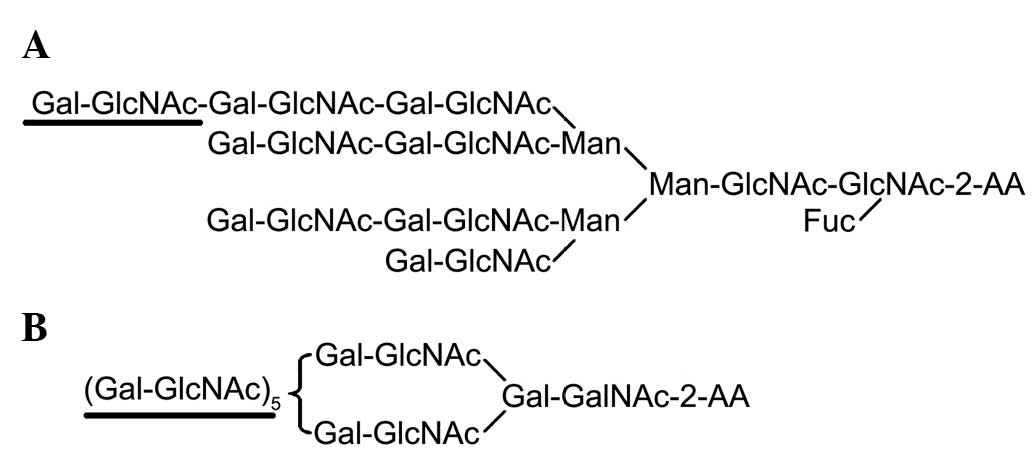Introduction
Glycosylation is one of the most frequently
occurring post-translational modifications of proteins and is
suggested to be involved in the regulation of a variety of
biological and physical processes, including the secretion,
stability, folding and solubility of proteins (1). It has also been observed that the
majority of proteins in the serum and the plasma membrane are
glycosylated (2). In addition,
several previous studies have suggested that aberrant glycosylation
of cell surface glycoproteins results in significant alterations in
the invasive and metastatic abilities of cancer cells (3,4). For
example, the synthesis of polylactosamine chains in colon cancer
cells has been demonstrated to be associated with metastasis
(5).
Polylactosamine is a unique glycan composed of
tandem repeating units of Galβ1-4GlcNAc at the nonreducing terminal
(Fig. 1). A previous study
observed that polylactosamine-type N-glycans were highly expressed
in certain types of cancer cells, including U937 (histiocytic
lymphoma), ACHN (human kidney glandular cancer), MKN45 (human
gastric cancer), A549 (human lung cancer) and Jurkat cells (acute
T-cell leukemia) cell lines (6).
Togayachi et al (7)
reported that the polylactosamine residues on glycoproteins
influenced basal levels of lymphocyte and macrophage activation.
Additionally, 24 glycoproteins that possess polylactosamine-type
N-glycans have been demonstrated to be present in malignant cells
(8).
Cluster of differentiation 147 (CD147), which is
also termed Basigin or extracellular matrix metalloproteinase
inducer (EMMPRIN), is a glycoprotein that carries polylactosamine
on its N-glycosylation sites (9).
CD147 is a transmembrane glycoprotein and its extracellular region
contains three N-linked glycosylation sites (Asn44, Asn152 and
Asn186), which make similar contributions to form the highly and
lowly glycosylated forms (HG- and LG-CD147, respectively). The
present study demonstrated that LG-CD147 contains a series of
high-mannose structures and HG-CD147 contains complex-type N-linked
glycans, including β1,6-branched polylactosamine (9). LG-CD147 does not self-aggregate and
is not able to induce the production of MMPs, whereas HG-CD147
molecules have been reported to self-aggregate and activate MMP
production in tumor cells (9,10).
MMP2 has been demonstrated to degrade and destroy the extracellular
matrix and basement membrane close to the tumor surface, which aids
in the infiltration of tumor cells into surrounding tissues, and
promotes tumor cell invasion and metastasis. CD147 is present in
tissues with increased MMP expression levels, which suggests that
CD147-mediated MMP induction may be involved in the physiological
or pathological mechanisms of cancer progression (11,12).
