Physiol. 278:F361–F368. 2000.PubMed/NCBI
|
|
10
|
Cerri MF, Rezende LC, Paes FM, Silva IV
and Rangel LBA: The cotransporter NaPi-IIb: characteristics,
regulation and its role in carcinogenesis. Applied Cancer Res.
30:197–203. 2010.
|
|
11
|
Shibasaki Y, Etoh N and Hayasaka M:
Targeted deletion of the tybe IIb Na+dependent
Pi-co-transporter, NaPi-IIb, results in early embryonic lethality.
Biochem Biophys Res Commun. 381:482–486. 2009.PubMed/NCBI
|
|
12
|
Kopantzev EP, Monastyrskaya GS,
Vinogradova TV, et al: Differences in gene expression levels
between early and later stages of human lung development are
opposite to those between normal lung tissue and non-small lung
cell carcinoma. Lung Cancer. 62:23–34. 2008. View Article : Google Scholar
|
|
13
|
el-Deiry WS, Nelkin BD, Celano P, Yen RW,
Falco JP, Hamilton SR and Baylin SB: High expression of the DNA
methyltransferase gene characterizes human neoplastic cells and
progression stages of colon cancer. Proc Natl Acad Sci USA.
88:3470–3474. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lengauer C, Kinzler KW and Vogelstein B:
DNA methylation and genetic instability in colorectal cancer cells.
Proc Natl Acad Sci USA. 94:2545–2550. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Kho AT, Kohane IS and Sun Y:
Predicting survival within the lung cancer histopathological
hierarchy using a multi-scale genomic model of development. PLoS
Med. 3:e2322006. View Article : Google Scholar
|
|
16
|
Ramirez MI, Pollack L, Millien G, Cao YX,
Hinds A and Williams MC: The alpha-isoform of caveolin-1 is a
marker of vasculogenesis in early lung development. J Histochem
Cytochem. 50:33–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hnasko R and Ben-Jonathan N: Developmental
regulation of PV-1 in rat lung: association with the nuclear
envelope and limited colocalization with Cav-1. Am J Physiol Lung
Cell Mol Physiol. 288:L275–L284. 2005. View Article : Google Scholar
|
|
18
|
Wiliams TM and Lisanti MP: Caveolin-1 in
oncogenic transformation, cancer, and metastasis. Am J Physiol Cell
Physiol. 288:C494–C506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sunaga N, Miyajima K, Suzuki M, Sato M,
White MA, Ramirez RD, Shay JW, Gazdar AF and Minna JD: Different
roles for caveolin-1 in the development of non-small cell lung
cancer versus small cell lung cancer. Cancer Res. 64:4277–4285.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bélanger MM, Gaudreau M, Roussel E and
Couet J: Role of caveolin-1 in etoposide resistance development in
A549 lung cancer cells. Cancer Biol Ther. 3:954–959.
2004.PubMed/NCBI
|
|
21
|
Traebert M: Expression of type II Na-P(i)
cotransporterin alveolar type II cells. Am J Physiol.
277:L868–L873. 1999.PubMed/NCBI
|
|
22
|
Griffiths MJ, Bonnet D and Janes SM: Stem
cells of the alveolar epithelium. Lancet. 366:249–260. 2005.
|
|
23
|
Kinnard WV, Tuder R, Papst P and Fisher
JH: Regulation of alveolar type II cell differentiation and
proliferation in adult rat lung explants. Am J Respir Cell Mol
Biol. 11:416–425. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ten Have-Opbroek AA, Benfield JR, Hammond
WG and Dijkman JH: Alveolar stem cells in canine bronchial
carcinogenesis. Cancer Lett. 101:211–217. 1996.PubMed/NCBI
|
|
25
|
Ten Have-Opbroek AA, Benfield JR, van
Krieken JH and Dijkman JH: The alveolar type II cell is a
pluripotential stem cell in the genesis of human adenocarcinomas
and squamous cell carcinomas. Histol Histopathol. 12:319–336.
