Introduction
Airway smooth muscle (ASM) cells are the main
components of the respiratory tract system, which have important
physiological roles, including maintaining the entire airway
structure and keeping airway tension normal. In recent years,
studies have indicated that cell function is closely correlated
with the pathophysiological changes of respiratory diseases,
including the proliferation of airway smooth muscle cells, airway
inflammation and airway remodeling. ASM is important in almost all
the pathophysiological and clinical aspects of respiratory illness,
including bronchial asthma and chronic obstructive pulmonary
disease (COPD) (1).
A better understanding of the regulatory factors in
airways may be beneficial for elucidating the pathogenesis of
respiratory illnesses. The function of ASM cells is regulated via
intracellular signaling pathways, involving Ca2+
handling, enzymatic activity as well as protein phosphorylation.
Among all the factors, intracellular Ca2+ may be
crucial. An increase in the intracellular Ca2+
concentration is a key signal in initiating and maintaining a
variety of cellular responses, including ASM contraction, cell
proliferation and migration and gene expression in ASM cells
(2). Studies have demonstrated
that Ca2+, which was released from the sarcoplasmic
reticulum (SR), was mainly regulated by the stimulated inositol
1,4,5-trisphosphate receptor (IP3R) and the ryanodine
receptor (RyR) (3,4). Stimulation of G protein-coupled
receptors leads to the activation of phospholipase C (PLC), which
then induces the production of the second messengers inositol
1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). When
IP3 binds to its receptor IP3R located on the
SR, the released Ca2+ is able to activate
Ca2+-calmodulin-dependent myosin light chain kinase,
which finally leads to the contraction of respiratory tract smooth
muscles (5). Furthermore, RyR is
also involved in calcium channel modulation. It is able to generate
self-sustaining intracellular calcium oscillations via a mechanism
called calcium-induced calcium release (6).
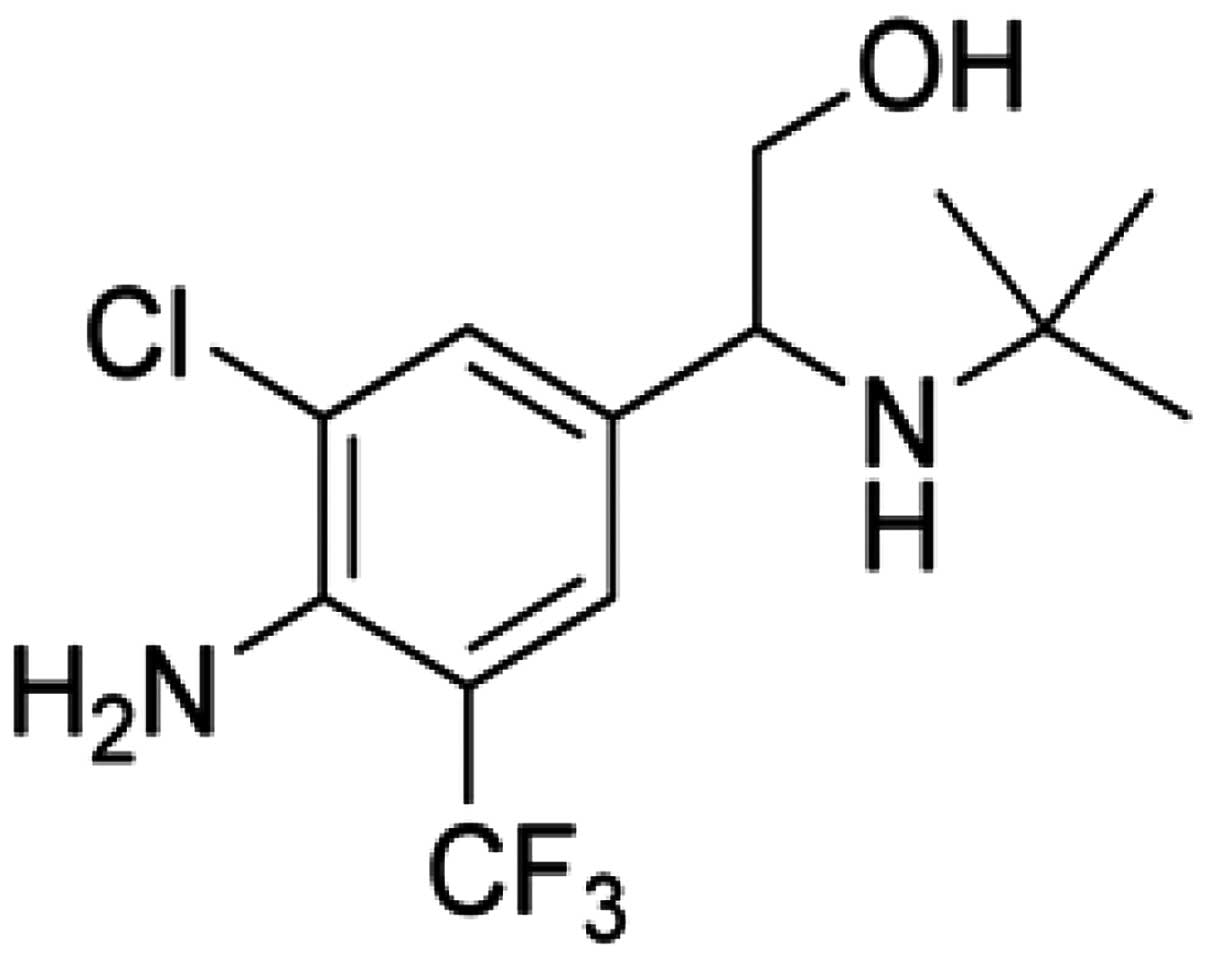
2-(4-amino-3-chloro-5-trifluomethyl-phenyl)-2-tert-butylamino-ethanol
hydrochloride (SPFF), a novel β2-adrenoceptor agonist, was
synthesized and developed by the chemical synthetic laboratory of
Shenyang Pharmaceutical University (Shenyang, Liaoning, China). The
molecular structure of SPFF is displayed in Fig. 1. Previous studies demonstrated that
SPFF was a potent, long-acting bronchodilator with relatively high
β2-adrenoceptor selectivity (7).
