Introduction
Cryptorchidism, also termed undescended testes, is
the failure of one or two testes to descend into the scrotum. The
testes first develop in the abdomen prior to birth and then descend
into the scrotum. As the most common disorder of the male endocrine
glands, cryptorchidism affects 2–4% of male infants, particularly
premature infants (1–3). It is a well-established risk factor
for later sub-fertility and testicular cancer in adulthood
(2,4). The failure of the testes to descend
into the scrotum results in histological changes in the undescended
testes (5,6). With the exception of standard
inguinal orchidopexy, cryptorchidism is treated using hormonal
therapy. Treatment of cryptorchidism with human chorionic
gonadotropin (HCG) was introduced in 1930 (7) and has been used for a number of
years, particularly in Europe (1).
However, there is increasing evidence that it has side effects on
the germ cells (7,8).
Cryptorchidism is a multifactorial disease involving
multiple genes (9,10). The mRNA and protein expression of
β-nerve growth factor (NGF) and homeobox A10 (HoxA10) have been
observed to decrease in the undescended testes (11,12).
NGF is considered to be one of the most important regulators of the
growth, proliferation, differentiation and survival of nerves
(10,11,13).
Furthermore, NGF has been detected in the testis (14–16)
and is produced by male germ cells (17). The administration of exogenous NGF
has been applied in certain animal models of the nervous system for
>35 years, and specific clinical applications have been
performed to investigate NGF delivery and its effects on the adult
or developing nervous system (18,19).
To the best of our knowledge, whether exogenous NGF
alters the endogenous levels of NGF and HoxA10 in cryptorchidism in
rats remains to be elucidated. The purpose of the present study was
to establish a unilateral mechanical cryptorchidism model in
Sprague-Dawley (SD) rats and to investigate the mRNA and protein
expression of β-NGF and HoxA10 using electromicroscopical
observation in the experimental cryptorchidism model following
treatment with exogenous NGF.
Materials and methods
Animals
Male 21-day-old SD rats (n=30) were obtained from
the Experimental Animal Center of Nantong University (Nantong,
China). The animals were maintained at controlled temperature
(25±2°C) and constant photoperiodic conditions (12 h light:12 h
dark). The rats were given ad libitum access to food and
water. The experimental procedure was designed in compliance with
the Recommendations for the Care and Use of Laboratory Animals of
the Local Ethical Committee at the Experimental Animals Center of
Nantong University.
Unilateral cryptorchidism was induced by the
surgical procedure described previously (12,20).
In brief, SD rats (n=24) were anesthetized using Nembutal (40 mg/kg
body weight; concentration, 1%; BOC Sciences, Shirley, NY, USA)
and, following a small incision to the abdomen, the gubernaculum
was cut on one side to displace the testis into the abdomen. Testis
descent was prevented by closure of the inguinal canal on one side
by suturing (Hualikang Medical Instrument Co., Nantong, China).
Following surgery, the animals were housed and fed routinely with
analgesics (Brufen, 1 mg/ml; GlaxoSmithKline, Shanghai, China). The
rats were then divided into four groups, a high-dose NGF group (HN
group; n=6), low-dose NGF group (LN group; n=6), human chorionic
gonadotropin (HCG) group (HC group; n=6) and negative control group
(NC group; n=6). In the HN and LN groups, 9,000 and 45,00 IU murine
NGF (NOBEX; Xiamen Bioway Biotech, Xiamen, China) were injected
intramuscularly each day for 5 days, respectively. This commenced
from the second day following surgery. The HC group were
administered with 400 IU HCG intramuscularly and the NC group were
administered with normal saline intramuscularly. A total of six
normal SD rats without surgery were used as a blank control group
(B group) and were housed in the same cage.
The normal and undescended testes were removed and
decapsulated 39 days following surgery at sexual maturity, which
was calculated using the website, http://www.taletn.com/rats/age/. The samples were
either stored at −70°C or fixed for further use. All surgical
procedures were performed under ether anesthesia (2–4%; Jiangsu
Qiangshen Chemical Co., Jiangsu, Chinag) and the animals were
sequentially sacrificed by CO2 asphyxiation.