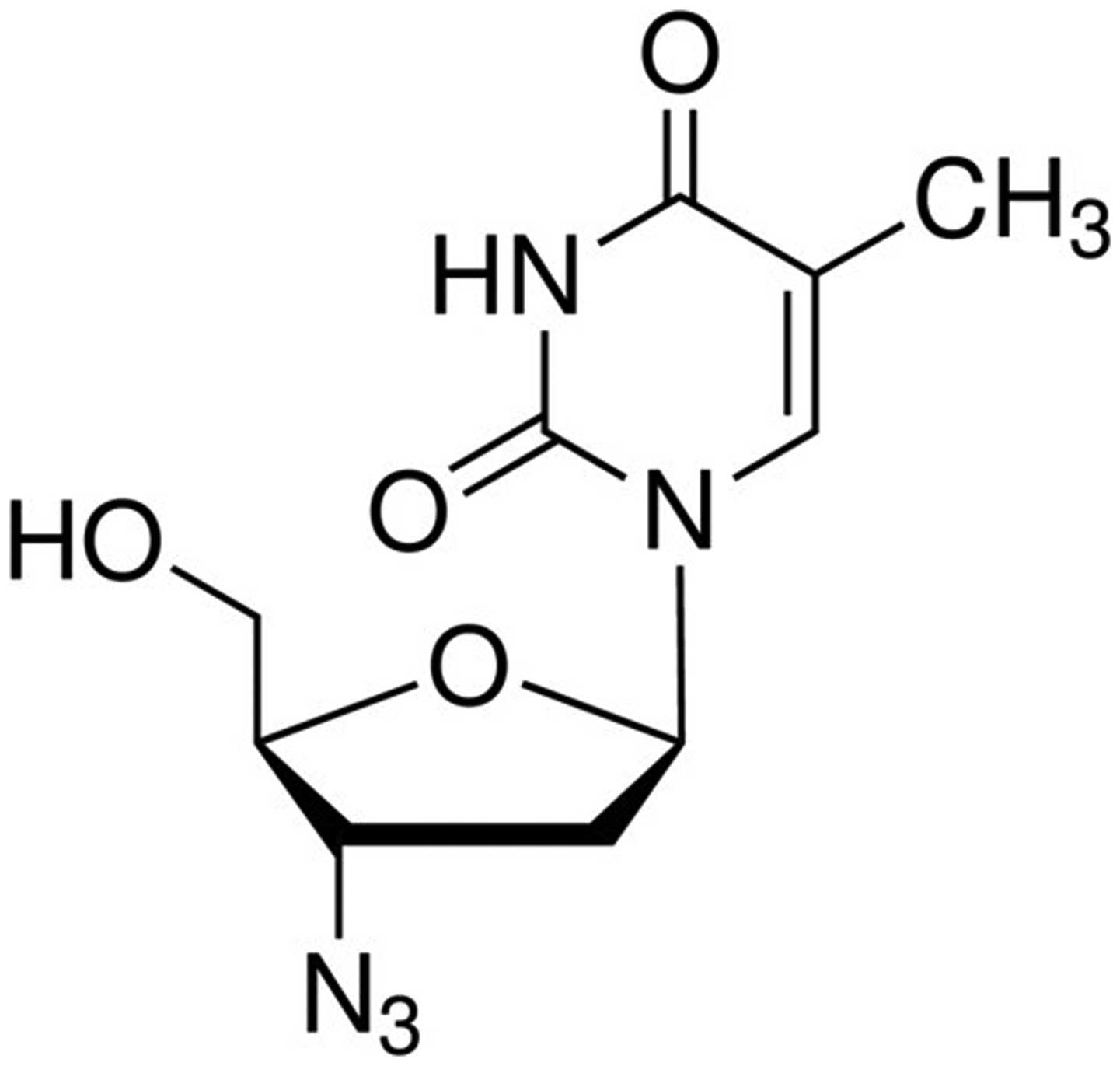
Previous studies have demonstrated that
3′-azidothymidine (AZT; Fig. 2)
inhibits the biosynthesis of β1,6-branched N-linked
oligosaccharides and polylactosamine chains in cells (13,14).
Additional studies have observed that glycosyltransferase β3GnT8
was involved in the synthesis of the polylactosamine chains on the
β1,6-branched N-glycans, and influenced the invasion and growth of
gastric cancer cells by regulating the expression levels of MMP2
(4,15). However, it remains unclear whether
the β1,6-branched polylactosamine chains are critical in this
mechanism. Therefore, in the present study, two different tumor
cell lines were treated with various concentrations of AZT, the
expression levels of HG-CD147 and MMP2 were measured, and the cell
cycle was analyzed to investigate cell proliferation in order to
elucidate how the β1,6-branched polylactosamine on HG-CD147 may
affect MMP2 expression levels and cell proliferation.
Materials and methods
Materials
The U251 human glioma cell line was obtained from
the American Type Culture Collection (Manassas, VA, USA). The
SGC-7901 human gastric cancer cells were obtained from the
Institute of Biochemistry and Cell Biology, Chinese Academy of
Science (Shanghai, China). Goat polyclonal anti-CD147 antibody
(cat. no. sc-9753) was purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA) and the mouse polyclonal anti-β-actin
antibody (cat. no. AA-128-1), and anti-rabbit-horseradish
peroxidase (HRP), anti-goat-HRP and anti-mouse-HRP secondary
antibodies were purchased from Beyotime Institute of Biotechnology
(Haimen, China). AZT was purchased from Sigma-Aldrich (St. Louis,
MO, USA). Other reagents were commercially available in China.
Cell culture
The U251 cells were cultured in Dulbecco’s modified
Eagle’s medium (Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS). The SGC-7901 cells
were cultured in RPMI-1640 (Gibco Life Technologies) medium
supplemented with 10% FBS (Gibco Life Technologies). The two cell
lines were cultured in a humidified atmosphere with 5%
CO2 at 37°C.
Semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR)
Total RNA was extracted from the two cancer cell
lines using TRIzol (Invitrogen Life Technologies, Carlsbad, CA,
USA), in accordance with the manufacturer’s instructions.
Complementary DNA (cDNA) was generated from total RNA using M-MLV
reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA).
The PCR reaction mix consisted of 12.5 μl Easy Taq PCR
Supermix (Beijing Transgen Biotech Co., Ltd., Beijing, China), 0.5
μl forward primer, 0.5 μl reverse primer, 1 μl
cDNA and 0.5 μl ddH2O. The PCR cycling
(Veriti® 96-well Thermal cycler; Applied Biosystems,
Foster City, CA, USA) conditions were as follows: Initial
denaturing at 95°C for 5 min, 30 cycles of denaturing at 95°C for
30 sec, annealing at 60°C for 45 sec, elongation at 72°C for 1 min
and at 72°C for 10 min. The annealing temperature of MMP2 was 55°C
and of β-actin was 53°C. Specific primers (Invitrogen Life
Technologies) used for the genes and expected product sizes were as
follows: Forward: 5′-AAC CCT CAG AGC CAC CCC TA-3′ and reverse:
5′-GTG CAT ACA AAG CAA ACT GC-3′ (286 bp) for MMP-2; and forward:
5′-GAG CTA CGA GCT GCC TGA CG-3′ and reverse: 5′-CCT AGA AGC ATT
TGC GGT GG-3′ (416 bp) for β-actin. The PCR products were separated
using electrophoresis on 10 g/l agarose gels and visualized using
ethidium bromide staining.