The present study established a method for guinea pig airway smooth
muscle cell culture in vitro and improved it in order to
acquire a sufficient quantity of the cells. The effect of SPFF on
the intracellular Ca2+ concentration and its potential
mechanisms were investigated in the cultured ASM cells.
Materials and methods
Animals
Male or female hartley guinea pigs weighing 150–200
g were provided by the Experimental Animal Center of Shenyang
Pharmaceutical University (Shenyang, Liaoning, China). Animals were
bred in a facility controlled by temperature (26±3°C), relative
humidity (50±5%) and light (14 and 10 h of light and dark), with
free access to food and water.
Drugs and reagents
SPFF was supplied by the School of Pharmaceutical
Engineering, Shenyang Pharmaceutical University (Shenyang,
Liaoning, China; ee>99%). Dulbecco’s modified Eagle’s medium
(DMEM) and Hanks’ balanced salt solution (HBSS) were purchased from
Gibco-BRL (Carlsbad, CA, USA). Triton X-100 and MTT were obtained
from Ameresco (Solon, OH, USA). 2-Aminoethyl diphenylborinate
(2-APB) and dantrolene sodium salt were obtained from Sigma (St.
Louis, MO, USA). Fura-2/AM was purchased from the Jiangsu Beyotime
Institute of Biotechnology (Haimen, Jiangsu, China). Mouse
anti-α-smooth muscle actin (α-SMA), the streptavidin-biotin complex
immunohistochemical staining kit and the diaminobenzidine staining
kit were purchased from Wuhan Boster Biological Technology, Ltd.
(Wuhan, China) and fetal bovine serum (FBS) was obtained from
Tianjin Hualida Biotechnology Co., Ltd. (Tianjin, China). Mabuterol
was provided by the School of Pharamceutical Engineering, Shenyang
Pharmaceutical University (Shenyang, Liaoning, China).
Method
Guinea pig ASM cell culture
ASM bundles were isolated from guinea pig tracheas
(five males and five females each time), which underwent surgical
resection in accordance with procedures approved by the ethics
committee of Shenyang Pharmaceutical University. Guinea pig ASM was
cut into pieces of 1 mm3 using iridectomy scissors
following washing in pre-cooled Hank’s balanced salt solution
(HBSS). The pieces were then transferred into a 100 cm2
cell culture flask coated preliminarily with FBS. The flask was
agitated to allow the pieces to firmly attach to the flask wall and
placed in a humidified incubator at 37°C and 5% CO2. The
flask was turned over following 16–20 h. DMEM containing 15 % FBS,
100 IU/ml penicillin, 100 IU/ml streptomycin and 2 mg/ml
l-glutamine were added to the flask, and the cells were further
incubated. In accordance with the routine culture procedure, the
medium was replaced every three days.
Once the cells migrated out of tissue blocks and
grew to 90% confluence, they were passaged using 0.25% trypsin in
0.02% EDTA. Cells at passage 3–10 were used in the subsequent
experiments.
Identification of the guinea pig ASM
cells
Cell morphology, including dimension and
arrangement, was observed under an optical microscope (IX71;
Olympus, Tokyo, Japan).
Immunocytochemical identification by α-SMA was
performed. In brief, cells at passages 5–8 were digested by 0.25%
trypsin and 95% viability was identified by trypan blue exclusion.
The cells were seeded in 24-well culture plates preliminarily
coated with poly-l-lysine in a humidified incubator at 37°C and 5%
CO2 for 24 h. The plates were rinsed three times using
0.01 mol/l of phosphate-buffered saline and fixed in 4%
phosphate-buffered paraformaldehyde for 30 min at room temperature.
