Introduction
Astrocytes are a type of macroglial cell, and are
the most abundant type of cell in the central nervous system (CNS)
(1). Astrocytes were previously
considered to have predominantly supportive roles to assist in
appropriate neuronal functions; however, with the development of
molecular neuroscience, studies have revealed several active roles
of astrocytes, including the regulation of blood flow (2,3),
involvement in synaptic functions (1,4) and
maintenance of the blood-brain barrier (BBB) integrity (5).
Reactive gliosis is an astrocytic response to a wide
range of CNS pathologies, which results in morphological and
molecular changes in the resting glia. Although the extent of the
changes in astrocytes due to astrogliosis vary with the nature and
severity of an insult, there are certain changes that astrocytes
commonly undergo in response to all forms of CNS insult, including
hypertrophy, proliferation and the upregulation of molecules,
including glial fibrillary acidic protein (GFAP) and S100β
(6). In certain severe case of CNS
injury, a physical barrier, referred to as a ‘glial scar’ is formed
by glial cells undergoing reactive gliosis, to isolate the damaged
tissue from the healthy region and to prevent a cascade of
inflammation, caused by factors released from the lesion site
(7). However, it is difficult to
categorize the effect of the glial scar as beneficial or harmful in
terms of clinical outcome, since this tight physical barrier also
inhibits the regeneration of damaged neurons at a later stage of
injury (8–10). Therefore, determining the
mechanisms by which reactive gliosis is regulated may improve
clinical outcomes, however, the molecular and cellular mechanisms
underlying the initiation, progression and resolution of this
process remain to be fully elucidated.
Meteorin is a secreted protein, which was initially
screened as a retinoic-acid-responding molecule (11). In the developing rodent CNS,
meteorin is highly expressed in the neuroepithelium and remains in
subpopulations of the glia, including the cortical astrocytes,
following completion of CNS development (11,12).
Our previous study reported that meteorin promotes glial
differentiation in neural stem cells (NSCs) via activation of the
janus kinase (Jak)-signal transducer and activator of trascription
3 (STAT3) signaling pathway (12),
and that astrocytic meteorin promotes the maturation of the BBB by
stimulating endothelial cells (13). Regarding its involvement in CNS
pathologies, previous studies have reported on the neuroprotective
effects of meteorin in rat models of neuropathic pain and in
quinolic acid-induced neuropathology (14,15).
In addition, a previous study identified a novel function of
meteorin in promoting neuroblast migration in a stroke model
(16). Despite emerging evidence
regarding the crucial functions of meteorin in neuropathologies,
its role in the glial response to CNS insults remains to be
elucidated.
The predominant source of meteorin in the adult
brain are astrocytes, and meteorin is known to promote glial cell
differentiation and the expression of GFAP in NSCs (12,17);
therefore, the present study aimed to demonstrate that meteorin was
involved in the reactive gliosis process. The expression levels of
meteorin were analyzed in reactive astrocytes from a
photothrombotic (PT) ischemia mouse model, as well as following
stimulation of transforming growth factor (TGF)-β, which led to the
activation of astrocytes in vitro. A small interfering RNA
(siRNA)-mediated meteorin-knockdown was performed to identify the
role of meteorin in reactive gliosis.
Materials and methods
Induction of PT ischemia in vivo
C57BL/6 mice (3–6 months old) were purchased from
Samtako (Daejeon, Korea) and PT ischemia was induced, as described
previously (18). Briefly, under
deep anesthesia by intraperitoneal injection of Zoletil®
(30 mg/kg) and Rompun® (10 mg/kg) (Virbac, Carros,
France), rose bengal photosensitive dye (Sigma-Aldrich; 0.1 ml of a
10% solution/25 g body weight) was injected into the tail vein and
allowed to circulate for 5 min. The skull was then exposed to a
cold light source (150 W, 1 mm diameter; Zeiss FL1500 LCD; Carl
Zeiss AG) for 20 min, 2.5 mm to the left and 2.5 mm to the back of
the bregma. A total of 7 days following PT induction, the mice were
deeply anesthesized and cardiac tissue was perfused with PBS,
followed by 4% paraformaldehyde/PBS solution for
immunohistochemical analysis. Alternatively, fresh brain tissue was
isolated, without cardiac perfusion, and the lesion sites were
dissected to isolate total RNA. Contralateral hemisphere tissue was
considered as a control.
The animal experiments of the present study were
approved by the Committee for Care and Use of Laboratory Animals at
the Seoul National University (Seoul, Korea), according to the
Guide for Animal Experiments edited by the Korean Academy for
Medical Sciences. The mice were maintained in a specific
pathogen-free room in the animal-housing facilities at Seoul
National University under a 12-h dark/light cycle with a provision
of chow and water ad libitum.