Western blot analysis
Protein was extracted from cell lysates using
ice-cold radio immunoprecipitation assay lysis buffer (50 mmol/l
Tris (pH 7.4), 150 mmol/l sodium chloride, 1% NP-40, 0.5% sodium
deoxycholate, 0.1% SDS, 1 mM sodium or thovanadate, 10 mM sodium
fluoride, 5 mM EDTA, 10 μg/ml leupeptin; Beyotime Institute
of Biotechnology) supplemented with 1 mmol/l phenylmethanesulfonyl
fluoride (Beyotime Institute of Biotechnology). The protein
concentration in cell lysates was determined using a protein assay
kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). An equal
quantity of protein from each sample was mixed with 4X loading
buffer (containing 250 mmmol/l Tris-hydrochloric acid, 40%
glycerol, 5% SDS, 0.005% bromophenol blue and 100 mmol/l
dithiothreitol; Beyotime Institute of Biotechnology) and was
denatured for 5 min at 100°C. Total proteins were then separated by
SDS-PAGE (10% acrylamide gel) and transferred onto polyvinylidene
fluoride membranes (Pall Corporation, Beijing, China) that had been
pretreated with methanol. The membranes were then blocked for 1 h
at room temperature in PBST [phosphate-buffered saline (PBS) with
0.05% Tween-20] (Gibco Life Technologies) containing 5% skimmed
milk. The proteins were analyzed using the specific antibodies
mentioned. The blots were incubated overnight at 4°C with the
primary antibodies against CD147 (1:300) and β-actin (1:1,000).
Subsequent to removal of the primary antibodies, the blots were
then incubated for 1 h at room temperature with goat anti-rabbit,
donkey anti-goat or rabbit anti-mouse HRP-conjugated secondary
antibodies (1:1,000). ECL Plus Detection system (Beyotime Institute
of Biotechnology) was used according to the manufacturer’s
instructions to detect chemiluminescence.
Regulation of CD147 and MMP2 expression
by various concentrations of AZT
The U251 and SGC-7901 cells were seeded into a
6-well plate and incubated overnight. Subsequently, the cells were
washed once with PBS and cultured for 48 h in fresh culture medium
in the absence or presence of various concentrations of AZT (0,
0.23 and 0.46 mmol/l). The cells were then harvested, and western
blotting and RT-PCR analysis were conducted to measure CD147 and
MMP2 expression.
Analysis of the cell cycle by flow
cytometry
The SGC-7901 cells were plated at a density of