Following that, they were rinsed a further three times and treated
with 0.25% Triton X-100 for 10 min at room temperature. Then, they
were blocked with 5% bovine serum albumin (Ameresco, Solon, OH,
USA) for 30 min at room temperature following being rinsed three
times. Then they were rinsed three times again and incubated with
α-SMA (1:200) at 4°C overnight. Following 12 h, the plates were
rinsed three times and treated according to the manufacturer’s
instructions of the SABC immunohistochemical staining kit and then
those of the DAB staining kit. The results from the immunoreactive
bands were determined according to the manufacturer’s instructions
and images were captured.
Identification of the growth
characteristics of guinea pig ASM cells
The cells were seeded in 96-well culture plates
following digestion with 0.25% trypsin. The viability was
determined using an MTT assay as previously described (8) following day 1, 2, 3, 4, 5, 6 and 7,
respectively. Cell viability and doubling time reflected the growth
characteristics of guinea pig ASM cells.
Determination of intracellular calcium
levels and their impairment by SPFF
The cells were digested by 0.25% trypsin when they
grew to 90% confluence. Following being resuspended by fresh DMEM,
they were seeded in black 96-well culture plates for 48 h in a
humidified incubator at 37°C and 5% CO2. The plates were
rinsed twice with HBSS following the removal of DMEM. The cells
were loaded with 5 μM Fura-2/AM for 45 min on a microscale shaker
at room temperature following treatment with SPFF or Mabuterol for
30 min at 37°C. Then, the loading buffer was removed and cells were
rinsed twice with HBSS to remove excess fluorescence dye. A
suitable quantity of HBSS was added to maintain the cells in a
basic physiological state. Calcium fluorescence intensity was
determined by a multi-function microplate reader (3925; Corning
America, Corning, NY, USA) at an excitation wavelength of 340 nm
and an emission wavelength of 510 nm. The relative calcium
inhibition rate was determined using the following formula:
(fluorescence intensity in the control group-fluorescence intensity
in the tested group)/fluorescence intensity in the control
group.
Investigation of the correlation between
SPFF intervention, IP3R and RyR
The cells were treated with 2-APB, an
IP3R blocker, and dantrolene, a RyR blocker, for 30 min
at 37°C following the addition of SPFF. Then the reaction system
was loaded with 5 μM Fura-2/AM for 45 min on a microscale shaker at
room temperature. The calcium fluorescence intensity of the
different treated groups was determined using a multi-function
microplate reader. The potential inhibitive effect of SPFF on the