One step reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from the decapsulated testes was extracted
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. To avoid
possible genomic DNA contamination, all the RNA samples were
treated using RNase-free DNase (Promega Corporation, Madison, WI,
USA). One step RT-qPCR was performed using a SensiMixTM One-Step
kit (Quantace, London, UK) according to the manufacturer’s
instructions of the Rotor-Gene 3000 real-time DNA analysis system
(Corbett Research, Sydney, Australia). For RT-qPCR examination of
the temporal expression of β-NGF and Hox10, the first-strand cDNA
was synthesized using an Omniscript reverse transcription kit
(Qiagen, Hilden, Germany) in a 20 μL reaction system containing 2
μg total RNA, 0.2 U/μL M-MLV reverse transcriptase, 0.5 mM dNTP mix
and 1 μM Oligo-dT primer. The cDNA was diluted 1:5 prior to use in
the RT-qPCR assays. Subsequently, the one step RT-qPCR was
performed using a SensiMixTM One-Step kit (Quantace, London, UK)
according to the manufacturer’s instructions of the Rotor-Gene 3000
real-time DNA analysis system (Corbett Research). The mRNA
expression levels of β-NGF and Hox10 were calculated using
Rotor-Gene Real-Time Analysis software 6.1 (Build 90; Corbett
Research). The oligonucleotide primers of β-NGF, Hox10 and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are listed in
Table I. The amplification program
consisted of 30 min at 42°C for reverse transcription and 2 min at
95°C for Taq activation, followed by 45 cycles of 95°C for
15 sec, 58°C for 30 sec and elongation at 72°C for 40 sec. GAPDH
was used as an endogenous control gene for normalization.
 | Table IOligonucleotide primers of β-NGF, Hox10 and GAPDH. |
Table I
Oligonucleotide primers of β-NGF, Hox10 and GAPDH.
| Target | Sequence (5′-3′) |
|---|
| β-NGF | Forward:
ACCTCTTCGGACACTCTG
Reverse: GTGGCTGTGGTCTTATCTC |
| Hox10 | Forward:
ACAAGCACACCACAATTCTCC
Reverse: ATCCAAACAATGTCTCCCTTCTC |
| GAPDH | Forward:
TCGTGGAGTCTACTGGCGTCT
Reverse: CAACCTGGTCCTCAGTGTAG |
In situ hybridization histochemistry with
digoxigenin (DIG)-labeled-β-NGF RNA sense and antisense probes
The DIG-labeled-β-NGF RNA sense and antisense probes
were generated using a DIG RNA Labeling kit (Roche Diagnostics
GmbH, Mannheim, Germany) according to the manufacturer’s
instructions. Briefly, the total RNA from the brain tissue of three
SD rats was extracted using TRIzol reagent, as previously
mentioned, and the cDNA fragment of β-NGF was obtained from the
total RNA using RT-qPCR with the following primers:
5′-GATCGGCGTACAGGCAGAAT-3′ and 5′-GGCTCGGCACTTGGTCTCAA-3′. The
resulting product was then recovered from agarose gel and cloned
into a pGEM-T easy vector (Promega Corporation) using T4 DNA ligase
(Promega Corporation). The recombinant plasmid was transformed into
the Escherichia coli strain, DH5a (Biomiga, Inc., San Diego,
CA USA) and positive clones, confirmed by DNA sequencing, were
transformed into E. coli Top10 (Biomiga, Inc.). The
successfully-transformed E. coli Top10 was collected from a
single colony and was then grown overnight at 37°C in Luria-Bertan
medium supplemented with ampicillin (Sigma-Aldrich, St. Louis, MO,
USA). Following plasmid extraction, the plasmid was 1inearized
using the restriction enzymes, Apa I and Sal. Using
Sp6 and T7 RNA polymerase, respectively, the DIG-labeled sense and
antisense probes were transcribed in vitro using a DIG RNA
Labeling kit according to the manufacturer’s instructions. The
sense probe was synthesized by T7 RNA polymerse, while the
antisense probe by T3 RNA polymerse. In order to confirm the
quality of the probes, an agarose gel was used, which revealed the
antisense probe as a clear specific band and the sense probe also
as a bright band
In situ hybridization (ISH) histochemistry