5×105 cells/well on 6-well plates and incubated in
serum-free medium for 24 h in order to arrest the cells at the same
stage of the cell cycle. Subsequent to exposure to various
concentrations of AZT for 24 h, the cells were harvested and fixed
with cold 70% ethanol overnight. The fixed cells were incubated in
PBS containing 50 μg/ml RNase A (Sigma-Aldrich), 0.25%
Triton X-100 (Sigma-Aldrich) and 0.1 mmol/l EDTA (Thermo Fisher
Scientific) for 30 min at 37°C. Propidium iodide (PI) was added to
the cell suspension at a concentration of 100 μg/ml and
incubated for 15 min at room temperature in the dark. The cell
cycle was analyzed using flow cytometry with Cell Quest Pro
software (FACScan; BD Biosciences, San Jose, CA, USA).
Statistical analysis
All results are expressed as the mean ± standard
deviation. Statistical significance was evaluated with data from
three independent experiments using Student’s t-test. Statistical
analyses were conducted using SPSS version 19.0 (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Regulation of N-glycosylation on CD147 by
AZT in SGC-7901 and U251 cells
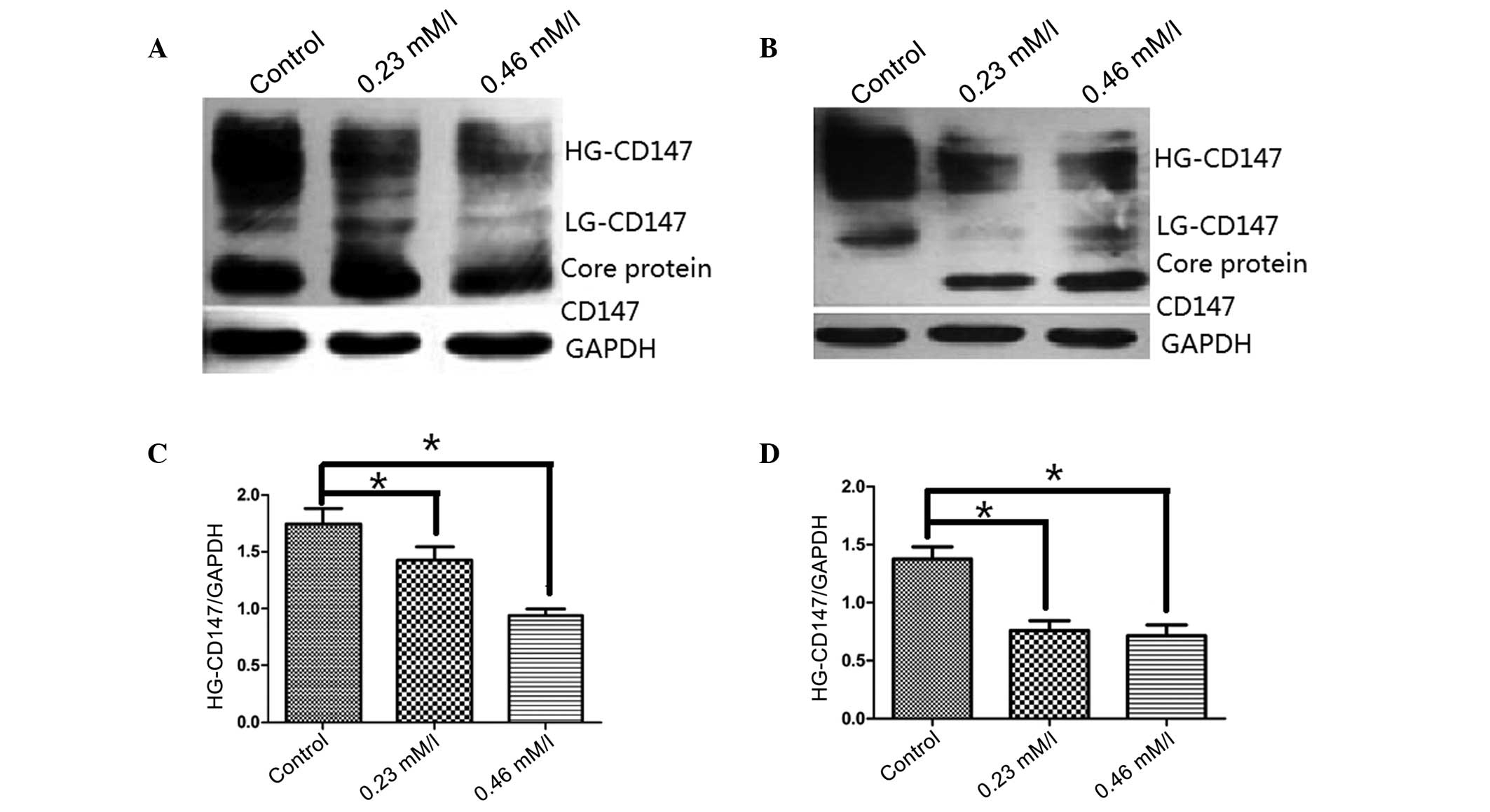
To examine the effect of AZT on CD147
N-glycosylation, the two cell lines were treated with various AZT
concentrations for 48 h. Proteins from these treated cell lines
were assessed using western blot analysis. As demonstrated in
Fig. 3, CD147 was clearly
expressed in SGC-7901 and U251 cells; however, subsequent to
treatment with AZT (0.23 and 0.46 mmol/l) the glycosylation level
of HG-CD147 located at 45–65 kDa was significantly reduced compared
with the controls in the two cell lines (P<0.05). Furthermore,
the glycosylation level of LG-CD147 was also significantly reduced
in the treated cells, as compared with the two control cell lines.
The results suggest that AZT may inhibit the biosynthesis of
polylactosamine chains on N-glycans of CD147.
Regulation of MMP2 expression by AZT in
SGC-7901 and U251 cells
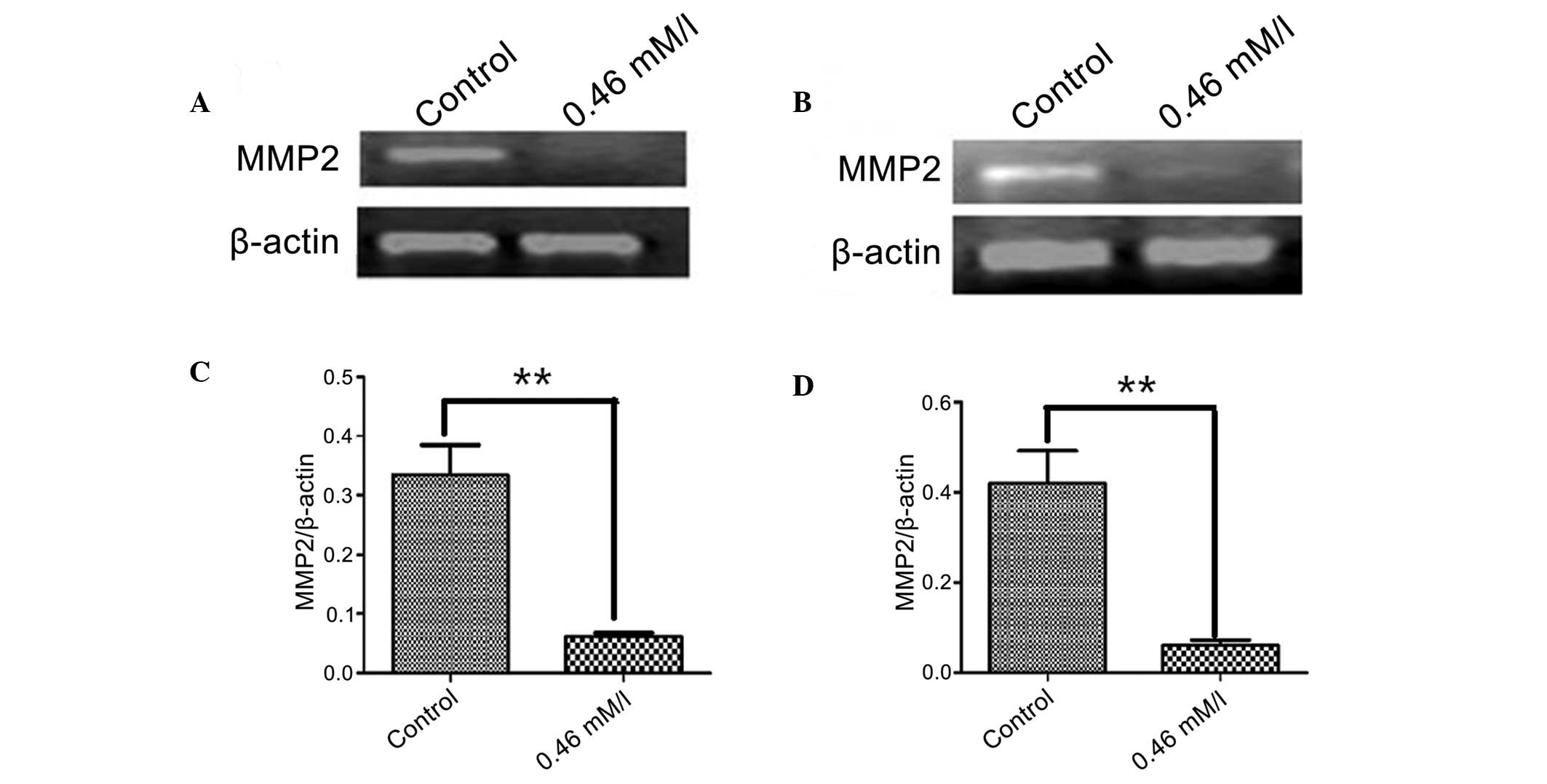
To examine the effect of AZT on the mRNA levels of
MMP2, the two cell lines were treated with 0.46 mmol/l AZT for 48 h
and RT-PCR analysis was conducted. As demonstrated by agarose gel
electrophoresis in Fig. 4,
following AZT treatment in SGC-7901 and U251 cells, MMP2 expression
was almost eradicated, compared with the control group (P<0.01).