Cell culture and purification of
recombinant meteorin
Primary cultures of mouse cortical astrocytes were
prepared from the brains of 2 day-old mice (Samtako). The mice were
sacrificed by decapitation, and the brains were isolated. The
cerebral cortices were dissected from the mouse brains and the
meninges were removed. The cortices were then diced into 1–2 mm
sections and incubated in 0.25% trypsin (Life Technologies,
Carlsbad, CA, USA) and 20 mg/ml DNase I (Sigma-Aldrich, St. Louis,
MO, USA) at 37°C for 20 min. The sections were then dissociated
into single cells by pipetting. The cells were plated and cultured
in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies),
supplemented with 10% fetal bovine serum (FBS; Life Technologies)
and 1% penicillin/streptomycin (Life Technologies). After 7–10 days
of culture, the cells were agitated at 250 rpm for 16 h, in order
to remove the microglia and oligodendrocytes. Once the cells had
reached 80% confluence they were treated with the indicated amount
of TGF-β1 (10 or 50 ng/ml; PeproTech, Rocky Hill, NJ, USA) for 24
h.
Primary NSCs were cultured from E13.5 mouse embryos,
as described previously (12).
Briefly, embryos were dissected from pregnant female mice (Samtako)
and neurospheres were allowed to form in complete neurobasal medium
(Invitrogen Life Technologies) containing 2% B27 (Invitrogen Life
Technologies), human epidermal growth factor (20 ng/ml, R&D
Systems, Minneapolis, MN, USA), and basic fibroblast growth factor
(10 ng/ml, Invitrogen Life Technologies) for 4 days, by plating
neuroepithelial cells on non-coated culture dishes. For in
vitro differentiation, the neurospheres were dissociated with
trypsin-EDTA and plated onto poly-L-ornithine
(Sigma-Aldrich)-coated dishes in complete medium. The neurospheres
were treated with 200 ng/ml recombinant meteorin for 48 h following
an overnight deprivation of growth factors.
Recombinant mouse meteorin was purified from the
conditioned medium of CHO-K1 Chinese hamster ovary cells (Korean
Cell Line Bank, Seoul, Korea) stably expressing meteorin tagged
with myc-His6 at C-terminus, as described previously (13). CHO-K1 cells were maintained in DMEM
supplemented with 10% FBS at 37°C in a humidified atmosphere
containing 5% CO2.
siRNA preparation and transfection
The following siRNAs were synthesized and were used
to target mouse meteorin: siMeteorin #1, 5′-GTTCAGCCGTGTCTATTCA-3′;
and siMeteorin #2, 5′-GTCTTCGCTGAACGTATGA-3′. Non-targeting siRNAs
were used as a control (GE Dharmacon, Lafayette, CO, USA). The
astrocytes were transfected with the siRNAs using oligofectamine
(Invitrogen Life Technologies, Carlsbad, CA, USA) once they had
reached ~50% confluence.
Western blot analysis and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The astrocytes were lysed using 1X cell lysis buffer
(Cell Signaling Technology Inc., Beverly, MA, USA). Protein
concentration was determined using bicinchoninic acid protein assay
kit (Invitrogen Life Technologies). The protein samples (40
μg) were then separated by 12% SDS-PAGE and transferred to
nitrocellulose membranes (GE Healthcare Life Sciences, Piscataway,
NJ, USA). After blocking with 5% skim milk/phosphate-buffered
saline (PBS) with 0.1% Tween-20, the membranes were immunoblotted
with the following antibodies overnight at 4°C: Rabbit
anti-phospho-STAT3 (Tyr705) (1:2,000 cat. no. 9131; Cell Signaling
Technology, Inc.), rabbit anti-STAT3 (1:5,000; cat. no. sc-482;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit anti-GFAP
(1:1,000; cat. no. s0334; DAKO, Glostrup, Denmark) and rabbit
anti-actin (1:10,000; cat. no. A2668; Sigma-Aldrich). The membranes
were then probed with anti-rabbit secondary antibodies conjugated
to horseradish peroxidase (1:5,000; cat. no. HAF008; R&D
Systems) for 1 h at room temperature, and proteins were visualized
using an enhanced chemiluminescence system (Intron Biotechnology,
Gyeonggi-do, Korea). Band intensities were quantified using
MultiGauge v.3.0 software (Fujifilm, Tokyo, Japan).
RT-qPCR was performed, as described previously
(12). Briefly, total RNA was
isolated using TRIzol® reagent (Invitrogen Life
Technologies), according to the manufacturer’s instructions. To
isolate total RNA from brain tissue, the samples were homogenized
in TRIzol by passing through a 30 gauge needle of a 1 ml syringe.
First-stranded cDNA was synthesized from 2 μg total RNA
using a murine leukemia virus reverse transcriptase (Promega
Corporation, Madison, WI, USA). A total of 2 μl cDNA was
then amplified by PCR using the following thermocycling conditions:
95°C for 30 sec, 55°C for 30 sec, 72°C for 60 sec, 25 cycles on a
thermal cycler (Applied Biosystems, Foster City, CA, USA). The PCR
products were visualized following separation by 1.5% agarose gel
electrophoresis and the band intensities were quantified using
MultiGauge v.3.0 software (Fujifilm). The following primers
(Bioneer Corporation, Daejeon, Korea) were used to amplify each
gene: Meteorin, forward 5′-ATGCTGGTAGCCACGCTTCTTT-3′ and reverse
5′-GTCCAGTGCCATCTCACATGGG-3′), Gfap, forward
5′-GGCCGGGGCGCTCAA-3′ and reverse 5′-GCCGACTCCCGCGCAT-3′),
S100β, forward 5′-GGTTGCCCTCATTGATGTCT-3′, and reverse
5′-GTCCAGCGTCTCCATCACTT-3′ and Gapdh, forward
5′-ACCACAGTCCATGCCATCAC-3′ and reverse
5′-TCCACCACCCTGTTGCTGTA-3′).
