Introduction
Radiation-induced pulmonary injuries are important
complications of chest irradiation. Clarifying the detailed
molecular mechanisms underlying these injuries, and developing
effective methods for their prevention and treatment are required
for more effective and safer chest irradiation. Fibroproliferative
changes resulting in fibrosis are considered to dynamic and
reversible processes involving constant remodeling and long-term
fibroblast activation. Fibroproliferative lesions contain
infiltrating inflammatory cells, including macrophages/monocytes
and specific fibroblasts termed myofibroblasts (1–3).
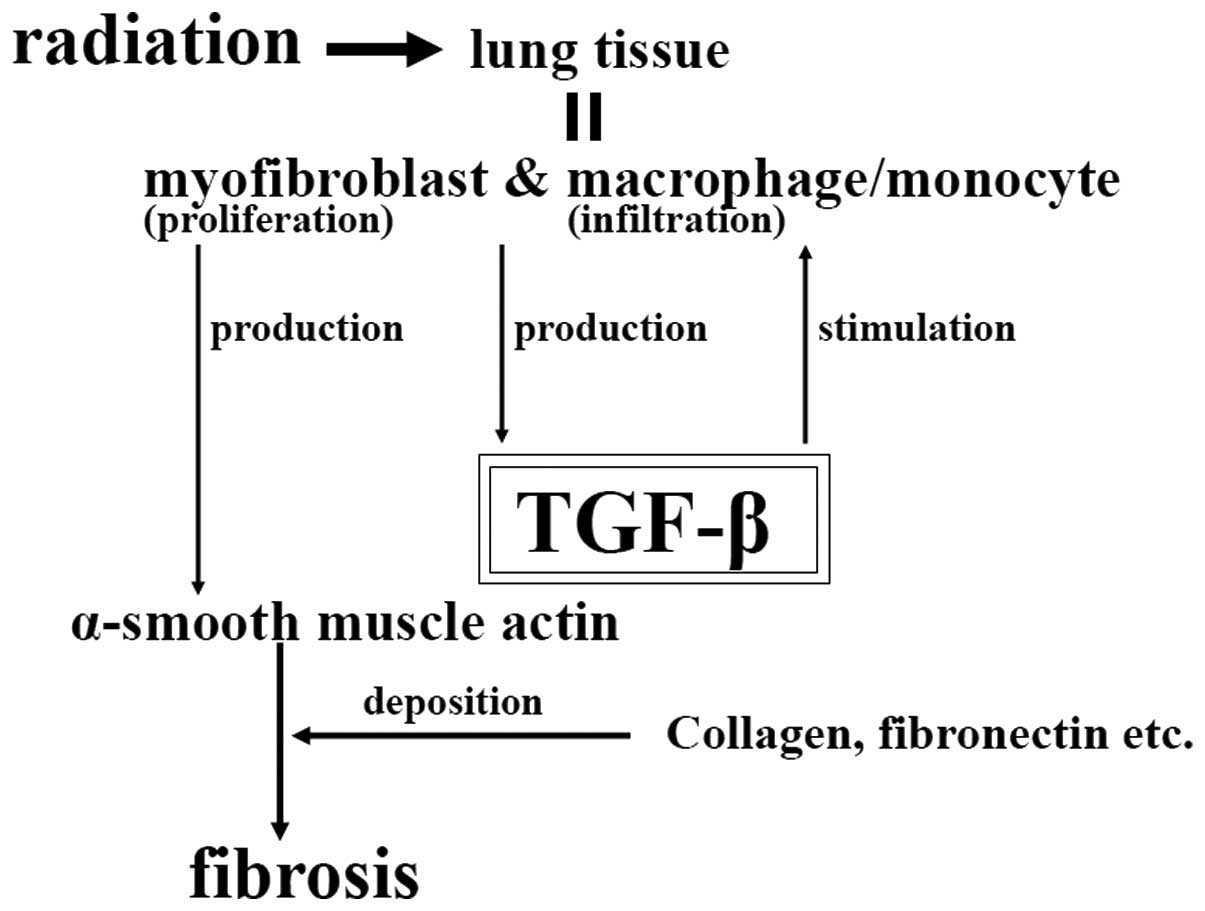
Transforming growth factor-β (TGF-β) is considered
to be a master switch for fibroproliferative changes in irradiated
lung tissue (4–6). That is, overexpression or activation
of TGF-β for a prolonged period may induce myofibroblast
proliferation, macrophage/monocyte infiltration and extracellular
matrix deposition, resulting in pulmonary fibrosis (Fig. 1).