release of intracellular calcium was analyzed according to changes
in calcium fluorescence intensity.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The significance between groups was determined using one-way
analysis of variance with SPSS 16.0 software (SPSS Inc, Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
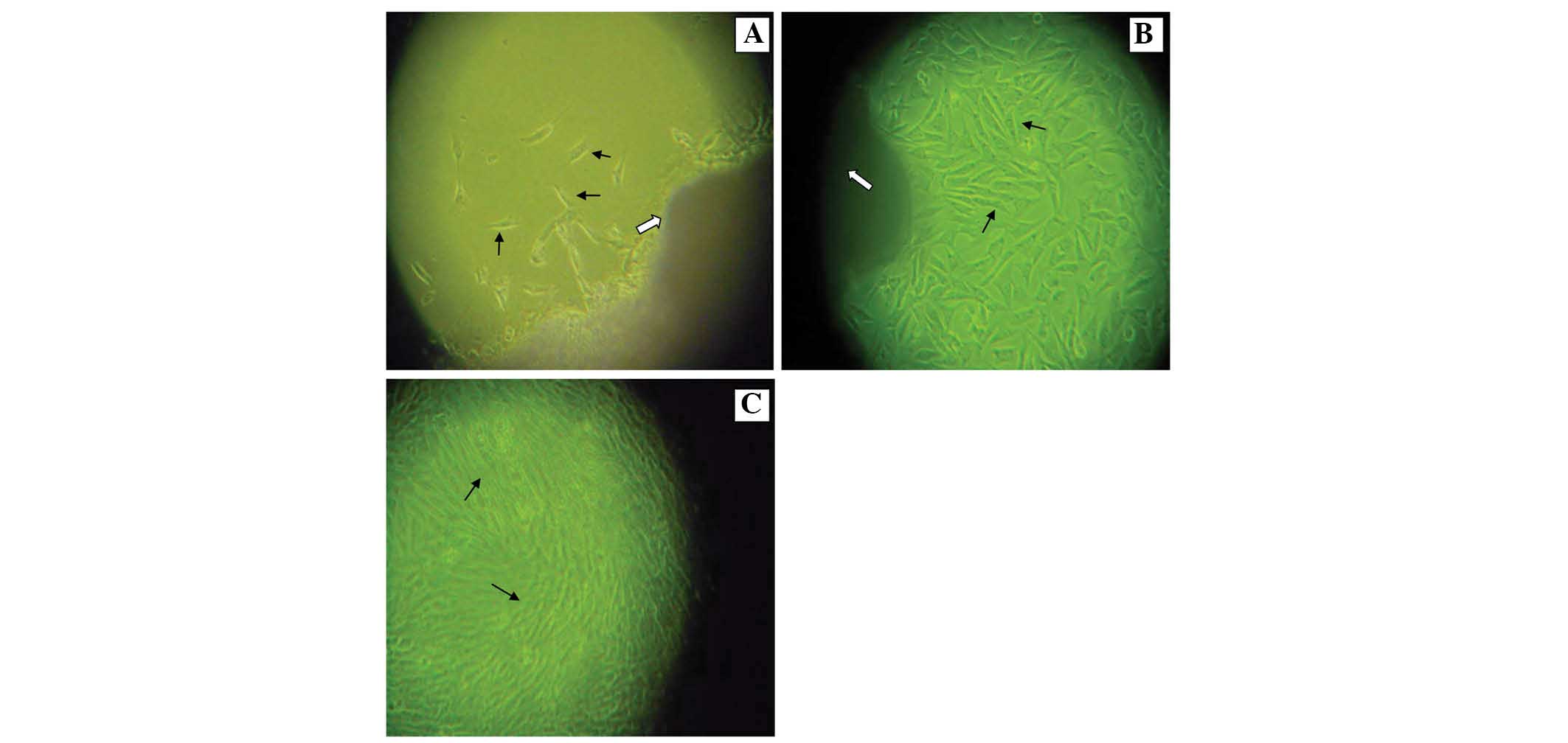
Primary guinea pig ASM cells
The cells migrated out from the tissue masses
(indicated by a hollow arrowhead in Fig. 2A and B) grew and spread out in a
radiating shape (indicated by short arrowheads in Fig. 2A) following 5–7 days of using the
improved abovementioned method. The number of cells was
significantly increased and fine confluence was able to be observed
among adjacent cells 8–10 days later (indicated by short arrowheads
in Fig. 2B). Cell confluence
gradually became apparent 11–14 days later. Furthermore, interlaced
reticulation around cells began to appear and then gradually
demonstrated numerous branches when the density of the cells was
highly increased (indicated by short arrowheads in Fig. 2C).
Guinea pig ASM cells following
passage
The cells that grew up to 80% confluence gradually
underwent morphological changes following treatment with 0.25%
trypsin in 0.02% EDTA, including shrinking into a ball and becoming
bright under the optical microscope, which indicated their
cytoplasmic rebound (indicated by short arrowheads in Fig. 3A). Cells that were firstly
subcultured and then partly transferred into a new cell culture
flask attached on the lining surface of the culture flask 12 h
later (indicated by short arrowhead in Fig. 3B). Within the next couple of days,
the cells returned to their original morphology and were fusiform
or polygonal (long arrowheads indicate fusiform cells and short
arrowheads indicate polygonal cells in Fig. 3C). In addition, a typical
peak-valley pattern of ASM cells was able to be observed under the
optical microscope around 5–7 days following subculture (long
arrowheads indicate peaks and short arrowheads indicate valleys in
Fig. 3D).
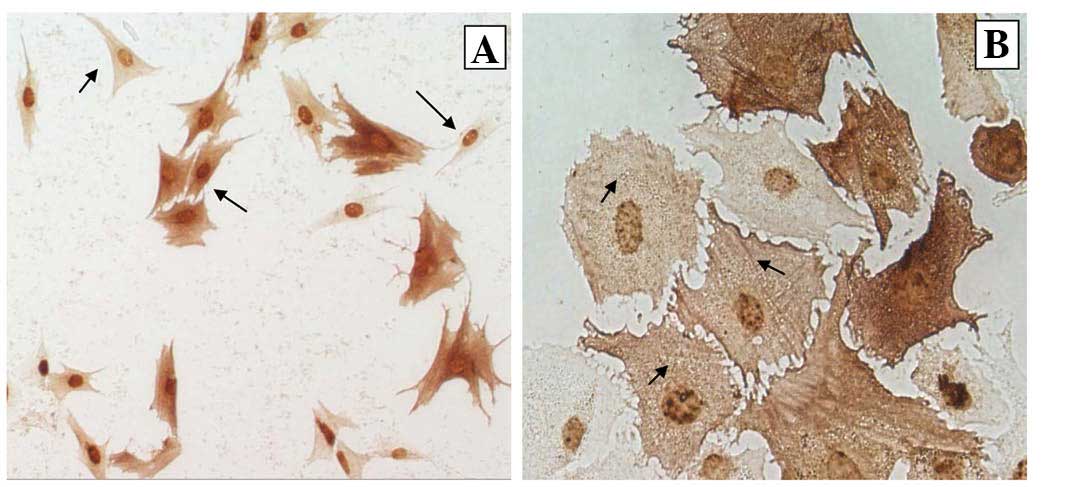
Guinea pig ASM cells identified by
immunocytochemistry
Data from immunocytochemical staining with specific
α-SMA antibodies demonstrated that the cultured cells were guinea
pig ASM cells. Immunoreactive products displaying a brown-yellow
color located in the cytoplasm were evident. Various shapes in the
brown-yellow positive cells, including fusiform, polygonal and
irregular triangles were observed under the optical microscope (the
longest arrowhead indicates fusiform cells, the long arrowhead
indicates polygonal cells and the short arrowhead indicates
irregular triangle cells in Fig.