was performed to detect the mRNA expression of β-NGF mRNA in the
4-μm frozen sections from the decapsulated testes, which were fixed
in freshly prepared 4% paraformaldehyde (pH 7.4) and stored at 4°C
for 8 h. The sections were then dried at 43°C overnight and washed
twice with 0.1% diethypyrocarbonate-treated water. Following
pre-hybridization for 2 h, the sections were hybridized with DIG
labeled-β-NGF RNA probes (1 μg/ml) in a humid chamber at 42°C for
18 h. These sections were then washed twice with 2X saline sodium
citrate (SSC)/0.1% sodium dodecyl sulfate (SDS) buffer at 25°C for
5 min and with 1X SSC/0.1% SDS at 60°C for 15 min. Following
incubation in inhibiting solution (100 mM maleic acid, 150 mM NaCl
and 1% blocking reagent) at 37°C for 30 min, the sections were
incubated with the anti-DIG-AP (Roche Diagnostics, GmbH) at 1:5,000
dilutions in the inhibiting solution at 4°C overnight. The sections
were then washed twice using the 100 mM maleic acid buffer (pH 7.5)
and 0.3% solution of Tween-20 (15 min each at 25°C), followed by
the addition of the detection buffer, containing 100 mM Tris-HCl
(pH 9.5) and 100 mM NaCl, for 5 min. The ISH signals were detected
using a coloring solution consisting of NBT/BCIP (Roche Diagnostics
GmbH) in the dark for 3–12 h. The coloring reaction was terminated
using 100 mM phosphate-buffered saline (PBS; Hyclone Laboratories,
Inc., Logan, UT, USA) and images were captured under a BX51 Olympus
microscope (Olympus, Tokyo, Japan). A control experiment was
performed in parallel using a sense probe. No significant β-NGF
mRNA signal (blue) was observed. Bar =100 μm.
Immunofluorescence (IF)
The frozen decapsulated testicular tissues were cut
into 4-μm slices. The sections were then inhibited in PBS with 5%
goat serum (Gibco-BRL, Carlsbad, CA, USA) for 15 min at room
temperature and incubated with goat anti-rat-β-NGF polyclonal
antibody (1:400, R&D Systems, Minneapolis, MN, USA) containing
1% bovine serum albumin (BSA) at 4°C for 24 h. Following washing
with PBS, these sections were incubated with monoclonal
anti-fluorescein isothiocyanate-conjugated mouse anti-goat
immunoglobulin G antibody (1:1,000; Sigma-Aldrich, St Louis, MO,
USA) for 2 h at room temperature. The samples were then washed with
PBS and the samples were observed using fluorescence microscopy
(Zeiss Axioskop 40; Zeiss, Oberkochen Germany). As mentioned above,
the IF experiments were repeated at least three times. Negative
controls were included by replacement of the primary antibody with
PBS.
Immunohistochemistry (IHC)
The decapsulated testicular tissues were fixed in
formalin and embedded in paraffin. The serial sections were cut at
4 μm on a manual rotary microtome (Leica RM2235; Leica Microsystems
GmbH, Wetzlar, Germany). IHC was performed on the sections using an
Autostainer Universal Staining system (LabVision, Fremont, CA,
USA). The sections were deparaffinized and peroxidase was quenched
using methanol and 3% H2O2 for 15 min. For
antigen retrieval, the sections were boiled under pressure in
citrate buffer (pH 6.0) for 3 min. Nonspecific binding was
inhibited using 5% goat serum in PBS for 15 min and the tissues
were incubated with rabbit anti-HoxA10 antibody (1 μg/ml; H-90;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) containing 1%
BSA at 4°C for 24 h. Following washing with PBS, the sections were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
antibody (DakoCytomation, Carpinteria, CA, USA) for 15 min at room
temperature. To develop the colour, the sections were incubated for
15 min with diaminobenzidine solution (Kem-En-Tec Diagnostics,
Taastrup, Denmark) and the sections were weakly counterstained
using hematoxylin. Negative controls were included by replacement
of the primary antibody with PBS.