Immunohistochemistry
Coronal brain sections (30 μm thickness)
isolated from mice 7 days following PT induction were prepared
using a cryotome and collected in PBS. Free-floating sections were
then permeabilized with PBS-0.25% Triton-X, blocked with 5% FBS,
and treated with the following primary antibodies: Rabbit anti-GFAP
(1:750; DAKO), goat anti-meteorin (1:100; R&D Systems,
Minneapolis, MN, USA), and mouse anti-S100β (1:500; Sigma-Aldrich)
in 5% FBS overnight at 4°C. Following extensive washing with
PBS-0.25% Triton-X, the sections were incubated with Alexa
Fluor-conjugated secondary antibodies (1:100) for 2 h at room
temperature, and the tissues were mounted using FluorSave™ Reagent
(EMD Millipore, Billerica, MA, USA). Images of the tissues were
captured using an M200 ApoTome microscope (Carl Zeiss AG,
Oberkochen, Germany) and a LSM700 confocal microscope (Carl Zeiss
AG).
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. Statistical significance was determined using an
unpaired two-tailed Student’s t-test in Microsoft Excel 2010
(Microsoft Corporation, Redmond, WA, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Meteorin is upregulated in reactive
astrocytes following PT insult
To determine the functions of meteorin in reactive
gliosis, the present study examined its expression in reactive
astrocytes using immunofluorescent staining. The PT ischemia mouse
model was used as a brain injury model, in which photochemical
occlusion of the irradiated vessels with secondary tissue ischemia
is induced by photosensitive dyes like rose-bengal and a local
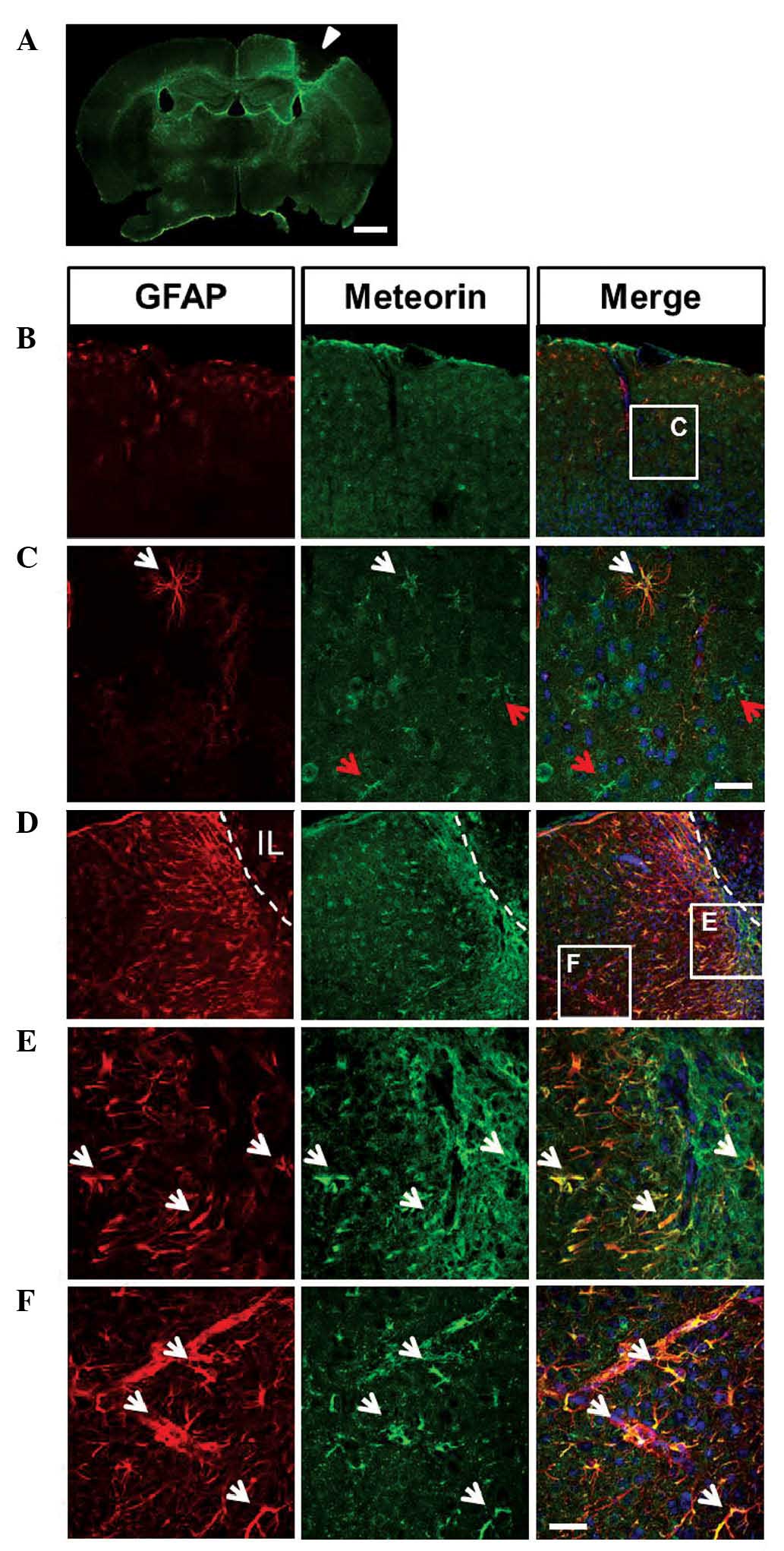
irradiation of cold light through the skull (19). At 7 days following PT induction,