4A). The brown-yellow immunoreactive products were displayed in
a skeletal pattern under a high-multiplied microscope (indicated by
short arrowheads in Fig. 4B). The
immunocytochemical characteristics clearly demonstrated that the
cultured cells were typical guinea pig ASM cells.
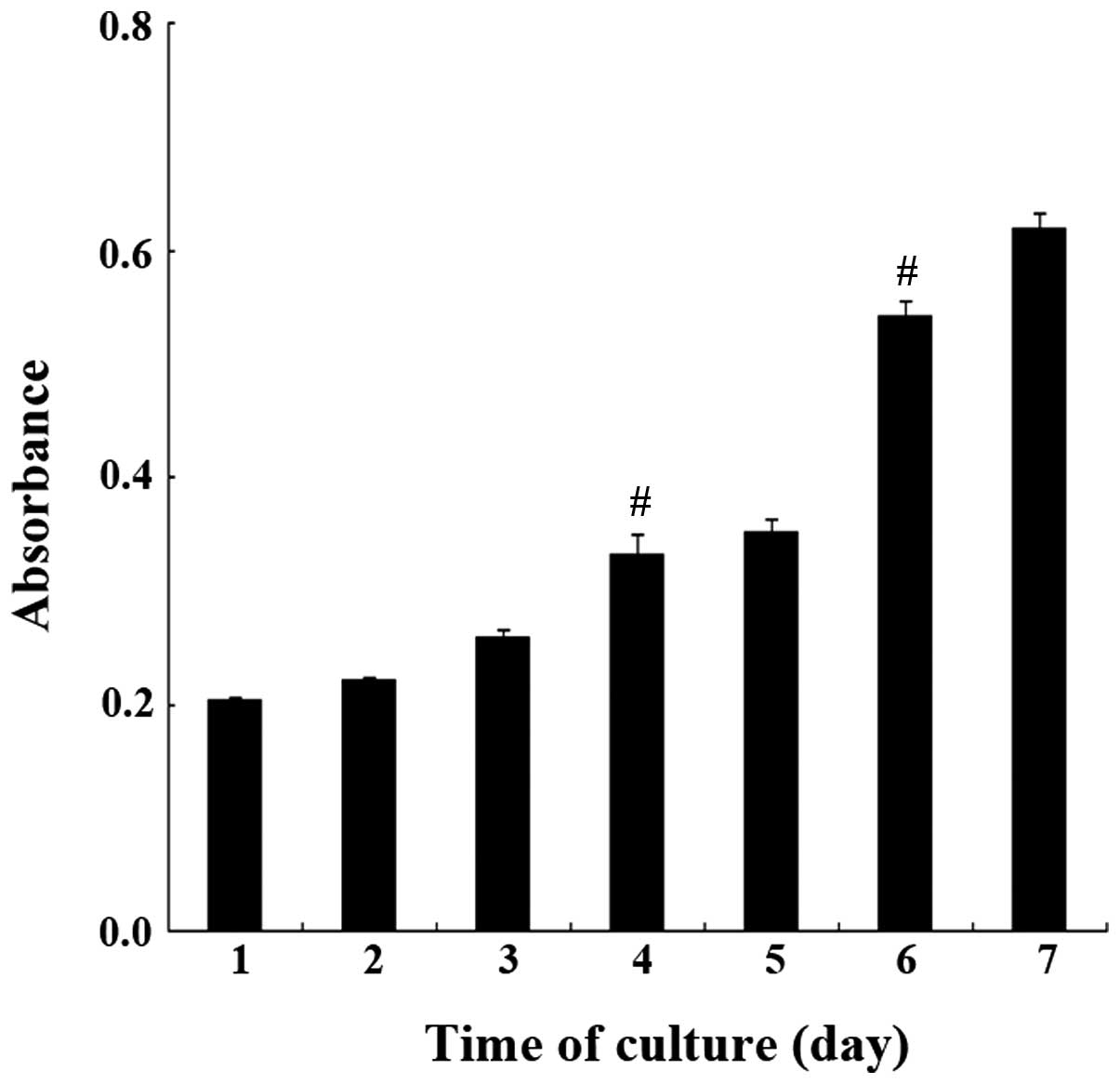
Growth characteristics of guinea pig ASM
cells
As displayed in Fig.
5, guinea pig ASM cells demonstrated a good growth status and
the number of cells significantly increased from day 4 to 6 when
compared with the previous days. Their doubling time was 3.00±0.5
days. The results demonstrated that cells were in a good condition
and were able to be employed in the follow-up studies.