The decapsulated testicular tissues were fixed in 1%
paraformaldehyde/1.25% glutaraldehyde in 0.1 M PBS, post-fixed in
1% buffered osmium tetroxide, dehydrated through a graded ethanol
series and embedded in Epon 812 epoxy resin. The 1.0 μm sections
for orientation were stained using toluidine blue. The ultra-thin
sections (50 nm) were stained using uranyl acetate and lead
citrate, followed by examination using a transmission electron
microscope (JEM-1230; JEOL Ltd., Tokyo, Japan).
Statistical analysis
The mRNA expression levels of HoxA10 and β-NGF mRNA
in the testis, measured using one step RT-qPCR, were assessed using
a one way analysis of variance test paired with a t-test. P<0.05
was considered to indicate a statistically significant difference
and the data were analyzed using STATA 9.0 software (Stata
Corporation, College Station, TX, USA).
Results
Expression of HoxA10 and β-NGF mRNA in
the testes measured by one step RT-qPCR
Total RNA was extracted from the testis and
subjected to one-step RT-qPCR to investigate the mRNA expression
levels of HoxA10 and β-NGF in the groups with various treatments.
The expression of HoxA10 and β-NGF were individually normalized to
GAPDH, expressed as the ratio in the same sample at day 39
following surgery (Fig. 1A and B).
Among the groups, the highest levels of mRNA expression of the two
genes were observed in the HN group (HoxA10, F=125.58, P<0.001;
and β-NGF, F=49.03, P<0.001). Compared with the testes in the NC
group, the mRNA expression of HoxA10 and β-NGF was clearly
upregulated in the HN and LN groups.
mRNA expression of β-NGF analyzed by ISH
with DIG-labeled-β-NGF RNA probes
The mRNA signals of β-NGF were observed as blue