infarct lesions were observed and a glial scar surrounding the
lesions had formed, as revealed by GFAP staining (Fig. 1A). In the cortex of the
contralateral hemisphere, in which the majority of astrocytes are
at a resting state, only a subset of astrocytes, predominantly
beneath the meningeal epithelium or surrounding blood vessels
exhibited GFAP immunoreactivity. Double immunostaining of GFAP and
meteorin demonstrated that, not only GFAP-positive, but also
GFAP-negative astrocytes exhibited weak expression levels of
meteorin (Fig. 1B and C). This
pattern of meteorin staining in the normal brain was concordant
with the findings of our previous study and of others (11,13,17).
By contrast, a marked increase in the number of
GFAP-positive cells was observed in the cortex exhibiting infarct
lesions, and these cells exhibited significantly more marked GFAP
staining (Fig. 1A and D–F).
Furthermore, a typical glial scar formed at the edge of the infarct
lesion, where the GFAP-positive cells had built a dense network.
The meteorin staining pattern in the infarct side was similar to
that of GFAP, being more marked in the glial scar region and
exhibiting a gradual decrease in the areas distant from the infarct
lesion (Fig. 1D–F). To further
confirm the upregulation of meteorin induced by PT, the cortex of
the infracted hemisphere and the contralateral hemisphere were
dissected, and total RNAs were extracted to compare the mRNA
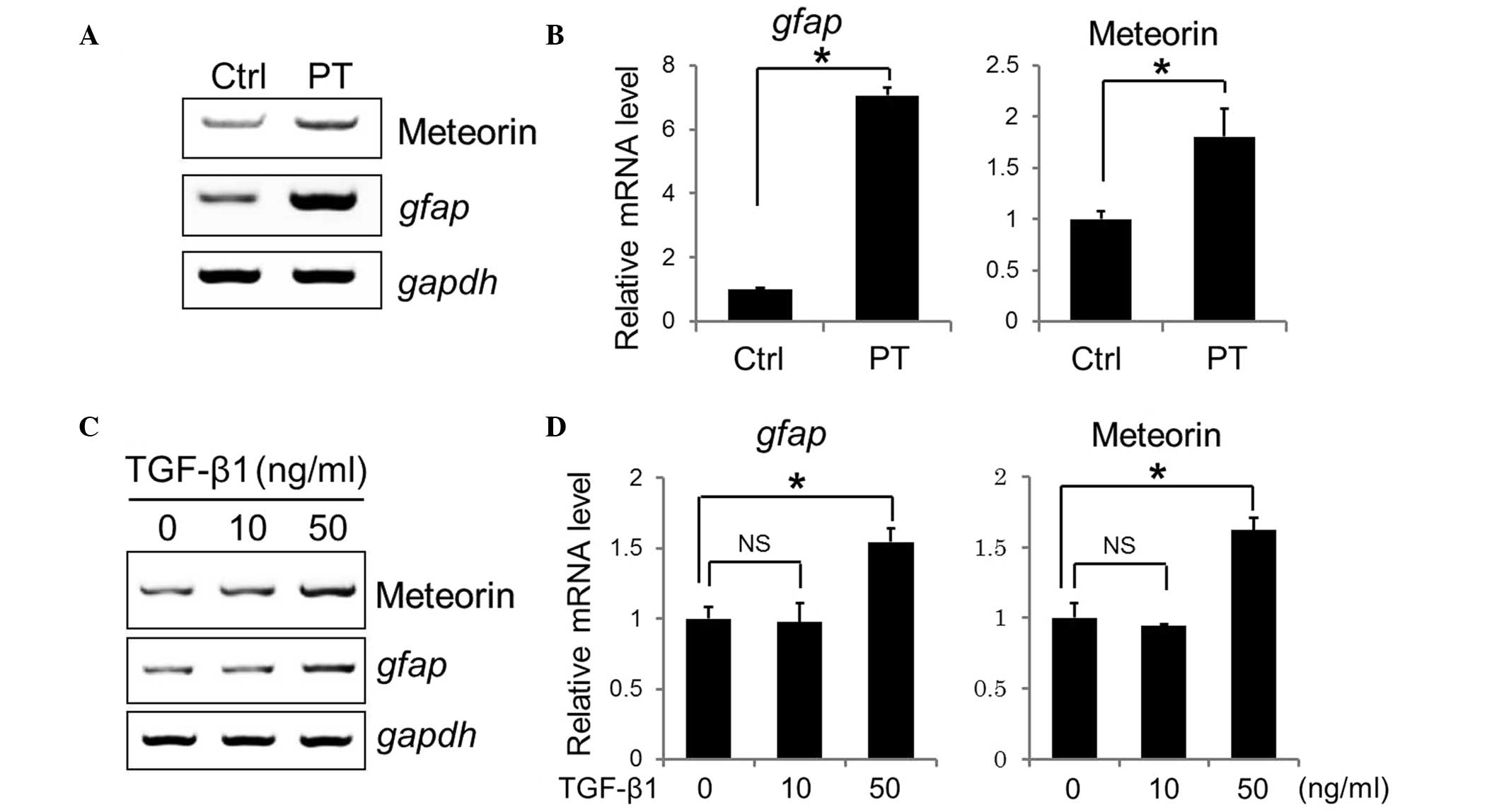
expression levels of meteorin and Gfap. RT-qPCR
revealed a 1.7-fold increase in the expression of meteorin
and a 7-fold increase in Gfap (Fig. 2A and B). These data indicated that
the expression of meteorin was increased in the reactive
astrocytes, activated by PT insult.
