Introduction
Bladder cancer is an important issue globally. Its
incidence ranks ninth among malignant tumors, of which >90% of
cases are transitional cell carcinomas (TCCs) (1). In China, bladder cancer is the most
common type of urinary system tumor. Furthermore, the recurrence
rate of bladder cancer is 60–70%, and 11% of patients who relapse
progress to metastatic cancer (2).
The occurrence of bladder cancer is the result of
the interaction between genetic and environmental factors, and is
associated with a number of different genes (3). Two of the most significant risk
factors for bladder cancer are smoking and exposure to chemicals,
such as aromatic amines (3). The
formation of DNA adducts in response to aromatic amine chemical
exposure, results in transitional mutations, which may be
associated with the development of bladder carcinogenesis (4). Liver enzymes, such as cytochrome p450
1A2 (CYP1A2), N-acetyltransferase 2 (NAT2) and glutathione
S-transferase M1 (GSTM1) affect the formation of these DNA adducts
(5). Cigarette smokers with slow
NAT2/rapid CYP1A2 phenotypes are more likely to develop bladder
cancer than those with rapid NAT2/slow CYP1A2 phenotypes (6). Smokers who are homozygous for
deletions in the detoxifying enzyme, GSTM1, are at a 1.8 fold
greater risk of developing bladder cancer than those with 1–2
copies of GSTM1 (7).
Recent studies have demonstrated that a number of
microRNAs (miRNAs), which are involved in the biological regulation
process of tumor cells, indirectly act as proto-oncogenes and
anti-oncogenes, and are associated with tumor initiation and
development (8). Using
oligonucleotide microarray analysis, Chiyomaru et al
(9) transfected a bladder cancer
cell line with miRNA-145/miRNA-133a, and demonstrated that the
expression of ~200 genes decreased. The most marked downregulation
was observed in beam protein 1 (FSCN1) gene. Ichimi et al
(10) demonstrated that keratin 7
expression was greater in a bladder cancer cell line transfected
with miRNA-30a-3p/-133a/-199a, which inhibited tumor cell growth,
compared with a control cell line. A separate study showed that the
ratios of miRNA-126:miRNA-152 and miRNA-182:miRNA-152 in urine, may
be useful markers of bladder cancer in asymptomatic patients, with
high sensitivity and specificity (11).
A number of tumor suppressor genes have been
detected in bladder cancer tissues. The retinoblastoma tumor
suppressor gene encodes a nuclear phosphoprotein (pRb), which acts
as a cell cycle regulator. Unphosphorylated pRb influences tumors
by negatively regulating and binding with E2F, which is a protein
transciption factor (12).
Phosphorylated pRb is not able to bind with E2F, and Rb gene
mutations were shown to be <30% in bladder cancer samples,
compared with control samples (13). The p53 tumor suppressor gene, which
encodes a 53kDa transcription factor, is associated with DNA repair
and apoptosis. p53 expression was found to be upregulated in 50% of
locally invasive TCCs (14),
resulting in a 2-fold increase in bladder cancer mortality rates
(15). Chromosome 9 is the only
chromosomal aberration at the initiation of the disease, and is not
associated with disease progression (16). Multiple tumor suppressor 1 on
chromosome 9 encodes for p16 and cyclin-dependent kinase inhibitor
2A (CDKN2), which have been previously identified to inhibit
cyclin-dependent kinase 4 (17).
Although a number of studies have been conducted in order to
investigate bladder cancer, the mechanisms underlying this disease
remain unclear.
In the present study, DE-miRNAs and DEGs associated
with bladder cancer were identified. Target genes of DE-miRNAs were
screened. The associations of the DEGs were analyzed using Search
Tool for the Retrieval of Interacting Genes/Proteins (STRING) and
interaction networks were constructed using Cytoscape. Furthermore,
functional and pathway enrichment analyses were conducted for the
DEGs from the interaction network.
Materials and methods
Microarray data
The GSE40355 expression profile was obtained from
the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), which was based on
the platforms of GPL8227-Agilent-019118 human miRNA microarray 2.0
G4470B (miRNA ID version) and GPL13497-Agilent-026652 whole human
genome microarray 4×44K v2 (probe name version). This dataset was
deposited by Hecker et al (18). Using healthy bladder tissue samples
(n=8) and urothelial carcinoma samples (low-grade, n=8; high-grade,
n=8), miRNA and mRNA microarray expression profiling analyses were
performed.
DE-miRNAs and DEGs screening
miRNAs and genes in the probe-level data from
Affymetrix CEL files were matched and a Log2 conversion was then
conducted (19). Probes without
corresponding gene symbols were filtered. The limma package in R
was used to identify DEGs of low- and high-grade bladder cancer
samples, compared with healthy bladder tissue samples (20). The Benjamin and Hochberg method in
multtest package was used to adjust the raw P-values into false
discovery rate (FDR) values (21).
FDR <0.01 and |logFC| >1 were used as cut-off criteria.