staining in the cytoplasm of the germ cells in the testes. Compared
with the NC group, the mRNA expression of β-NGF in the undescended
testes increased on administering a high dose of exogenous NGF
(Fig. 2A–E). This result was
consistent with that of the above-mentioned one-step RT-qPCR.
Specific expression of β-NGF protein
detected by IF
The β-NGF protein was stained in light green using
IF, which revealed that the majority was localized in the cytoplasm
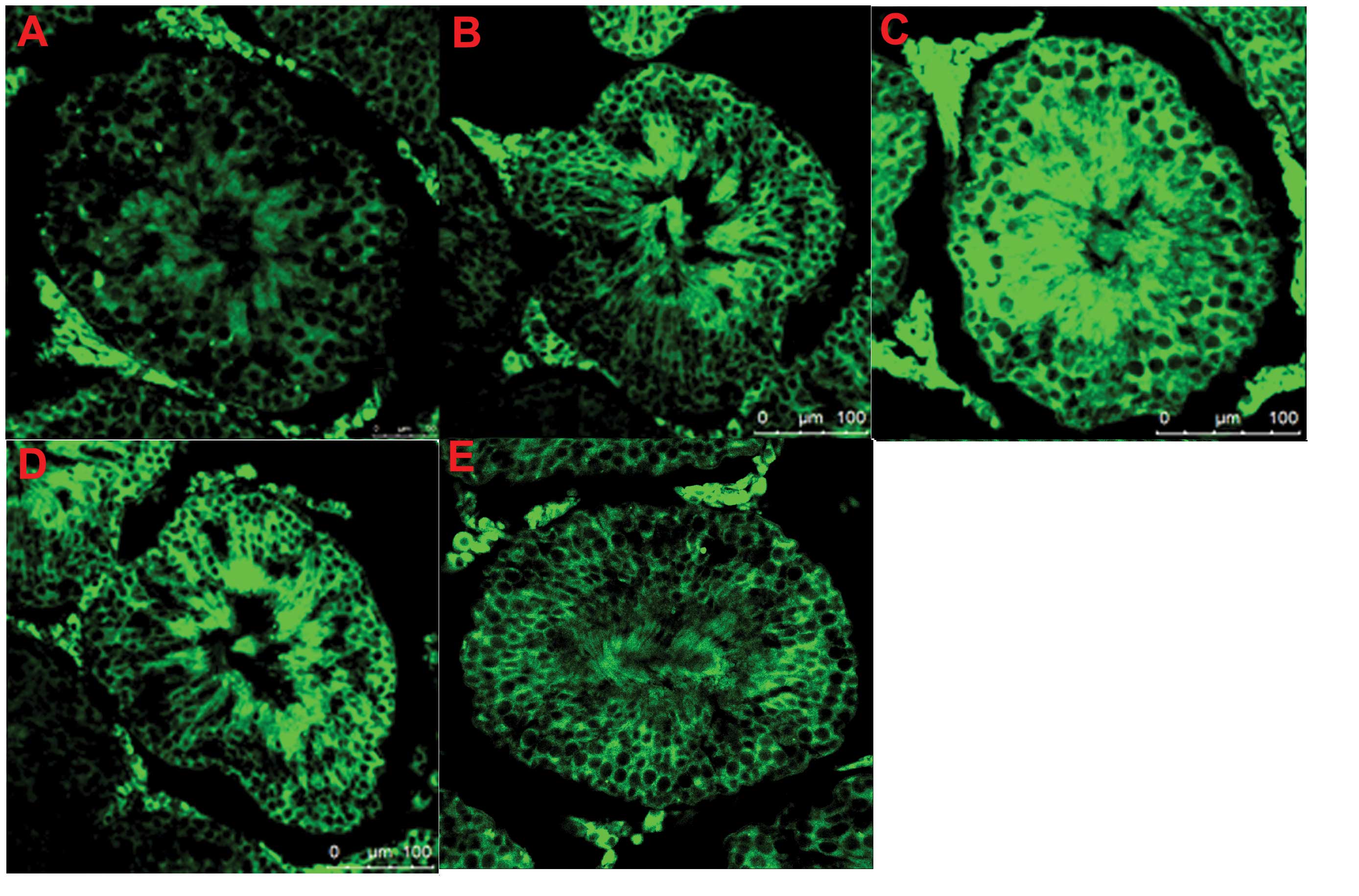
of the germ cells, interstitial cells and leydig cells. The β-NGF
protein was expressed in all the testicular tissues in each group,
including the B group. It was notable that the protein expression
of β-NGF in the undescended testis of the HN group was markedly
higher compared with the NC, HC, LN and B groups(Fig. 3A–E).
Specific protein expression of HoxA10
measured by IHC
The positive staining, colored brown, of HoxA10 was
mainly localized in the cell nucleus of the germ cells,
interstitial cells and leydig cells in the testes. Notably, the
protein expression of HoxA10 in the cryptorchidism of the HN group
was markedly higher compared with the NC, HC, LN and B groups
(Fig. 4A–E).
Transmission electron microscopy
Compared with the cells of the B group, the NC group
exhibited pathological modifications, including reduced nuclear
chromatin, vacuolar degeneration in ribosomes, thickened and
damaged nuclear membrane, widening of the perinuclear space and
nuclear pyknosis. Following treatment with a high dose of NGF, the
testes of the HN group appeared to resemble normal testes, with
fewer of the pathological changes mentioned above (Fig. 5A–E).