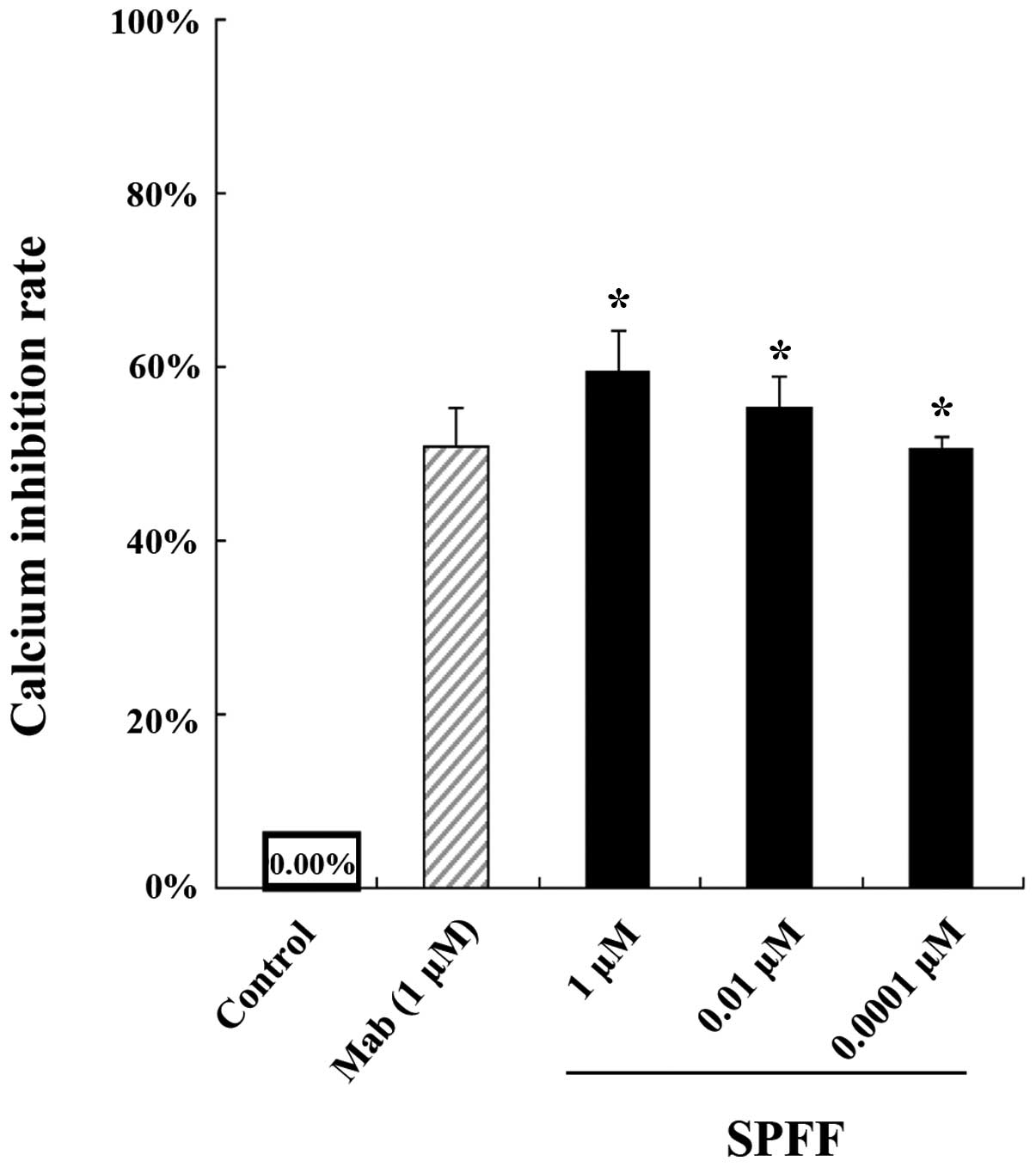
Suppressive effect of SPFF on
intracellular Ca2+ release
As shown in Fig. 6,
the release of intracellular Ca2+ was clearly suppressed
by SPFF and the inhibition rate of the drug at 1, 0.01 and 0.0001
μM was 59.54, 55.30 and 50.46%, respectively. The effect was
concentration-dependent when compared with the control. It was
clear that SPFF had a better pharmacological activity than
mabuterol, the positive drug.