Expression of meteorin is increased in
response to TGF-β stimulation in vitro
The activation of astrocytes can be triggered by
various factors, including cytokines and nitric oxide, which are
produced by microglia and other immune cells in infarct lesions
(7). To determine whether the
in vitro stimulation of astrocyte activation leads to
changes in the expression of meteorin, primary mouse cortex
astrocytes were cultured and treated with increasing doses of
TGF-β1, which is a well-known reactive gliosis-promoting factor
(20). Treatment with recombinant
TGF-β1 at a concentration of 50 ng/ml resulted in ~1.5-fold and
1.7-fold increases in the mRNA expression levels of Gfap and
meteorin, respectively. However, no effects were observed
following treatment of the cells with 10 ng/ml TGF-β1 (Fig. 2C and D).
Meteorin activates different signaling
pathways in astrocytes and NSCs
Our previous study demonstrated that the Jak-STAT3
pathway is a downstream signaling unit of meteorin in neural
progenitor cell differentiation (12). Therefore, the present study aimed
to analyze the effects of recombinant meteorin treatment on
astrocyte activation in comparison to its effects on NSCs.
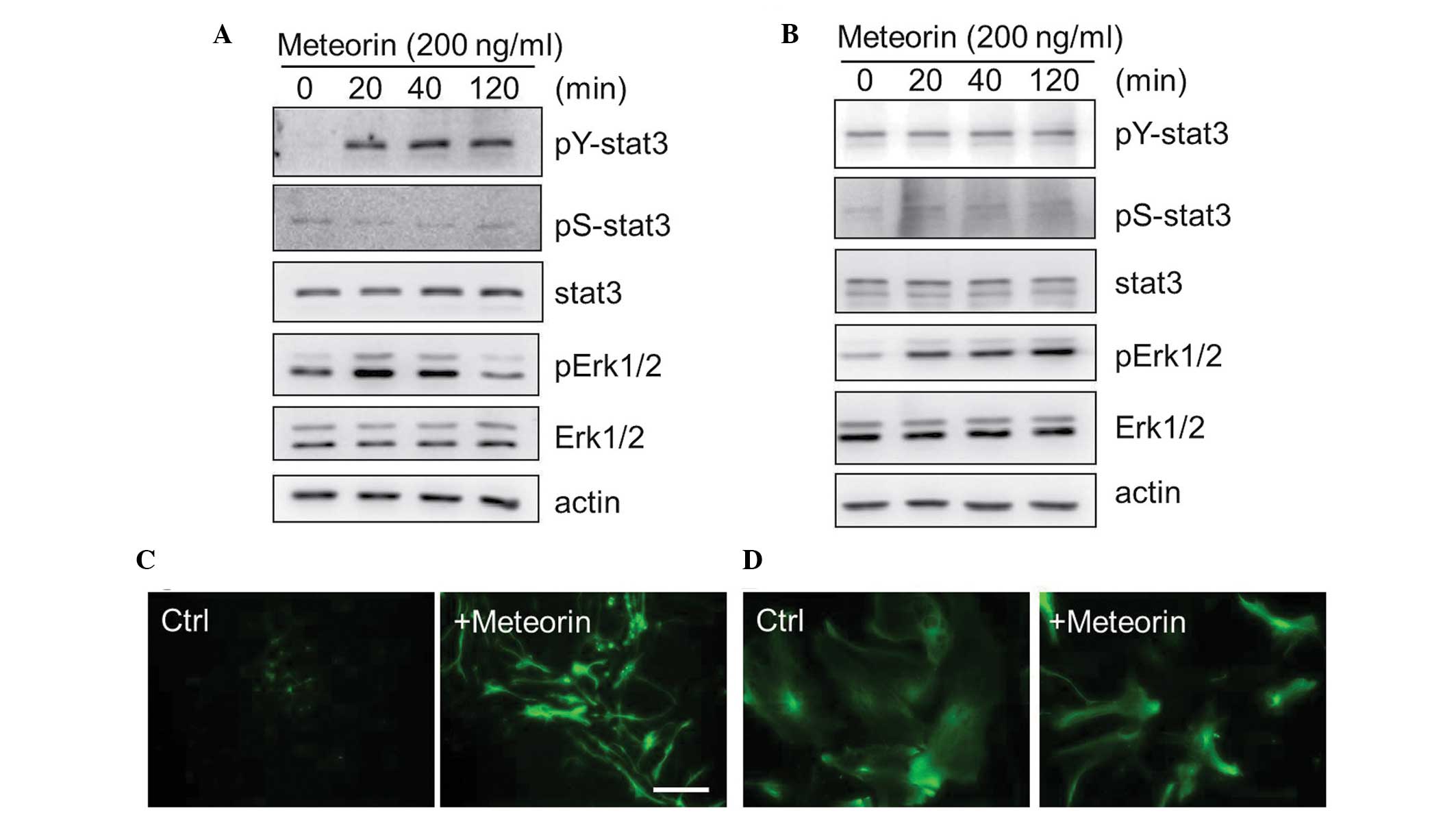
Concordant with the findings of our previous report, treatment of
the NSCs, from embryonic mouse brain tissues cultured with
recombinant meteorin (200 ng/ml) induced the tyrosine
phosphorylation of STAT3 and activation of extracellular
signal-regulated kinase (Erk)1/2, as early as 20 min after
treatment (Fig. 3A). The
phosphorylation of STAT3 by meteorin was not detected in the
astrocytes, although subsequent Erk1/2 activation was observed
(Fig. 3B). The NSCs and astrocytes
were also treated with recombinant meteorin for 3 days, followed by
GFAP staining, to determine whether meteorin treatment increased
the expression of GFAP. Treatment of NSCs with meteorin resulted in
an increase in the number of GFAP-positive cells, possibly due to
hyperactivation of STAT3 signaling (Fig. 3C). However, no significant changes
in GFAP staining were observed in the astrocyte cultures treated
with the same dose of meteorin (Fig.
3D). These results indicated that meteorin activated different