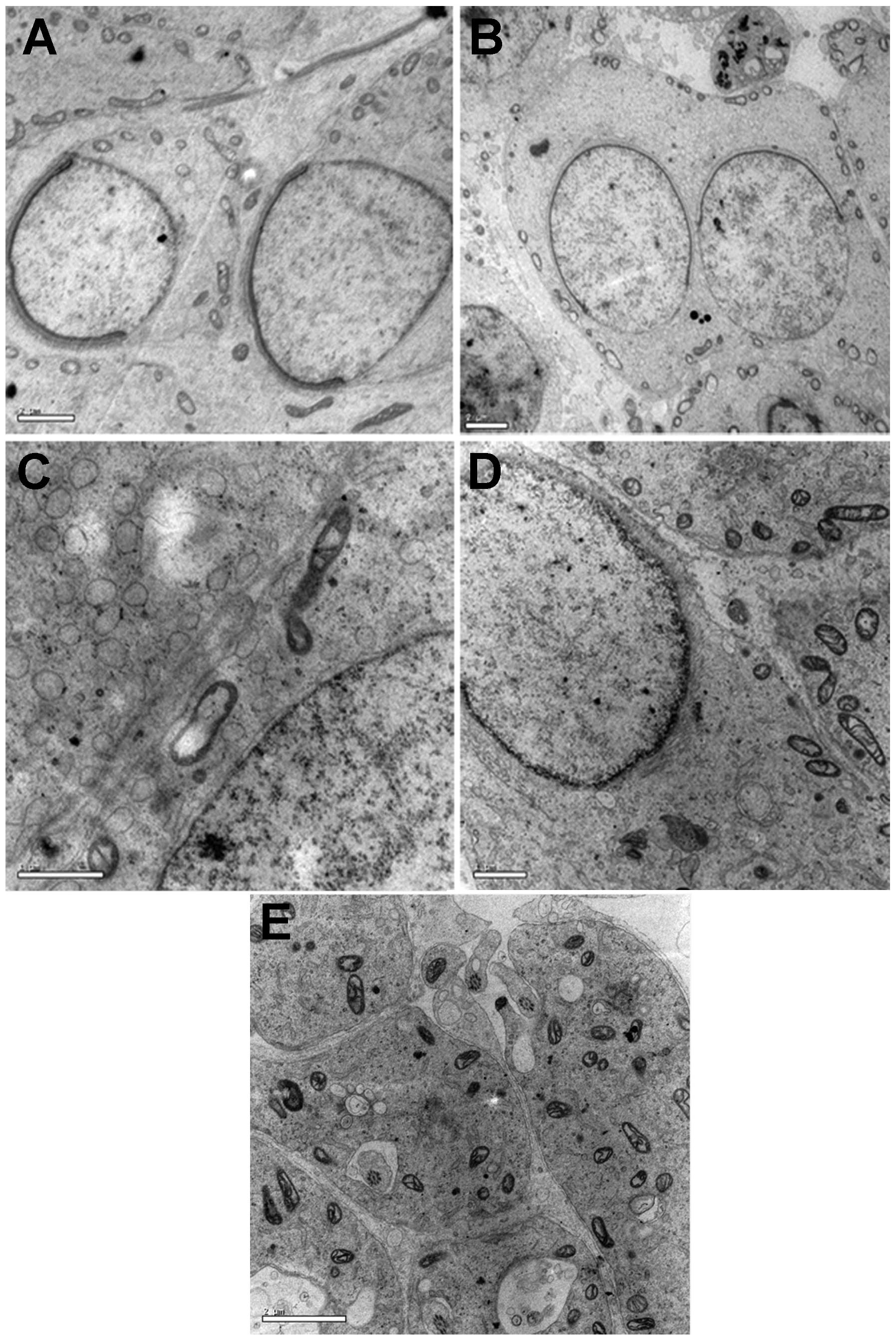 | Figure 5Microstructural changes in the testes
observed in transmission electron microscope analysis following
staining with uranyl acetate and lead citrate. (A) Reduced nuclear
chromatin, vacuolar degeneration of ribosomes, widening of
perinuclear space, nuclear pyknosis and damaged nuclear membrane
were observed in the testicular tissues of the NC group compared
with the B group. (B) Degree of injury in the testicular tissues of
the human chorionic gonadotropin group was improved compared with
the NC group. (C) Increased nuclear chromatin, decreased nuclear
pyknosis, narrowing perinuclear space and an intact nuclear
membrane were observed in the testicular tissues of the HN group,
which was improved compared with the NC and LN groups. (D) Level of
testicular tissue injury in the LN group was greater compared with
the HN group. (E) Characteristic features of normal mature
testicular tissues were observed in the B group. (Scale bar, 100
μm) HN. high-dose NGF; LN, low-dose NGF; NC, negative control; B,
blank control. |
Discussion
In the majority of mammals, the testes are situated
in the scrotum at the testicular temperature required in order to
function (21). The unilateral
mechanical cryptorchidism of SD rats, in which he distal tip of the
gubernaculum was exposed extra-abdominally and was fixed to the
fascia of the groin, is widely used as an experimental model to
investigate the histopathologic changes and the molecular genetics
of cryptorchidism (5,20–22).