Suppression of Ca2+ release by
SPFF is regulated by IP3R and RyR antagonists
As shown in Fig. 7,
the release of intracellular calcium was clearly suppressed by SPFF
(1 μM) alone, SPFF (1 μM) plus 2-APB (40 μM, an IP3
receptor antagonist) as well as SPFF (1 μM) plus dantrolene (40 μM,
a ryanodine receptor antagonist) and the calcium inhibition rate
was 56.52, 58.91 and 71.10%, respectively. The effect of SPFF on
the Ca2+ levels was almost the same as that in the SPFF
plus 2-APB group. However, when SPFF was used in combination with
dantrolene, its calcium inhibition rate increased to 71.10%.
 | Figure 7Inhibition of SPFF (1 μM) on the
release of intracellular calcium was regulated by 2-APB (40 μM, an
IP3R antagonist) or dantrolene (40 μM, a ryanodine
receptor antagonist) in guinea pig ASM cells. As shown, the effect
of SPFF on Ca2+ was almost the same as SPFF plus 2-APB,
which indicated that the inhibitory effect of the two overlapped;
however, when SPFF was combined with dantrolene, its calcium
inhibition rate clearly increased. The results implied that the
potential mechanism of SPFF on the inhibition of intracellular
calcium may be independent of the RyR pathway, but may have a close
association with the IP3R pathway. Data are expressed as
the mean ± standard deviation (n=3). #P<0.05 was
considered to indicate a statistically significant difference when
compared with SPFF alone. SPFF,
2-(4-amino-3-chloro-5-trifluomethyl-phenyl)-2-tert-butylamino-ethanol
hydrochloride; ASM, airway smooth muscle; IP3R, inositol
1,4,5-trisphosphate receptor; RyR, ryanodine receptor; 1-APB,
2-aminoethyl diphenylborinate. |
Discussion
Cultured ASM cells are important and useful as an
in vitro model for studying the molecular mechanisms
underlying either normal or diseased airways. However, it is not
easy to obtain in vitro cultured primary guinea pig ASM
cells in order to study the pharmacological effects and mechanisms
of drugs acting on respiratory diseases. The first reason is the
technical obtaining of adequate tissue. Isolating ASM from tissues
is difficult. Secondly, it is difficult to remove other cells,
including myofibroblasts (1).
Therefore, it is important to establish a good method for ASM cell
primary culture which is simple, efficient and able to produce
repeatable results. The present study described and improved method
for the isolation, culture, passage and characterization of guinea
pig ASM cells. Trypsin digestion and tissue adherence are two
frequently used cultural methods to obtain primary ASM cells. The
former may readily cause cells a certain amount of damage due to
their vulnerable properties and thus, the trypsin digestion time is
difficult to control. Consequently, on the basis of present cell
culture techniques, a tissue adherence method was applied and the
primary ASM cells were successfully cultured via optimizing certain
crucial conditions: a) The cell culture flask was preliminarily
coated with FBS, which allowed for the tissue masses to easily
attach and provided nutritional support for the culture as well; b)
the time for turning over the flask was reasonably controlled,
which avoided shedding of tissue masses from the cell culture
flask; c) connective tissue, bloodstains and tracheal cartilage
were removed to the greatest extent, which ensured that a large
number of primary ASM cells with high purity were obtained and d)
ASM from guinea pigs weighing 150–200 g may provide primary ASM
cells with better viability. The whole operation for obtaining
guinea pig ASM tissue masses was at 4°C and was sterile.
When guinea pig ASM cells migrated out from tissue
blocks and grew to two or three generations, there was still a
small amount of mixed cells, particularly myofibroblasts, in the
culture. Therefore, the process of subculturing was subsequently
used to purify the ASM cells from the mixed culture. ASM cells were
isolated and purified with a difference-speed adherence method.
Proliferation advantage was also able to be utilized to inhibit the
growth of myofibroblasts when there was a large proportion of ASM
cells.
A previous study by our group demonstrated that SPFF
had a relaxing effect on tracheal smooth muscles. A pharmacodynamic
in vivo study of the drug demonstrated an evident increase
in lung overflow, lung compliance and cyclic adenosine
monophosphate levels in airway tissues when compared with the
traditional β2-adrenoceptor agonist (9). SPFF also exhibited a new and
potential mechanism involving the non-selective potassium channel
and the large conductance-activated potassium channel, which
differs from traditional β-adrenoceptor agonists.
The present study further confirmed the effect of
SPFF on reducing intracellular calcium release in guinea pig ASM
cells cultured by the improved abovementioned method. Furthermore,
the potential mechanism of the inhibition of intracellular calcium
release by the drug, which ultimately led to the relaxation of
tracheal smooth muscles, was researched. The results demonstrated
that the effect of SPFF on the release of intracellular calcium was
concentration-dependent. Furthermore, the IP3 receptor
blocker 2-APB and the ryanodine receptor blocker dantrolene were
used to block the two pathways of regulating intracellular calcium
release. It was demonstrated that the calcium inhibition rate of
SPFF on guinea pig ASM cells was almost the same when incubated
with or without 2-APB. However, the calcium inhibition rate of the
combined use of SPFF and dantrolene was clearly higher than that of
SPFF alone. Accordingly, it was suggested that the inhibitory
effects of SPFF and 2-APB were additive and the mechanism of
inhibiting intracellular calcium release of the two drugs may be
achieved by IP3R blocking. By contrast, the inhibitory
effect of SPFF and dantrolene may be individual and independent.