1997.PubMed/NCBI
|
|
26
|
Kitinya JN, Sueishi K, Tanaka K and
Katsuda Y: Immunoreactivity of surfactant-apoprotein
adenocarcinomas, large cell and small cell carcinomas of the lung.
Acta Pathol Jpn. 36:1271–1278. 1986.PubMed/NCBI
|
|
27
|
Gazdar FA, Linnoila R, Kurita Y, et al:
Peripheral airway cell differentiation in human lung cancer. Cancer
Res. 50:5481–5487. 1990.PubMed/NCBI
|
|
28
|
Holm BA, Matalon S, Finkelstein JN and
Notter RH: Type II pneumocyte changes during hyperoxic lung injury
and recovery. J Appl Physiol. 1985.65:2672–2678. 1988.PubMed/NCBI
|
|
29
|
Kim CF, Jackson EL, Woolfenden AE, et al:
Identification of bronchioalveolar stem cells in normal lung and
lung cancer. Cell. 121:823–835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu CX, Jin H, Chung YS, et al: Low dietary
inorganic phosphate affects the lung growth of developing mice. J
Vet Sci. 10:105–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsao MS, Zhu H and Viallet J: Autocrine
growth loop of the epidermal growth factor receptor in normal and
immortalized human bronchial epithelial cells. Exp Cell Res.
223:268–273. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lieber M, Smith B, Szakal A, Nelson-Rees W
and Todaro G: A continuous tumor-cell line from a human lung
carcinoma with properties of type II alveolar epithelial cells. Int
J Cancer. 17:62–70. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Q, Lu A, Xiao G, et al: Transcriptional
profiling of midgut immunity response and degeneration in the
wandering silkworm, Bombyx mori. Plos one. 7:e437692012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hill LD, Sun L, Leuschen MP and Zach TL:
C3 synthesis by A549 alveolar epithelial cells is increased by
interferon-γ and dexamethasone. Immunology. 79:236–240.
1993.PubMed/NCBI
|
|
35
|
Neal C, Neal M and Wickham H: Phosphate
measurement in natural waters: two examples of analytical problems
associated with silica interference using phosphomolybdic acid
methodologies. Sci Total Environ. 252:511–512. 2000. View Article : Google Scholar
|
|
36
|
Gałeza M, Zebracka J, Szpak-Ulczok S, et
al: Expression of selected genes involved in transport of ions in
papillary thyroid carcinoma. Endokrynol Pol. 57:26–31. 2006.(In
Polish).
|
|
37
|
Blanchard A, Shiu R, Booth S, et al: Gene
expression profiling of early involuting mammary gland reveals
novel genes potentially relevant to human breast cancer. Front
Biosci. 12:2221–2232. 2007. View
Article : Google Scholar
|
|
38
|
Yin BW, Kiyamova R, Chua R, Caballero OL,
et al: Monoclonal antibody MX35 detects the membrane transporter
NaPi2b (SLC34A2) in human carcinomas. Cancer Immun.
8:32008.PubMed/NCBI
|
|
39
|
Cuzin M: DNA chips: a new tool for genetic
analysis and diagnostics. Transfus Clin Biol. 8:291–296. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Strunk RC, Eidlen DM and Mason RJ:
Pulmonary alveolar type II epithelial cells synthesize and secrete
proteins of the classical and alternative complement pathways. J
Clin Invest. 81:1419–1426. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Niculescu F, Rus HG, Retegan M and Vlaicu
R: Persistent complement activation on tumor cells in breast
cancer. Am J Pathol. 140:1039–1043. 1992.PubMed/NCBI
|
|
42
|
Magyarlaki T, Mosolits S, Baranyay F and
Buzogany I: Immunohistochemistry of complement response on human
renal cell carcinoma biopsies. Tumori. 82:473–479. 1996.PubMed/NCBI
|
|
43
|
Clynes RA, Towers TL, Presta LG and
Ravetch JV: Inhibitory Fc receptors modulate in vivo cytoxicity
against tumor targets. Nat Med. 6:443–446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kierszenbaum F and Budzko DB: Cytotoxic
effects of normal sera on lymphoid cells. I. Antibody-independent
killing of heterologous thymocytes by guinea pig, rabbit, and human
sera: Role of the alternative pathway of complement activation.