It has been observed that β-NGF is involved in the regulation of
spermatocyte differentiation by inhibiting secondary spermatocytes
in metaphase. This leads to a reduction in round spermatid
formation in co-cultures of pachytene spermatocytes and Sertoli
cells (22). Furthermore, the NGF
and HoxA10 genes are closely associated with the development of the
testes (9,10).
In the present study, NGF and HoxA10 mRNA and
protein were observed in the undescended testes at sexual maturity.
Changes of the two genes in the cryptorchid tissues of the
different groups were identified using one step RT-qPCR, ISH with
DIG-labeled-β-NGF RNA probes, IF and IHC. As expected, the
expression levels of the two genes in the undescended testis were
lower than that in the normal testis. Notably, the expression
levels of these genes in the HN group were significantly higher
than those in the NC, HC, B and LN groups. As NGF and HoxA10 genes
are closely associated with the development of the testes, it was
hypothesized that the increased expression of the two genes was an
important effect of exogenous NGF in treating cryptorchidism in the
present study.
In addition, the present study revealed
microstructural changes in cryptorchidism using transmission
electron microscopy. These changes may affect the development,
maturation, apoptosis and necrosis of sperm organelles. The
characteristic features of immaturity, including altered acrosomes,
misshapen, round or elliptical nuclei with uncondensed chromatin,
and the presence of cytoplasmic droplets, as previously described
(23,24), were also observed in the
undescended testes of the present study. Additionally, less injury
was observed in the HN group treated with a high dose of exogenous
NGF compared with the groups treated with HCG or a low dose of
exogenous NGF. These observations indicated the potential for using
exogenous NGF to cure cryptorchidism instead of hormonal therapy
using HCG.
Further studies are required to confirm these
observations in the undescended testes. Investigation of the
association between the gene expression of NGF and HoxA10 and
factors, including caspase-3 and Fas in undescended testes are also
required to provide relevant information for elucidating the
mechanisms involved in treating cryptorchidism with exogenous NGF
administration.
Acknowledgements
The authors would like to thank the President of
Nantong University, Professor Fei Ding (Key laboratory of
Neuroregeneration), for critically reviewing this study. This study
was supported by the Social Development Project (nos. S2008046 and
HS2011047) in Nantong (Jiangsu, China).
References
|
1
|
Ashley RA, Barthold JS and Kolon TF:
Cryptorchidism: pathogenesis, diagnosis, treatment and prognosis.
Urol Clin North Am. 37:183–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wohlfahrt-Veje C, Boisen KA, Boas M, et
al: Acquired cryptorchidism is frequent in infancy and childhood.
Int J Androl. 32:423–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sijstermans K, Hack WW, Meijer RW and van
der Voort-Doedens LM: The frequency of undescended testis from
birth to adulthood: a review. Int J Androl. 31:1–11. 2008.
|
|
4
|
Hadziselimovic NO, de Geyter C, Demougin
P, Oakeley EJ and Hadziselimovic F: Decreased expression of FGFR1,
SOS1, RAF1 genes in cryptorchidism. Urol Int. 84:353–361. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zivkovic D, Bica DG and Hadziselimovic F:
Effects of hormonal treatment on the contralateral descended testis
in unilateral cryptorchidism. J Pediatr Urol. 2:468–472. 2006.