signaling pathways depending on the cell type, but appears unlikely
to act as an inducer of reactive gliosis.
Silencing the expression of meteorin
results in upregulation of reactive astrocyte markers
To further analyze the role of meteorin in astrocyte
activation, the gene expression of meteorin was silenced using
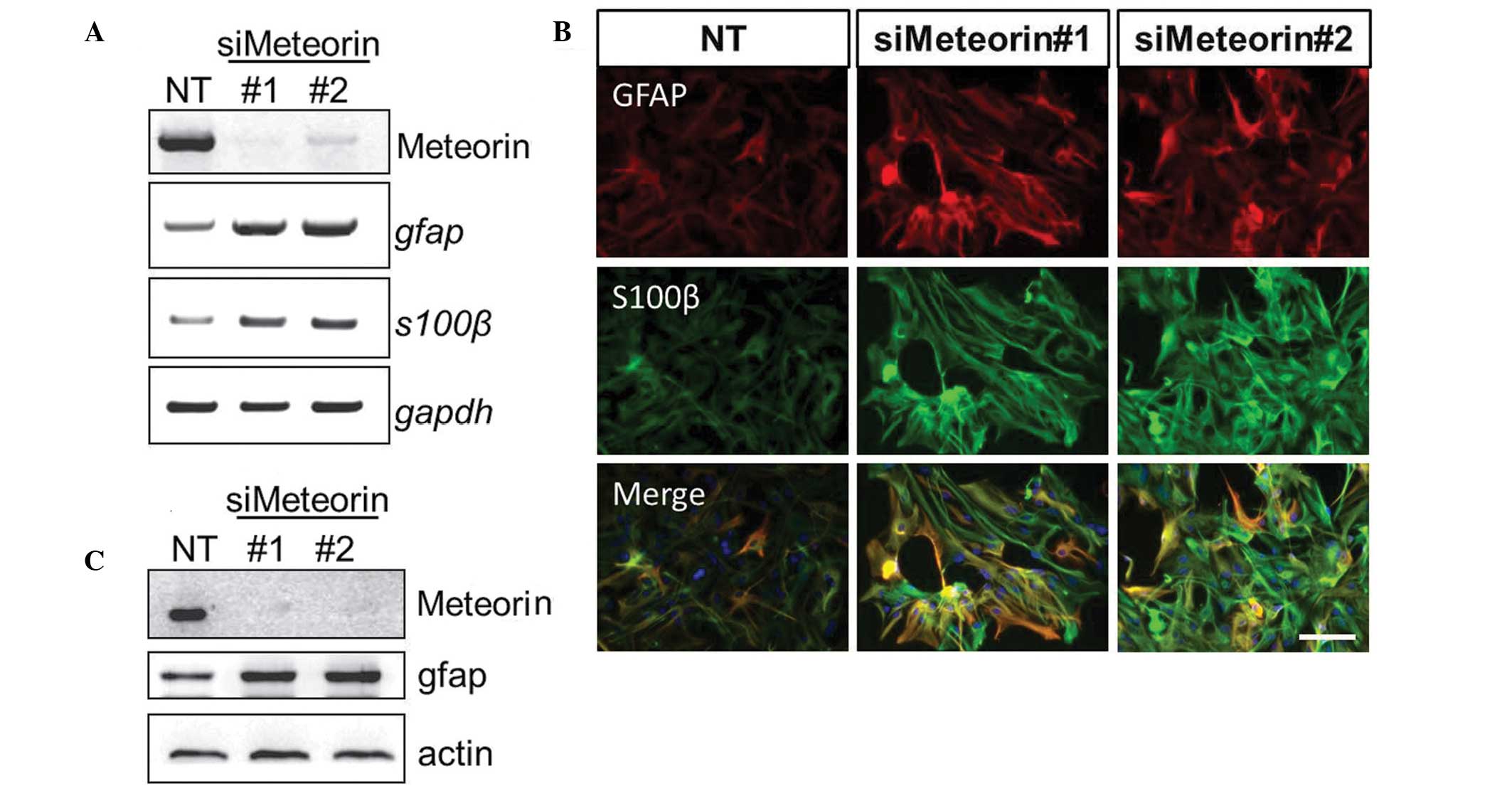
siRNAs specifically targeting mouse meteorin. Efficient knockdown
of the expression of metorin in astrocytes using two different
siRNAs was confirmed by RT-qPCR and western blotting. A correlation
between the expression of meteorin and astrocyte activation was
determined by comparing the expression levels of GFAP and S100β.
The two meteorin-targeting siRNAs resulted in increased mRNA and
protein expression levels of GFAP and S100β, determined by RT-qPCR
and western blotting, respectively (Fig. 4A and C). In addition,
immunocytochemical staining for GFAP and S100β was more marked in
the astrocytes transfected with meteorin-targeting siRNAs (Fig. 4B). These results suggested the
possible function of meteorin in a negative feedback loop in
reactive gliosis, during which glial activation is resolved and
cells revert to their resting state.
Discussion
The present study analyzed the expression profile
and functions of meteorin in reactive gliosis in the mouse brain.
The results demonstrated: i) Meteorin was expressed in cortical
astrocytes and its expression was upregulated in reactive
astrocytes; ii) exogenous application of meteorin to astrocytes did
not activate STAT3 signaling; and iii) silencing the expression of
meteorin in astrocytes led to the upregulation of reactive
astrocyte markers.
In the majority of CNS injuries, astrocytes undergo
reactive gliosis, which can be beneficial and detrimental. Although
a physical barrier, by formation of a glial scar, is the primary
defense against damage, the beneficial effects of reactive gliosis
are also mediated by the secretion of soluble factors (20). For example, the expression of
ciliary neurotrophic factor is known to be increased in reactive
astrocytes following ischemic insult, and its neuroprotective
effects have been demonstrated in a wide range of animal models of