These results indicate that AZT may also inhibit the expression of
MMP2.
Cell cycle analysis
To investigate the effect of AZT treatment on cell
cycle distribution in SGC-7901 cells, the cells were treated with
various concentrations of AZT (0.23, 0.46, 0.69, 0.92 and 1.15
mmol/l) for 24 h and subjected to flow cytometric analysis
subsequent to staining of the chromosomal DNA with PI. As
demonstrated in Fig. 5, AZT
treatment altered the cell cycle distribution of SGC-7901 cells.
The proliferation index
[%S+%G2/M)/(%G0/G1+%S+G2/M)]
values for G1 phase in the AZT-treated cells was 53.949,
54.716, 57.611, 59.852, 60.799 and 65.785% for the different AZT
concentrations, respectively. These results indicated that the
percentage of AZT-treated cells in the G1 phase
increased and the number of cells in the S, G2 and M
phases reduced in a dose-dependent manner. This suggests that AZT
may arrest the cell cycle in the G1 phase to inhibit
cell proliferation.
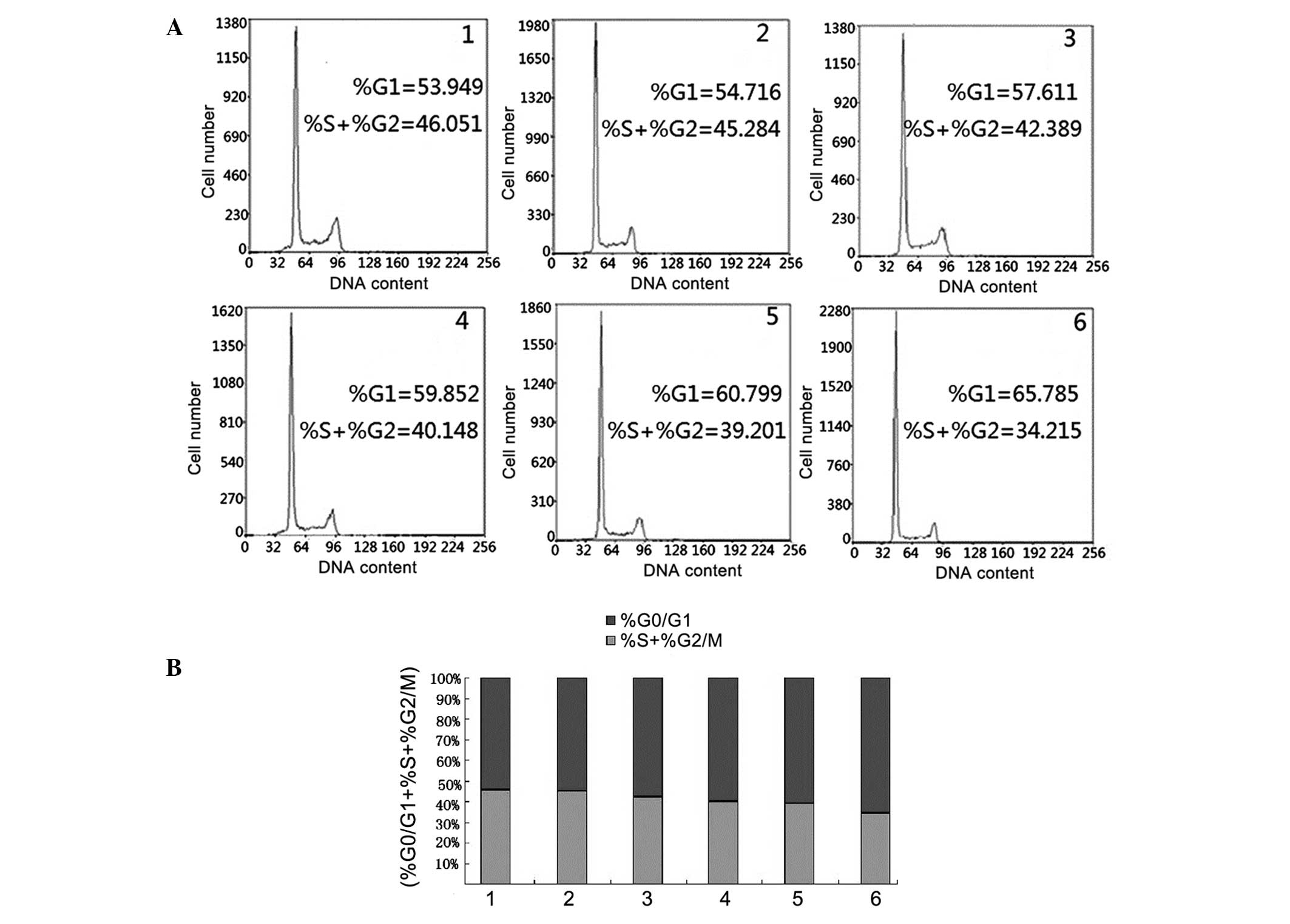 | Figure 5Flow cytometric analysis to examine
the cell cycle distribution of SGC-7901 cells undergoing AZT
treatment. (A) The results of the cell cycle distribution with
various concentrations of AZT. 1, no treatment; 2, 0.23; 3, 0.46;
4, 0.69; 5, 0.92; and 6, 1.15 mmol/l. The percentages of
AZT-treated cells in the G1 phase were 53.949, 54.716,
57.611, 59.852, 60.799 and 65.785%, respectively for the
concentrations of AZT listed above. (B) The relative percentages of
cells in different stages of the cell cycle with various AZT
concentrations. The experiments were representative of three
independent experiments. AZT, 3′-azidothymidine. |
Discussion
It has been reported that AZT may markedly alter the
profile of N-linked oligosaccharides in the K562 erythroleukemia
cell line and the SKSSK-MEL-30 melanoma cell line (13). A previous study demonstrated that
in these cell lines, AZT treatment markedly reduced the synthesis
of highly branched complex oligosaccharides and polylactosamine
chains (poly-LacNAc) (14).