Previously, based on the known molecular mechanisms,
the ability of adenovirus-mediated soluble TGF-β type II receptor
gene therapy to ameliorate active TGF-β expression and prevent
radiation-induced pulmonary injury was demonstrated (7). As the next step, using an
adenovirus-mediated soluble TGF-β type II receptor, which adsorbs
TGF-β and may act as a dominant-negative receptor to inhibit the
function of wild-type receptor, the present study investigated
whether established fibroproliferative changes in the irradiated
lung may be reduced.
Materials and methods
Adenoviral vectors
Replication-defective E1− and
E3− adenoviral vectors expressing the entire
extracellular domain of the type II human TGF-β receptor fused with
the Fc protein of human IgG1 (AdTβ-ExR), under the
control of a CA promoter comprising a cytomegalovirus enhancer and
a chicken β-actin promoter, was prepared by in vitro
homologous recombination in 293 cells (Kyushu University, Fukuoka,
Japan), as described previously (8,9). It
was confirmed that the soluble TGF-β type II receptor was secreted
from AdTβ-ExR-infected cells and was detectable in circulating
blood following AdTβ-ExR injection. It was also confirmed that the
soluble receptor bound to TGF-β and inhibited TGF-β activity in
vivo and in vitro (8,9). The
titer of the virus stock was assessed by a plaque formation assay
using 293 cells and is expressed in plaque-forming units (PFU).
Animal model
The present study was conducted under the Guidelines
for Animal Experiments of Kochi University, (Kochi, Japan). All of
the animals were treated following the procedures approved by the
Committee on Animal Research of Kochi University. Male Fisher-344
rats (SLC, Shizuoka, Japan) aged 12 weeks were divided into three
groups: An intact control group (C group), a radiation plus saline
group (R group) and a radiation plus soluble TGF-β type II receptor
group (R + T group). Each group consisted of three rats. The rats
in the C group did not receive irradiation or treatment. The rats
in the R and R + T groups each received 4-MV X-ray irradiation at a
dose of 30 Gy in a single fraction of the right lung using a linear
accelerator (ML-15MDX; Mitsubishi Electric Co., Ltd., Tokyo,
Japan). Eight weeks following irradiation, the rats in the R and R
+ T groups were treated with a single infusion of 0.5 ml saline or
AdTβ-ExR (1.0×109 PFU/ml), respectively, via the
external jugular vein. It was previously reported that marked TGF-β
expression, myofibroblast proliferation, macrophage/monocyte
infiltration and fibroproliferative changes were observed in the
irradiated lungs of rats at 8 weeks following 30 Gy irradiation
(7). The rats in all of the groups
were housed under the same conditions with ad libitum access
to food and water for 16 weeks after irradiation (8 weeks after
single infusion). The rats were then sacrificed with an
intraperitoneal injection of pentobarbital sodium (2.5 ml/rat) for
lung extraction.
Histopathological examination
For the histopathological investigation, the right
lungs of all of the rats were fixed in 10% neutral-buffered
formalin, embedded in paraffin and sectioned (5-μm sections) with a
microtome. A number of mounted sections were stained with
hematoxylin and eosin (H&E). To analyze TGF-β expression,
myofibroblast proliferation and macrophage/monocyte infiltration,
other tissue sections of the lung were immunohistochemically
stained using the avidin-biotin-horseradish peroxidase complex
(ABC) method with anti-TGF-β1 antibody (1:100; Promega Corporation,
Madison, WI, USA), anti-α-smooth muscle actin antibody (1:200; DAKO
A/S, Copenhagen, Denmark) and anti-CD68 antibody (1:100; KP1 clone;
DAKO A/S) as the primary antibodies. Immunoreactive materials were
visualized using biotinylated anti-mouse (or anti-rabbit)
immunoglobulin G secondary antibody (1:100; Vector Laboratories,
Inc., CA, USA), peroxidase-labeled streptavidin (Vector
Laboratories, Inc.) and diaminobenzidine (Sigma-Aldrich, St. Louis,
MO, USA). Firstly, the sections were incubated in the primary
antibodies at the appropriate dilutions for 1 h at room
temperature. They were then incubated in peroxidase blocking
solution (0.3% H2O2) for 10 min and in the
biotinylated secondary antibody for 30 min at room temperature.
Finally, the sections were incubated in peroxidase-labeled
streptavidin for 30 min at room temperature, and then visualized
with diaminobenzidine. The number of TGF-β-expressing cells within
a field at a magnification of ×400 (area, ~240×320 μm) were then
counted using a microscope (Eclipse 80i; Nikon Corporation, Tokyo,
Japan). Five fields were randomly selected for each rat and three
rats from each group were examined; therefore, a total of 15 fields
were analyzed for each group. Proliferating myofibroblasts or
infiltrating macrophages/monocytes were also counted using the same
method. Silver staining was performed for semi-quantitative
evaluation of fibroproliferative changes. The red-stained area in
the silver-stained sections was exhibited on a charge-coupled
device (CCD)-camera screen (magnification, ×400; Keyence Corp.,
Osaka, Japan), and the percentage of red-stained area in each field
(area, ~240×320 μm) was calculated using image analyzing software
(Lumina Vision and Mac Scope, Mitani Co., Ltd., Tokyo, Japan).