CNS injury (21–23).
It is noteworthy that meteorin has been reported to
have neuroprotective effects in various neuropathologiacal models
(14,15). A possible scenario, based on
previous studies (14,15,20)
and the present study, is that cytokines, including TGF-β1, trigger
reactive gliosis to promote the production of meteorin by
astrocytes, and meteorin subsequently acts on neurons to regenerate
axons in a paracrine manner. In addition, meteorin may signal to
astrocytes in an autocrine manner as a negative feedback factor,
which in turn leads to the resolution of reactive gliosis. Notably,
one of the beneficial effects of reactive gliosis is to restore the
BBB following brain injury (24,25),
and our previous study identified meteorin as a maturation factor
for brain vascular development (13). Therefore, analyzing the effects of
meteorin administration in vivo following brain injury, in
terms of neuroprotection, reactive gliosis and BBB integrity is
worthwile.
Since the first report regarding meteorin in 2004
(11), numerous lines of
investigation by independent groups have uncovered its novel
functions and characteristics (11–16).
However, the cellular receptor(s) of meteorin remain to be
elucidated. In our previous study, the Jak-STAT3 pathway was found
to be involved in the downstream signaling of meteorin in NSC
differentiation. In the present study, meteorin did not activate
the same pathway in astrocytes, indicating another layer of
complexity in meteorin signaling. One possible explanation is that
there is more than one meteorin receptor, which is differentially
expressed in distinct cell types and they activate distinct
signaling pathways; however, additional investigations are required
to confirm this hypothesis.
Acknowledgments
The present study was supported by the Global
Research Laboratory Program (grant no. 2011-0021874), the Global
Core Research Center Program (grant no. 2011-0030001), the National
Research Foundation grant, funded by the Ministry of Science, ICT,
and Future Planning ((grant no. 2013-036038)) and the Basic Science
Research Program through the NRF of Korea, funded by the Ministry
of Education (grant no. 2013R1A1A2058956).