Poly-LacNAc contains linear LacNAc (Galβ1-4GlcNAcβ1-3) N repeats,
which are formed by the addition of galactose (Gal) to terminal
GlcNAc residues in the Golgi complex (16). However, it has been reported that
the mechanism of AZT-mediated inhibition of poly-LacNAc synthesis
is via the inhibition of UDP-GalNAc movement into the Golgi complex
and blocking the addition of LacNAc repeats. In addition, AZT is
also reported to block the formation of the GlcNAc-β-1,6-Man
branch, a preferred initiation site for poly-LacNAc synthesis
(17,18).
Alterations in N-linked glycans are associated with
the progression of cancer. Of particular importance in tumor growth
and invasion is the synthesis of complex N-linked oligosaccharides
containing long polylactosamine chains (19). These outer branch modifications are
suggested to be critical in cell-cell recognition and communication
events, due to the modulation of the structural and the functional
properties of carrier glycoproteins associated with cancer
progression (20). Highly branched
complex glycan chains, for example the polylactosamine extension,
appear to be important for the oncogenic phenotype of tumor cells
(21). In addition,
polylactosamine side chains have been suggested to serve critical
roles in the adhesive properties of tumorigenic cells (22). Saitoh et al (4) demonstrated that lysosomal associated
membrane proteins isolated from a highly metastatic cancer cell
line contained markedly more polylactosamine side chains than one
with low metastatic activity. From this, it was concluded that
increased quantities of these side chains are characteristic of the
metastatic phenotype.
The glycoprotein HG-CD147 is a key carrier of
β1,6-branched polylactosamine sugars on N-glycans in tumor cells
(9).
β1,3-N-acetylglucosaminyltransferases (β3GnTs) may catalyze the
initiation and elongation of polylactosamine chains on O-glycans,
N-glycans and glycolipids (17,23,24).
A previous study identified that β3GnT8 was involved in the
biosynthesis of β1,6-branched polylactosamine sugars on N-glycans
in vitro and it may alter the glycosylation of HG-CD147 and
further regulate the metastatic potential of colorectal cancer
cells (25).
CD147 is composed of two domains in the
extracellular region, a single transmembrane domain and a short
cytoplasmic domain containing 39 amino acids. The extracellular
region contains three Asn glycosylation sites; however, the glycan
portion of the molecule differs depending on the source of CD147.
CD147 glycosylation has been previously demonstrated to determine
its ability to activate MMPs. Purified deglycosylated CD147 and
LG-CD147 failed to induce MMP activity, whereas HG-CD147
effectively induced MMP activity. Since the glycosylated regions of
CD147 serve an important role in MMP expression and HG-CD147
contains multiple β1,6-branched polylactosamine sugars, which are
catalyzed by β3GnT8 and may regulate the metastatic potential of
colorectal cancer cells. Therefore, AZT was used in the present
study to examine whether the β1,6-branched polylactosamine sugars
on CD147 are important in SGC-7901 and U251 cells.
Subsequent to treatment of SGC-7901 and U251 cells
with AZT, it was observed using western blot analysis that the
quantity of N-glycans of HG-CD147 was reduced, compared with that
in the control group (P<0.05; Fig.
3). This result indicated that the N-glycans of CD147 contain
β1,6-branched polylactosamine sugars and may be inhibited by AZT.
As HG-CD147 regulates MMP expression, AZT may additionally affect
MMP expression in SGC-7901 and U251 cells. MMPs are a class of
molecules that have been demonstrated to be integral to tumor
progression, facilitating cell migration and invasion through their
ability to degrade the extracellular matrix. As shown in Fig. 4, MMP2 expression was almost
eradicated compared with the control group subsequent to treatment
with AZT (P<0.01), it is thus suggested that AZT may prevent the
expression of MMP2. In addition, the cell cycle distribution of
SGC-7901 cells was altered by AZT, with a dose-dependent increase
in the percentage of cells in the G1 phase, indicating
that AZT may arrest the cell cycle in G1 phase to
inhibit cell proliferation (Fig.
5). These results suggest that AZT may inhibit the biosynthesis
of polylactosamine chains on CD147 and thus affect MMP2 expression
and cell cycle progression in SGC-7901 and U251 tumor cells.