Statistical analysis
A total of 15 fields (randomly selected as described
above) were analyzed for each group. Statistical analysis of
differences was performed using unpaired Student’s t-test.
P<0.01 was considered to indicate a statistically significant
difference.
Results
Histopathology results
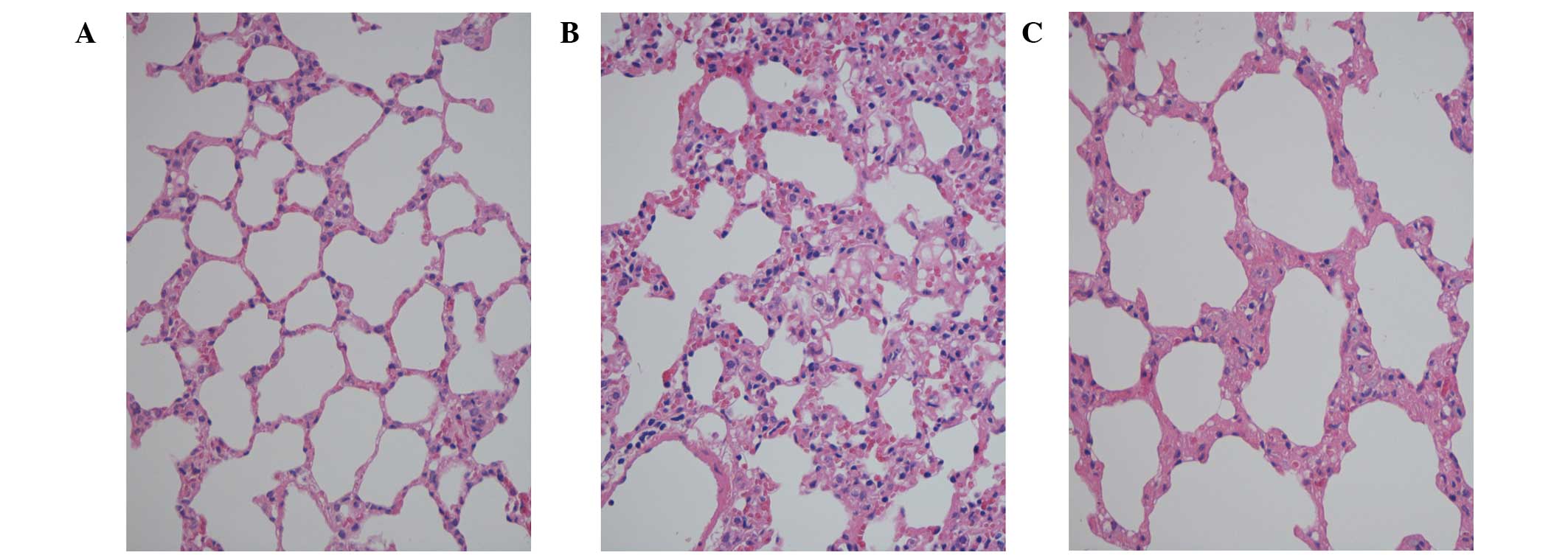
There were no notable changes in the H&E stained
lung sections in rats in the C group. In the lung sections from the
R group, widespread distortion of the architecture with thickening
of the alveolar wall and interstitium, and infiltration of
mononuclear cells were observed, which suggests the presence of
inflammatory and fibroproliferative change. The same findings noted
in the R group were observed in lung sections from the R + T group,
but the severity of these changes in the R + T group was reduced
when compared with that of the R group (Fig. 2).