References
|
1
|
Volterra A and Meldolesi J: Astrocytes,
from brain glue to communication elements: The revolution
continues. Nat Rev Neurosci. 6:626–640. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iadecola C and Nedergaard M: Glial
regulation of the cerebral microvasculature. Nat Neurosci.
10:1369–1376. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamby ME and Sofroniew MV: Reactive
astrocytes as therapeutic targets for CNS disorders.
Neurotherapeutics. 7:494–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seifert G, Schilling K and Steinhäuser C:
Astrocyte dysfunction in neurological disorders: A molecular
perspective. Nat Rev Neurosci. 7:194–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abbott NJ, Rönnbäck L and Hansson E:
Astrocyte-endothelial interactions at the blood-brain barrier. Nat
Rev Neurosci. 7:41–53. 2006. View
Article : Google Scholar
|
|
6
|
Sofroniew MV: Reactive astrocytes in
neural repair and protection. Neuroscientist. 11:400–407. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sofroniew MV: Molecular dissection of
reactive astrogliosis and glial scar formation. Trends Neurosci.
32:638–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silver J and Miller JH: Regeneration
beyond the glial scar. Nat Rev Neurosci. 5:146–156. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Giorgio FP, Carrasco MA, Siao MC,
Maniatis T and Eggan K: Non-cell autonomous effect of glia on motor
neurons in an embryonic stem cell-based ALS model. Nat Neurosci.
10:608–614. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagai M, Re DB, Nagata T, et al:
Astrocytes expressing ALS-linked mutated SOD1 release factors
selectively toxic to motor neurons. Nat Neurosci. 10:615–622. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishino J, Yamashita K, Hashiguchi H,
Fujii H, Shimazaki T and Hamada H: Meteorin: A secreted protein
that regulates glial cell differentiation and promotes axonal
extension. EMBO J. 23:1998–2008. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HS, Han J, Lee SH, Park JA and Kim KW:
Meteorin promotes the formation of GFAP-positive glia via
activation of the Jak-STAT3 pathway. J Cell Sci. 123:1959–1968.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park JA, Lee HS, Ko KJ, et al: Meteorin
regulates angiogenesis at the gliovascular interface. Glia.
56:247–258. 2008. View Article : Google Scholar
|
|
14
|
Tornøe J, Torp M, Jørgensen JR, et al:
Encapsulated cell-based biodelivery of meteorin is neuroprotective
in the quinolinic acid rat model of neurodegenerative disease.
Restor Neurol Neurosci. 30:225–236. 2012.PubMed/NCBI
|
|
15
|
Jørgensen JR, Xu XJ, Arnold HM, et al:
Meteorin reverses hypersensitivity in rat models of neuropathic
pain. Exp Neurol. 237:260–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, Andrade N, Torp M, et al: Meteorin
is a chemokinetic factor in neuroblast migration and promotes
stroke-induced striatal neurogenesis. J Cereb Blood Flow Metab.
32:387–398. 2012. View Article : Google Scholar :
|
|
17
|
Jørgensen JR, Thompson L, Fjord-Larsen L,
et al: Characterization of Meteorin-an evolutionary conserved
neurotrophic factor. J Mol Neurosci. 39:104–116. 2009. View Article : Google Scholar
|
|
18
|
Cha JH, Wee HJ, Seo JH, et al: AKAP12
mediates barrier functions of fibrotic scars during CNS repair.
PLoS One. 9:e946952014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watson BD, Dietrich WD, Busto R, Wachtel
MS and Ginsberg MD: Induction of reproducible brain infarction by
photochemically initiated thrombosis. Ann Neurol. 17:497–504. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
John GR, Lee SC and Brosnan CF: Cytokines:
Powerful regulators of glial cell activation. Neuroscientist.
9:10–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beurrier C, Faideau M, Bennouar KE, et al:
Ciliary neurotrophic factor protects striatal neurons against
excitotoxicity by enhancing glial glutamate uptake. PLoS One.
5:e85502010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang M, He D, Zhang F, et al:
Antineuroinflammatory and neurotrophic effects of CNTF and C16
peptide in an acute experimental autoimmune encephalomyelitis rat
model. Front Neuroanat. 7:442013. View Article : Google Scholar
|
|
23
|
Rhee KD, Nusinowitz S, Chao K, Yu F, Bok D
and Yang XJ: CNTF-mediated protection of photoreceptors requires
initial activation of the cytokine receptor gp130 in Müller glial
cells. Proc Natl Acad Sci USA. 110:E4520–E4529. 2013. View Article : Google Scholar
|
|
24
|
Bush TG, Puvanachandra N, Horner CH, et
al: Leukocyte infiltration, neuronal degeneration and neurite
outgrowth after ablation of scar-forming, reactive astrocytes in
adult transgenic mice. Neuron. 23:297–308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Faulkner JR, Herrmann JE, Woo MJ, Tansey
KE, Doan NB and Sofroniew MV: Reactive astrocytes protect tissue
and preserve function after spinal cord injury. J Neurosci.
24:2143–2155. 2004. View Article : Google Scholar : PubMed/NCBI
|