Cell Immunol. 29:137–146. 1977.
|
|
45
|
Rothman BL, Despins AW and Kreutzer DL:
Cytokine regulation of C3 and C5 production by the human type II
pneumocyte cell line, A549. J Immunol. 145:592–598. 1990.PubMed/NCBI
|
|
46
|
Rothman BL, Merrow M, Despins A, Kennedy T
and Kreutzer DL: Effect of lipopolysaccharide on C3 and C5
production by human lung cells. J Immunol. 143:196–202.
1989.PubMed/NCBI
|
|
47
|
Duerst RE, Ryan DH and Frantz CN:
Variables affecting the killing of cultured human neuroblastoma
cells with monoclonal antibody and complement. Cancer Res.
46:3420–3425. 1986.PubMed/NCBI
|
|
48
|
Jima DD, Shaha RN and Orcutta TM: Enhanced
transcription of complement and coagulation genes in the absence of
adaptive immunity. Mol Immunol. 46:1505–1516. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hickman-Davis JM, Fang FC and Nathan C:
Lung surfactant and reactive oxygen-nitrogen species: antimicrobial
activity and host-pathogen interactions. Am J Physiol Lung Cell Mol
Physiol. 281:L517–L523. 2001.PubMed/NCBI
|
|
50
|
Malmsten M, Lassen B, James MV and Nilsson
UR: Adsorption of complement proteins C3 and C1q. J Colloid
Interface Sci. 178:123–134. 1996. View Article : Google Scholar
|
|
51
|
Mold C: Effect of membrane phospholipids
on activation of the alternative complement pathway. J Immunol.
143:1663–1668. 1989.PubMed/NCBI
|
|
52
|
Takeda E, Yamamoto H, Nashiki K, Sato T,
Arai H and Taketani Y: Inorganic phosphate homeostasis and the role
of dietary phosphorus. J Cell Mol Med. 8:191–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Furukawa T, Sunamura M, Motoi F, Matsuno S
and Horii A: Potential tumor suppressive pathway involving
DUSP6/MKP-3 in pancreatic cancer. Am J Pathol. 162:1807–1815. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang Z, Kobayashi S, Borczuk AC, Leidner
RS, Laframboise T, Levine AD and Halmos B: Dual specificity
phosphatase 6 (DUSP6) is an ETS-regulated negative feedback
mediator of oncogenic ERK signaling in lung cancer cells.
Carcinogenesis. 31:577–586. 2010.
|
|
55
|
Li T, Yang W and Li M: Expression of
selenium binding protein 1 characterizes intestinal cell maturation
and predicts survival for patients with colorectal cancer. Mol Nutr
Food Res. 52:1289–1299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang KC, Park DC, Ng SK, et al: Selenium
binding protein 1 in ovarian cancer. Int J Cancer. 118:2433–2440.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen G, Wang H and Miller CT: Reduced
selenium-binding protein 1 expression is associated with poor
outcome in lung adenocarcinomas. J Pathol. 202:321–329. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang M and Sytkowski AJ: Differential
expression and androgen regulation of the human selenium-binding
protein gene hSP56 in prostate cancer cells. Cancer Res.
58:3150–3153. 1998.PubMed/NCBI
|
|
59
|
Parikh H, Carlsson E, Chutkow WA and
Johansson LE: TXNIP regulates peripheral glucose metabolism in
humans. PLoS Med. 4:e1582007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Capuano P, Bacic D and Stange G:
Expression and regulation of the renal Na/phosphate cotransporter
NaPi-IIa in a mouse model deficient for the PDZ protein PDZK1.
Pflugers Arch. 449:392–402. 2005. View Article : Google Scholar : PubMed/NCBI
|