TGF-β expression, myofibroblast
proliferation and immune cell infiltration
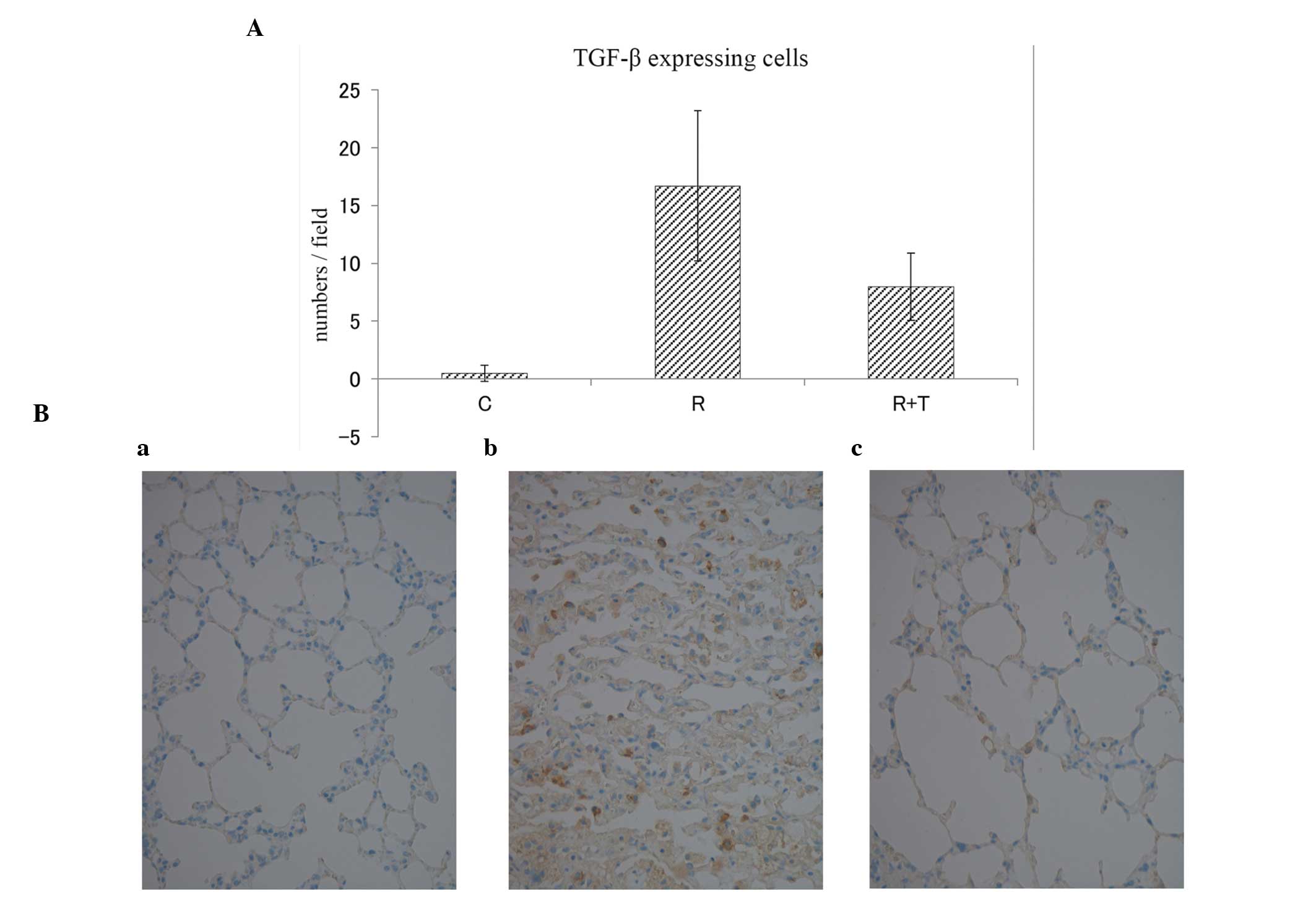
The numbers of TGF-β-expressing cells within a field
in the C, R and R + T groups were 0.5±0.7, 16.7±6.5 and 8.0±2.9
[mean ± standard deviation (SD)], respectively (Fig. 3A). No marked differences regarding
TGF-β expression were observed in the lungs of the C group, and
definitive TGF-β expression was observed in the lungs of the R
group. By contrast, in the lungs of the R + T group, TGF-β
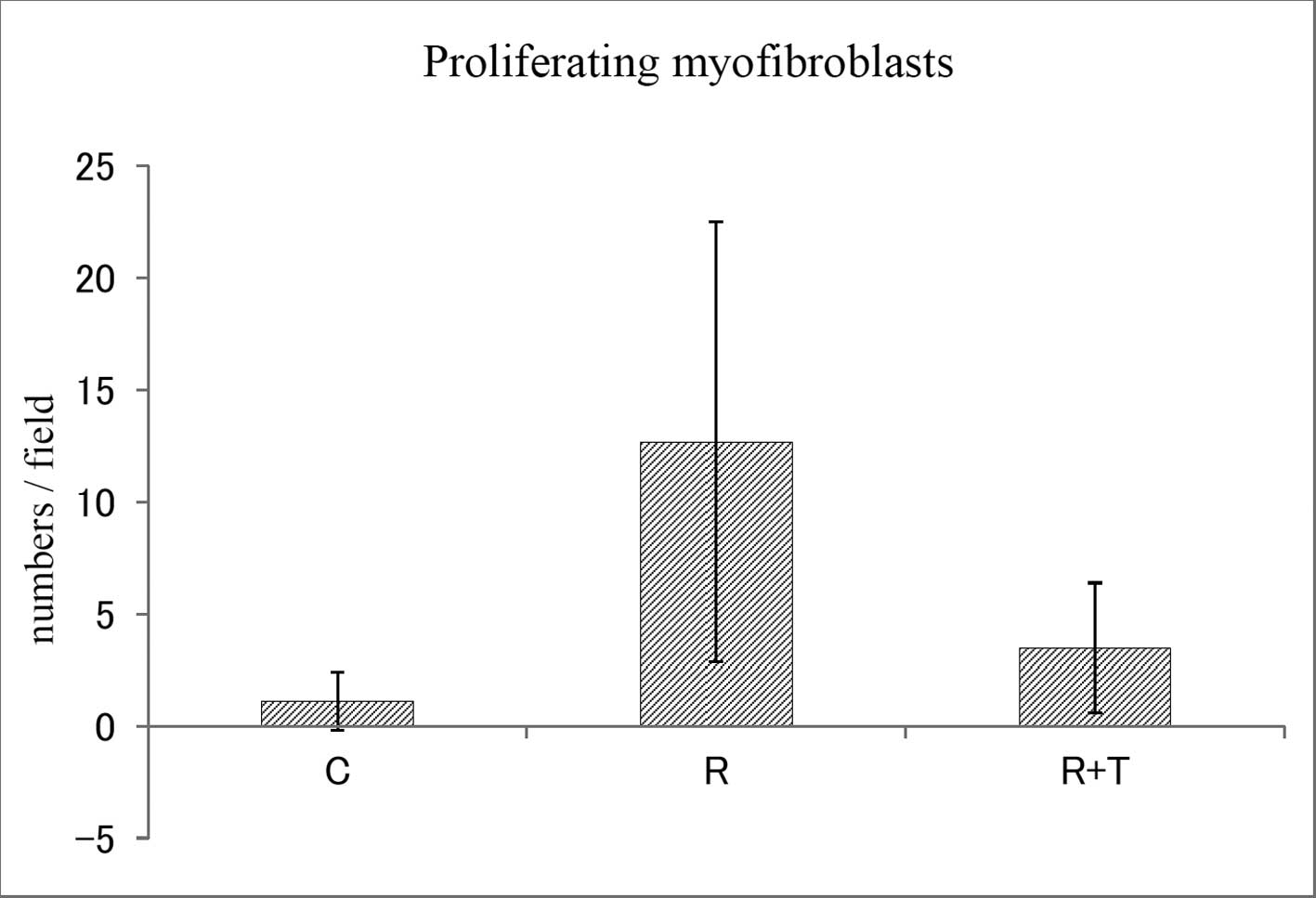
expression was significantly lower than in the R group (Fig. 3B). The numbers of proliferating
myofibroblasts within a field in the C, R and R + T groups were
1.1±1.3, 12.7±9.8 and 3.5±2.9 (mean ± SD), respectively (Fig. 4). The numbers of infiltrating
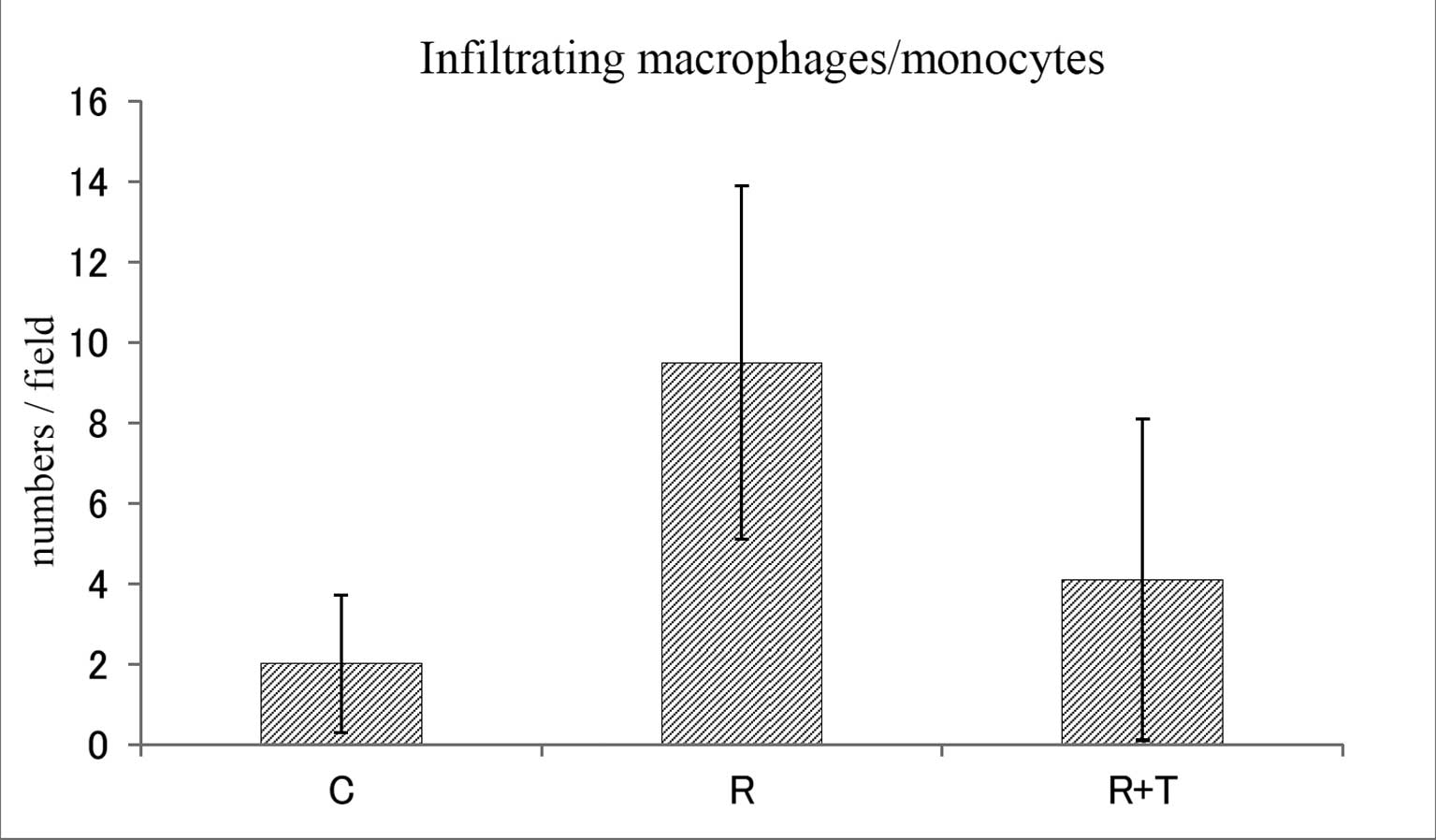
macrophages/monocytes within a field in the C, R and R + T groups
were 2.0±1.7, 9.5±4.4 and 4.1±4.0 (mean ± SD), respectively
(Fig. 5). The findings for
myofibroblast proliferation and macrophage/monocyte infiltration
were identical to those for TGF-β expression in the lungs of each