View Article : Google Scholar
|
|
6
|
Ferlin A, Zuccarello D, Zuccarello B,
Chirico MR, Zanon GF and Foresta C: Genetic alterations associated
with cryptorchidism. JAMA. 300:2271–2276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thorsson AV, Christiansen P and Ritzén M:
Efficacy and safety of hormonal treatment of cryptorchidism:
current state of the art. Acta Paediatr. 96:628–630. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaleva M and Toppari J: Cryptorchidism: an
indicator of testicular dysgenesis? Cell Tissue Res. 322:167–172.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Zheng XM, Hubert J, Zheng H, Yang
ZW and Li SW: Prenatal exposure to diaethylstilbestrol in the rat
inhibits transabdominal testicular descent with involvement of the
INSL3/LGR8 system and HOXA10. Chin Med J (Engl). 122:967–971.
2009.
|
|
10
|
Levi-Montalcini R and Hamburger V:
Selective growth stimulating effects of mouse sarcoma on the
sensory and sympathetic nervous system of the chick embryo. J Exp
Zool. 116:321–361. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levi-Montalcini R: The nerve growth factor
35 years later. Science. 237:1154–1162. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xian H, Xian Y, Jiang CY, et al: Decreased
expression of beta-nerve growth factor correlated with histological
changes in a cryptorchidism rat model. Chin Med J (Engl).
125:713–716. 2012.
|
|
13
|
Levi-Montalcini R, Dal Toso R, della Valle
F, Skaper SD and Leon A: Update of the NGF saga. J Neurol Sci.
130:119–127. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seidl K and Holstein AF: Evidence for the
presence of nerve growth factor (NGF) and NGF receptors in human
testis. Cell Tissue Res. 261:549–554. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ayer-LeLievre C, Olson L, Ebendal T,
Hallböök F and Persson H: Nerve growth factor mRNA and protein in
the testis and epididymis of mouse and rat. Proc Natl Acad Sci USA.
85:2628–2632. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin W, Tanaka A, Watanabe G, Matsuda H and
Taya K: Effect of NGF on the motility and acrosome reaction of
golden hamster spermatozoa in vitro. J Reprod Dev. 56:437–443.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Persson H, Ayer-Le Lievre C, Söder O, et
al: Expression of beta-nerve growth factor receptor mRNA in Sertoli
cells downregulated by testosterone. Science. 247:704–707. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams BJ, Eriksdotter-Jonhagen M and
Granholm AC: Nerve growth factor in treatment and pathogenesis of
Alzheimer’s disease. Prog Neurobiol. 80:114–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albeck D, Mesches MH, Juthberg S, et al:
Exogenous NGF restores endogenous NGF distribution in the brain of
the cognitively impaired aged rat. Brain Res. 967:306–310. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hou W, Hu J, Li Y, et al: Altered
expression of NDRG2 in the testes of experimental rat model of
cryptorchidism. Urology. 75:985–991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tena-Sempere M, Kero J, Rannikko A and
Huhtaniemi I: Experimental cryptorchidism induces a change in the
pattern of expression of LH receptor mRNA in rat testis after
selective Leydig cell destruction by ethylene dimethane sulfonate.
J Endocrinol. 161:131–141. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perrard MH, Chassaing E, Montillet G,
Sabido O and Durand P: Cytostatic factor proteins are present in
male meiotic cells and beta-nerve growth factor increases mos
levels in rat late spermatocytes. PLoS One. 4:e72372009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moretti E, Di Cairano G, Capitani S,
Scapigliati G, Baccetti B and Collodel G: Cryptorchidism and semen
quality: a TEM and molecular study. J Androl. 28:194–199. 2007.
View Article : Google Scholar
|
|
24
|
Absalan F, Movahedin M and Mowla SJ: Germ
cell apoptosis induced by experimental cryptorchidism is mediated
by molecular pathways in mouse testis. Andrologia. 42:5–12. 2010.
View Article : Google Scholar : PubMed/NCBI
|