The present study indicated that the potential mechanism of SPFF on
the inhibition of intracellular calcium was independent of the RyR
pathway, but may be closely associated with the IP3R
pathway.
Although the inositol 1,4,5-trisphosphate receptor
and the ryanodine receptor are thought to be important in the
regulation of intracellular Ca2+ release, the process is
intricate and affected by certain cytokines or is closely
associated with the cytokines. It has been demonstrated that
interleukin (IL)-4 inhibited the magnitude of calcium transients
stimulated by ionomycin, which depended on the function of RyR
calcium release channels and that the inhibitory effect of IL-4 on
calcium transients in ASM cells was able to modulate contraction,
proliferation, apoptosis, migration and secretion of mediators
(4,10). Furthermore, IL-8 is able to trigger
Ca2+-release and ASM cell contraction and migration,
which suggests that IL-8 may contribute to airway narrowing, airway
hyperresponsiveness and airway remodeling in airway diseases,
including asthma and COPD (6).
IL-13 is able to augment agonist-induced contraction of the
tracheal ring and enhance the frequency of leukotriene D4-induced
Ca2+ oscillations and finally increase intracellular
Ca2+ concentrations in human ASM cells, which may be
co-operatively modulated by the IP3R and/or RyR system
(11). Consequently, the factors
that lead to the fluctuation of the intracellular calcium
concentration are numerous and the exact mechanisms by which SPFF,
a novel β2-adrenoceptor agonist, inhibits the release of
intracellular calcium in ASM cells remains to be elucidated.
Although the potential mechanisms of SPFF on the inhibition of
intracellular calcium are only preliminarily discussed in the
present study, it provides useful information for follow-up
studies. In future studies, we endeavor to identify new targets
upstream or downstream of the IP3R signaling pathway and
to ultimately elucidate the exact mechanism of the inhibition of
intracellular calcium release and relaxation of ASM by SPFF.
In the present study, a method for guinea pig ASM
cell culture was successfully established and the purity and
viability of ASM cells obtained were better than that those
obtained via the traditional method. The drug SPFF, a novel
β2-adrenoceptor agonist, was able to concentration-dependently
decrease Ca2+ levels in the cells and the potential
mechanism of the inhibition of intracellular calcium by SPFF may be
closely associated with the IP3R pathway, which may be
conferred as one of the mechanisms of its relaxant effect. However,
factors that lead to decreases or fluctuation of intracellular
calcium levels and ASM relaxation or contraction are numerous and
the exact mechanism by which SPFF inhibits the release of
intracellular calcium involving the IP3R pathway and
eventually relaxes airway smooth muscles remains to be
elucidated.
Acknowledgements
This study was supported by grants from the
Education Department of Liaoning (no. L2011169).
References
|
1
|
Camoretti-Mercado B: Targeting the airway
smooth muscle for asthma treatment. Transl Res. 154:165–174. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woodruff PG: Gene expression in asthmatic
airway smooth muscle. Proc Am Thorac Soc. 5:113–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu QH, Zheng YM and Wang YX: Two distinct
signaling pathways for regulation of spontaneous local Ca2+ release
by phospholipase C in airway smooth muscle cells. Pflugers Arch.
453:531–541. 2007.PubMed/NCBI
|
|
4
|
Ethier MF and Madison JM: IL-4 inhibits
calcium transients in bovine trachealis cells by a ryanodine
receptor-dependent mechanism. FASEB J. 20:154–156. 2006.PubMed/NCBI
|
|
5
|
Pelaia G, Renda T, Gallelli L, et al:
Molecular mechanisms underlying airway smooth muscle contraction
and proliferation: implications for asthma. Respir Med.
102:1173–1181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Govindaraju V, Michoud MC, Al-Chalabi M,
Ferraro P, Powell WS and Martin JG: Interleukin-8: novel roles in
human airway smooth muscle cell contraction and migration. Am J
Physiol Cell Physiol. 291:C957–C965. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gan LL, Wang MW, Cheng MS and Pan L:
Trachea relaxing effects and beta2-selectivity of SPFF, a newly
developed bronchodilating agent, in guinea pigs and rabbits. Biol
Pharm Bull. 26:323–328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hao Z, Zhang Y, Pan L, et al: Comparison
of enantiomers of SPFF, a novel beta2-Adrenoceptor agonist, in
bronchodilating effect in guinea pigs. Biol Pharm Bull. 31:866–872.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Madison JM and Ethier MF: Interleukin-4
rapidly inhibits calcium transients in response to carbachol in
bovine airway smooth muscle cells. Am J Respir Cell Mol Biol.
25:239–244. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsumoto H, Hirata Y, Otsuka K, et al:
Interleukin-13 enhanced Ca2+ oscillations in airway smooth muscle
cells. Cytokine. 57:19–24. 2012. View Article : Google Scholar : PubMed/NCBI
|