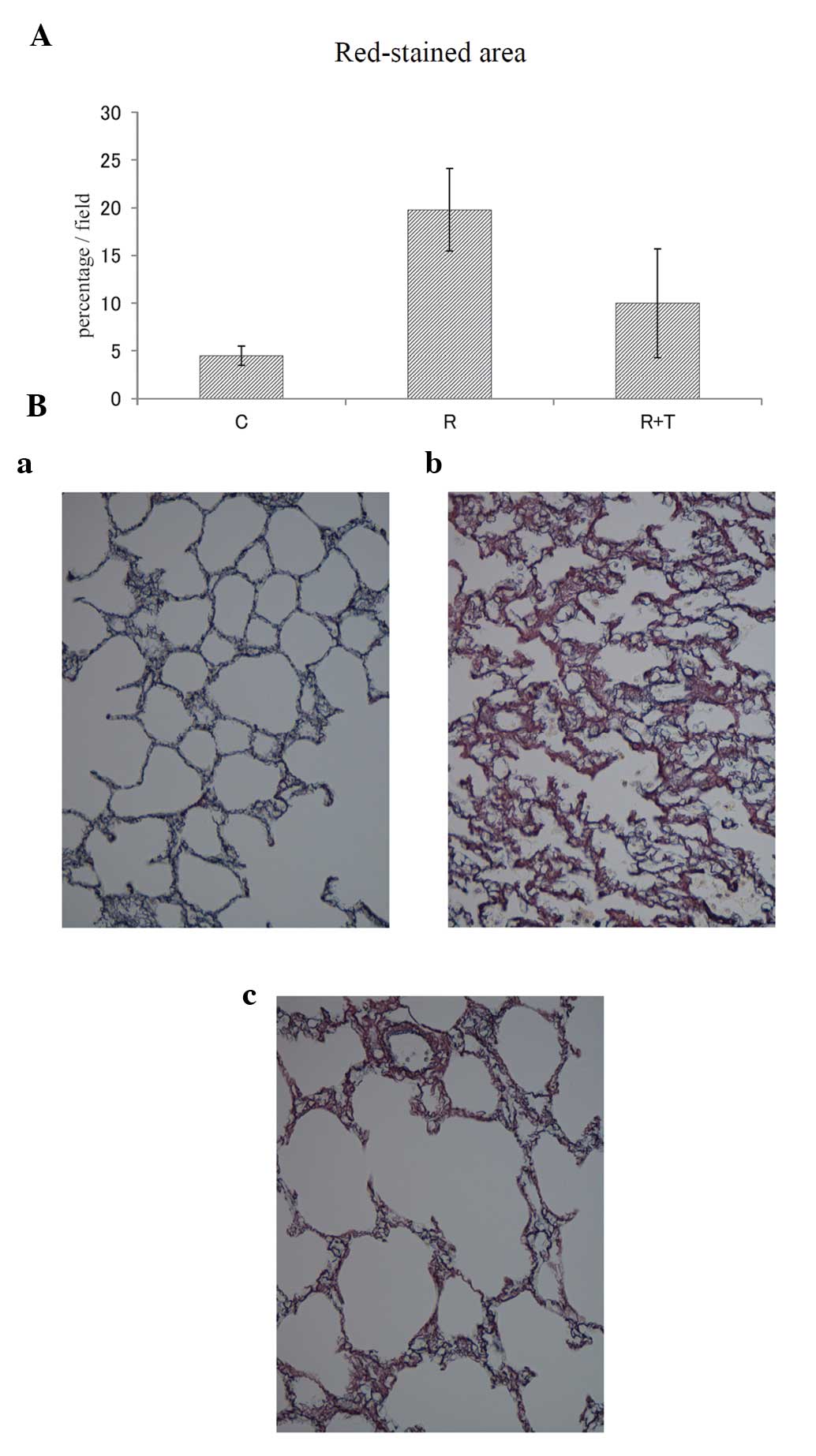
group. With respect to fibroproliferation, 4.5±1.0% (mean ± SD) of
the total area of the sections was stained red in the C group. In
the R group, 19.8±4.3% were stained red. The red-stained area in
the R + T group was 10.0±5.7%, which was markedly lower than that
in the R group (Fig. 6A and
B).
Discussion
For the management of radiation-induced
fibroproliferative changes, numerous drugs have been investigated
in experimental studies and clinical trials. These include
anti-inflammatory agents, such as steroids, drugs acting on blood
flow, interferons and superoxide dismutases (SODs). A number of
these were found to be effective for the prevention and treatment
of radiation-induced fibroproliferation. Using a porcine model,
Lefaix et al (10)
demonstrated that pentoxifylline combined with α-tocopherol is
effective for the treatment of fibrotic scar tissue development
following exposure to γ-rays. Delanian et al (11) revealed that liposomal Cu/Zn SOD is
clinically effective in reducing long-standing radiation-induced
fibrosis. Lefaix et al (12) also reported the effectiveness of