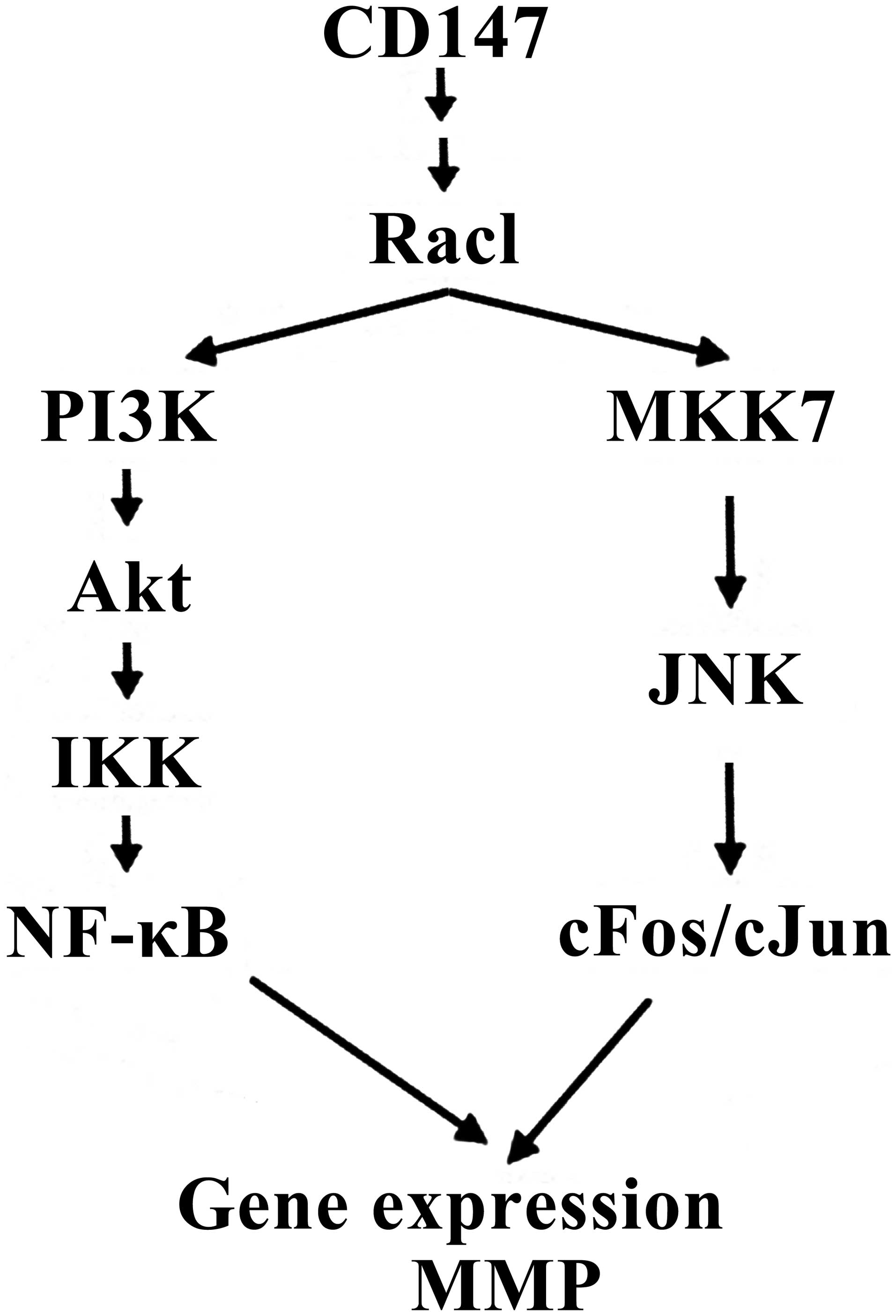
It has been reported that CD147 may activate several
transcription factors, including activator protein 1 (AP-1) and
nuclear factor-κB (NF-κB), which leads to the MMP-inducing activity
of CD147 (25). For example, MMP3,
7, 9, 10, 12 and 13 contain a minimum of one AP-1 binding site in
their proximal promoter region (17). MMP2, 3, 9 and 14 are also NF-κB
responsive in a cell- and stimulus-specific manner (26–28).
In addition, CD147 may also induce IκB kinase (IKK) activation in
the PI3K/Akt-dependent signaling pathway and induce c-Jun
N-terminal kinase-dependent c-Jun activation via the
mitogen-activated protein kinase 7 (MKK7) signaling pathway. Thus,
a previous study demonstrated that CD147 induced MMP expression
predominantly via PI3K/Akt/IKK-dependent NF-κB and
MKK7/JNK-dependent AP-1 signal transduction pathways (25) (Fig.
6). Therefore, it was hypothesized that the AZT-mediated
inhibition of MMP2 expression by reducing CD147 N-glycans in the
present study, may be via the above two pathways.
In conclusion, AZT may inhibit the biosynthesis of
polylactosamine chains on CD147 in tumor cells. The glycoprotein
CD147 is hypothesized to be an upstream modulator inducing MMP
production in tumor cells and AZT may be effective therapeutically
as an antineoplastic drug in certain types of cancer by altering
the glycosylation levels of HG-CD147.
Acknowledgments
The current study was supported by the National
Natural Science Foundation of China (grant no. 31170772) and Suzhou
Municipal Natural Science Foundation (grant no. SY201208).
References
|
1
|
Kato T, Suzuki M, Murata T and Park EY:
The effects of N-glycosylation sites and the N-terminal region on
the biological function of beta1,3-N-acetylglucosaminyltransferase
2 and its secretion. Biochem Biophys Res Commun. 329:699–705. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Narimatsu H: Human glycogene cloning:
focus on beta 3-glycosyltransferase and beta 4-glycosyltransferase
families. Curr Opin Struct Biol. 16:567–575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hakomori S: Glycosylation defining cancer
malignancy: new wine in an old bottle. Proc Natl Acad Sci USA.
99:10231–10233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saitoh O, Wang WC, Lotan R and Fukuda M:
Differential glycosylation and cell surface expression of lysosomal
membrane glycoproteins in sublines of a human colon cancer
exhibiting distinct metastatic potentials. J Biol Chem.
267:5700–5711. 1992.PubMed/NCBI
|
|
5
|
Kinoshita M, Mitsui Y, Kakoi N, Yamada K,
Hayakawa T and Kakehi K: Common glycoproteins expressing
polylactosamine-type glycans on matched patient primary and
metastatic melanoma cells show different glycan profiles. J
Proteome Res. 2013.PubMed/NCBI
|
|
6
|
Mitsui Y, Yamada K, Hara S, Kinoshita M,
Hayakawa T and Kakehi K: Comparative studies on glycoproteins
expressing polylactosamine-type N-glycans in cancer cells. J Pharm
Biomed Anal. 70:718–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Togayachi A, Kozono Y, Ishida H, et al:
Polylactosamine on glycoproteins influences basal levels of
lymphocyte and macrophage activation. Proc Natl Acad Sci USA.