Cu/Zn- or Mn-SOD for the reduction of radiation-induced fibrosis in
pigs. Gauter-Fleckenstein et al (13) identified that Mn porphyrin
[MnTE-2-PyP(5+)] was able to reverse lung damage when treatment was
initiated 8 weeks post-irradiation, at the time of lung injury
establishment, with thickening of the alveolar wall and
interstitium, and infiltration of mononuclear cells.
Based on the recent findings on the molecular
mechanisms of radiation-induced fibroproliferation, a number of
attempts have also been made to prevent radiation-induced
fibroproliferative changes in the lung via direct inhibition of
TGF-β function. Rabbani et al (14) demonstrated the ability of
adenovirus-mediated soluble TGF-β receptor gene therapy in
preventing radiation-induced lung injury. As mentioned above, the
usefulness of adenovirus-mediated soluble TGF-β type II receptor to
ameliorate active TGF-β expression and to prevent radiation-induced
pulmonary fibroproliferation was also reported (7). Anscher et al (15,16)
demonstrated that the anti-TGF-β antibody or small molecular
inhibitor of TGF-β ameliorates radiation-induced lung injury. To
date, however, few attempts have been made to reduce established
pulmonary fibroproliferative changes by radiation via direct
inhibition of TGF-β function.
In the present study, it was demonstrated that TGF-β
expression, myofibroblast proliferation, macrophage/monocyte
infiltration and fibroproliferation in the irradiated lungs of rats
were markedly elevated at 16 weeks following irradiation. It was
also demonstrated that these markers were significantly reduced by
treatment with an adenoviral vector (AdTβ-ExR) expressing a soluble
TGF-β type II receptor at 8 weeks following irradiation, when
marked TGF-β expression, myofibroblast proliferation,
macrophage/monocyte infiltration and fibroproliferation were
observed in 30-Gy irradiated lungs in the control rats. The present
data indicates that TGF-β has a critical role in the
fibroproliferative changes in the irradiated lung, and that the
fibroproliferative changes induced by radiation may be reversible.
The present results suggest that gene therapy with an adenoviral
vector expressing a soluble TGF-β receptor is effective in reducing
the established pulmonary fibrosis caused by radiation.