104:15829–15834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang W, Chang SB and Hemler ME: Links
between CD147 function, glycosylation, and caveolin-1. Mol Biol
Cell. 15:4043–4050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang W, Luo WJ, Zhu P, et al: Modulation
of CD147-induced matrix metalloproteinase activity: role of CD147
N-glycosylation. Biochem J. 449:437–448. 2013. View Article : Google Scholar
|
|
10
|
Gabison EE, Hoang-Xuan T, Mauviel A and
Menashi S: EMMPRIN/CD147, an MMP modulator in cancer, development
and tissue repair. Biochimie. 87:361–368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agrawal SM and Yong VW: The many faces of
EMMPRIN - roles in neuroinflammation. Biochim Biophys Acta.
1812:213–219. 2011. View Article : Google Scholar
|
|
12
|
Hall ET, Yan JP, Melançon P and Kuchta RD:
3′-Azido-3′-deoxythymidine potently inhibits protein glycosylation.
A novel mechanism for AZT cytotoxicity. J Biol Chem.
269:14355–14358. 1994.PubMed/NCBI
|
|
13
|
Steet RA, Melancon P and Kuchta RD:
3′-Azidothymidine potently inhibits the biosynthesis of highly
branched N-linked oligosaccharides and poly-N-acetyllactosamine
chains in cells. J Biol Chem. 275:26812–26820. 2000.PubMed/NCBI
|
|
14
|
Liu Z, Shen L, Xu L, Sun X, Zhou J and Wu
S: Down-regulation of β-1,3-N-acetylglucosaminyltransferase-8 by
siRNA inhibits the growth of human gastric cancer. Mol Med Rep.
4:497–503. 2011.PubMed/NCBI
|
|
15
|
van den Eijnden DH, Koenderman AH and
Schiphorst WE: Biosynthesis of blood group i-active
polylactosaminoglycans. Partial purification and properties of an
UDP-GlcNAc:N-acetyllactosaminide beta 1 - 3-N-acetylglucos
aminyltransferase from Novikoff tumor cell ascites fluid. J Biol
Chem. 263:12461–12471. 1988.PubMed/NCBI
|
|
16
|
Ishida H, Togayachi A, Sakai T, et al: A
novel beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8), which
synthesizes poly-N-acetyllactosamine, is dramatically upregulated
in colon cancer. FEBS Lett. 579:71–78. 2005. View Article : Google Scholar
|
|
17
|
Do KY and Cummings RD: 2,6-branched
mannose and the regulation of poly-N-acetyllactosamine biosynthesis
in N-linked oligosaccharides of Chinese hamster ovary cells. J Biol
Chem. 268:22028–22035. 1993.PubMed/NCBI
|
|
18
|
Hakomori S: Tumor malignancy defined by
aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer
Res. 56:5309–5318. 1996.PubMed/NCBI
|
|
19
|
Pierce M, Buckhaults P, Chen L and Fregien
N: Regulation of N-acetylglucosaminyltransferase V and Asn-linked
oligosaccharide beta(1,6) branching by a growth factor signaling
pathway and effects on cell adhesion and metastatic potential.
Glycoconj J. 14:623–630. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Datti A, Orlacchio A, Siminovitch KA and
Dennis JW: A coupled assay for UDP-GlcNAc:Gal beta 1-3GalNAc-R beta
1,6-N-acetylglucosaminyltransferase (GlcNAc to GalNAc). Anal
Biochem. 206:262–266. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sawada R, Lowe JB and Fukuda M:
E-selectin-dependent adhesion efficiency of colonic carcinoma cells
is increased by genetic manipulation of their cell surface
lysosomal membrane glycoprotein-1 expression levels. J Biol Chem.
268:12675–12681. 1993.PubMed/NCBI
|
|
22
|
Krishnan V, Bane SM, Kawle PD, Naresh KN
and Kalraiya RD: Altered melanoma cell surface glycosylation
mediates organ specific adhesion and metastasis via lectin
receptors on the lung vascular endothelium. Clin Exp Metastasis.
22:11–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishida H, Togayachi A, Sakai T, et al: A
novel beta1,3-N-acetyl-glucosaminyltransferase (beta3Gn-T8), which
synthesizes poly-N-acetyllactosamine, is dramatically upregulated
in colon cancer. FEBS Lett. 579:71–78. 2005. View Article : Google Scholar
|
|
24
|
Ni J, Jiang Z, Shen L, et al: β3GnT8
regulates the metastatic potential of colorectal carcinoma cells by
altering the glycosylation of CD147. Oncol Rep. 31:1795–1801.
2014.PubMed/NCBI
|
|
25
|
Venkatesan B, Valente AJ, Prabhu SD,
Shanmugam P, Delafontaine P and Chandrasekar B: EMMPRIN activates
multiple transcription factors in cardiomyocytes, and induces
interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB
and MKK7/JNK/AP-1 signaling. J Mol Cell Cardiol. 49:655–663. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li G, Zhang Y, Qian Y, Zhang H, et al:
Interleukin-17A promotes rheumatoid arthritis synoviocytes
migration and invasion under hypoxia by increasing MMP2 and MMP9
expression through NF-κB/HIF-1α pathway. Mol Immunol. 53:227–236.
2013. View Article : Google Scholar
|
|
27
|
Sun P, Mu Y and Zhang S: A novel
NF-κB/MMP-3 signal pathway involves in the aggressivity of glioma
promoted by Bmi-1. Tumour Biol. 35:12721–12727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han YP, Tuan TL, Wu H, Hughes and Garner
WL: TNF-alpha stimulates activation of pro-MMP2 in human skin
through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci.
114:131–139. 2001.
|