However, the present study only confirmed the
ability of the adenovirus-mediated soluble TGF-β receptor to reduce
radiation-induced pulmonary fibroproliferation at a
histopathological level. Therefore, whether direct inhibition of
TGF-β functions by an adenovirus-mediated soluble TGF-β receptor
actually preserves respiratory function in irradiated lungs
requires further investigation. In addition, although it was
confirmed that the adenoviral vector itself does not affect the
general condition, behavior or macroscopic appearance of the vital
organs for at least three months following injection, possible
long-term side-effects of TGF-β inhibition, such as carcinogenesis,
should also be carefully examined in future studies. If AdTβ-ExR is
to be used in gene therapy, efforts to optimize administration
methods and to minimize side effects directly associated with the
use of adenoviral vectors are also required (17).
References
|
1
|
Gabbiani G: Modulation of fibroblastic
cytoskeletal features during wound healing and fibrosis. Pathol Res
Pract. 190:851–853. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Desmoulière A: Factors influencing
myofibroblast differentiation during wound healing and fibrosis.
Cell Biol Int. 19:471–476. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Powell DW, Mifflin RC, Valentich JD, et
al: Myofibroblasts. I Paracrine cells important in health and
disease. Am J Physiol. 277:C1–9. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finkelstein JN, Johnston CJ, Baggs R, et
al: Early alterations in extracellular matrix and transforming
growth factor β gene expression in mouse lung indicative of late
radiation fibrosis. Int J Radiat Oncol Biol Phys. 28:621–631. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yi ES, Bedoya A, Lee H, et al:
Radiation-induced lung injury in vivo: Expression of transforming
growth factor-beta precedes fibrosis. Inflammation. 20:339–352.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Franko AJ, Sharplin J, Ghahary A, et al:
Immunohistochemical localization of transforming growth factor β
and tumor necrosis factor α in the lungs of fibrosis-prone and
‘non-fibrosing’ mice during the latent period and early phase after
irradiation. Radiat Res. 147:245–256. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishioka A, Ogawa Y, Mima T, et al:
Histopathologic amelioration of fibroproliferative change in rat
irradiated lung using soluble transforming growth factor-beta
(TGF-β) receptor mediated by adenoviral vector. Int J Radiat Oncol
Biol Phys. 58:1235–1241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakamoto T, Ueno H, Sonoda KH, et al:
Blockade of TGF-beta by in vivo gene transfer of a soluble TGF-beta
type II receptor in the muscle inhibits corneal opacification,
edema and angiogenesis. Gene Ther. 7:1915–1924. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ueno H, Sakamoto T, Nakamura T, et al: A
soluble transforming growth factor-beta receptor expressed in
muscle prevents liver fibrogenesis and dysfunction in rats. Hum
Gene Ther. 11:33–42. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lefaix JL, Delanian S, Vozenin MC, et al:
Striking regression of subcutaneous fibrosis induced by high doses
of gamma rays using a combination of pentoxifylline and
alpha-tocopherol: an experimental study. Int J Radiat Oncol Biol
Phys. 43:839–847. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delanian S, Baillet F, Huart J, et al:
Successful treatment of radiation-induced fibrosis using liposomal
Cu/Zn superoxide dismutase: clinical trial. Radiother Oncol.
32:12–20. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lefaix JL, Delanian S, Leplat JJ, et al:
Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD
and Mn-SOD: An experimental study. Int J Radiat Oncol Biol Phys.
35:305–312. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gauter-Fleckenstein B, Fleckenstein K,
Owzar K, et al: Early and late administration of
MnTE-2-PyP5+ in mitigation and treatment of
radiation-induced lung damage. Free Radic Biol Med. 48:1034–1043.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rabbani ZN, Anscher MS, Zhang X, et al:
Soluble TGFbeta type II receptor gene therapy ameliorates acute
radiation-induced pulmonary injury in rats. Int J Radiat Oncol Biol
Phys. 57:563–572. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anscher MS, Thrasher B, Rabbani Z, et al:
Antitransforming growth factor-beta antibody 1D11 ameliorates
normal tissue damage caused by high-dose radiation. Int J Radiat
Oncol Biol Phys. 65:876–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anscher MS, Thrasher B, Zgonjanin L, et
al: Small molecular inhibitor of transforming growth factor-beta
protects against development of radiation-induced lung injury. Int
J Radiat Oncol Biol Phys. 71:829–837. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishioka A, Ogawa Y and Ueno H: In
response to Drs. Ubrovskaya and Chukhlovin Re: Histopathologic
amelioration of fibroproliferative change in rat irradiated lung
using soluble transforming growth factor-beta (TGF-beta) receptor
mediated by adenoviral vector. Int J Radiat Oncol Biol Phys.
61:308–309. 2005. View Article : Google Scholar
|