Comparison of DE-miRNAs and DEGs
DE-miRNAs were compared with DEGs screened from low-
and high-grade bladder cancer samples, compared with healthy
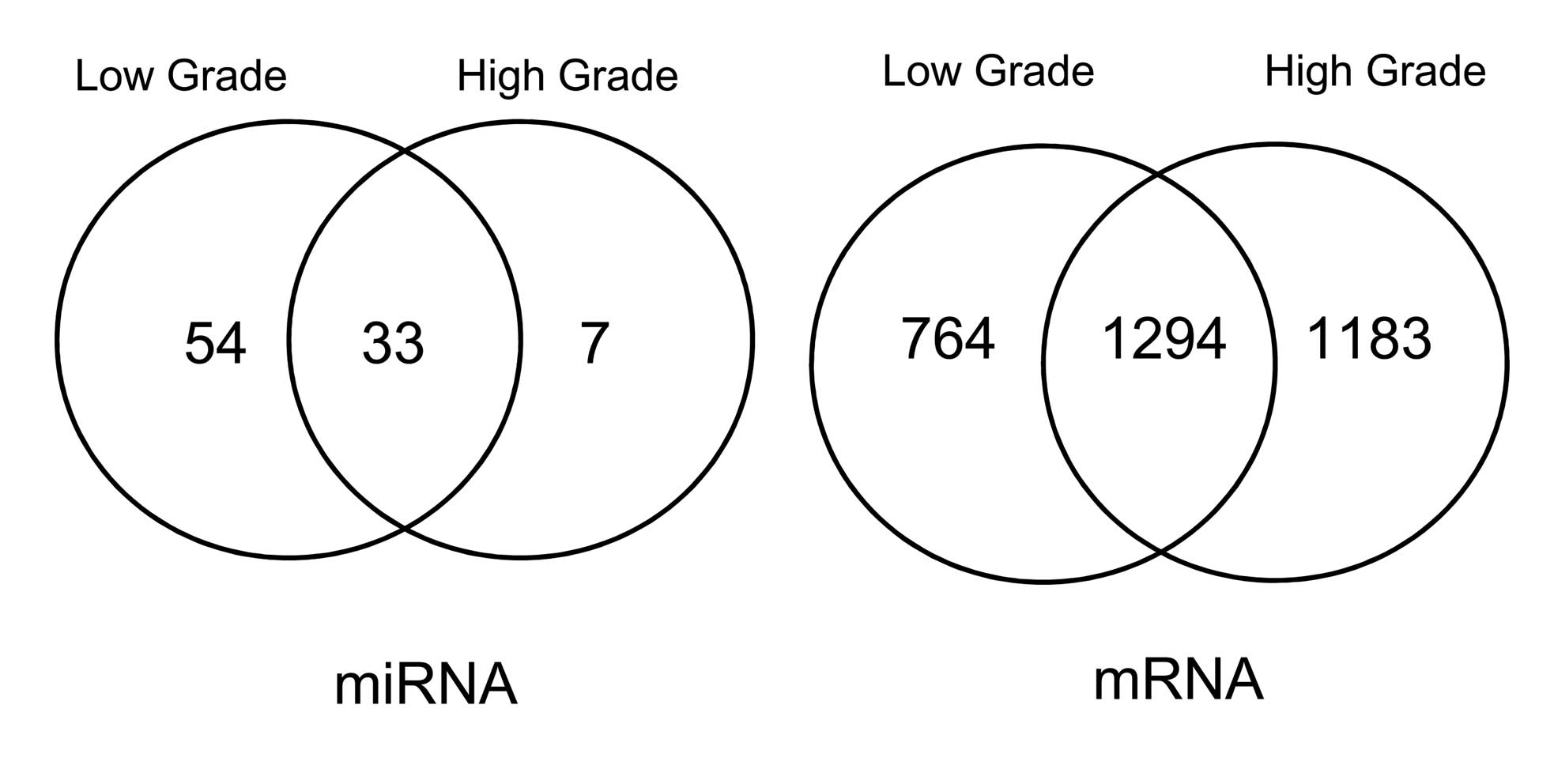
bladder tissue samples. The results are represented using a Venn
diagram.
Obtaining DE-miRNA target genes
Each miRNA has a number of corresponding target
genes. In order to elucidate reliable predicted target genes, two
miRNA databases, including miRDB (http://mirdb.org/miRDB/) and microRNA.org (http://www.microrna.org), were used. Only the target
genes that were identified by both databases were used as target
genes in the present study. MiRDB is an online database for animal
microRNA target prediction and functional annotations (22,23).
microRNA.org is a software and database developed by
researchers from the Memorial Sloan-Kettering cancer research
center (24). Following target
gene prediction, DEGs were mapped to the target gene set and target
genes with significantly differential expression, were
screened.
Network analysis
STRING online software was used to investigate
associations between the DEGs and to construct the interaction
network (25). Based on the
interactions between DE-miRNAs and DEGs, an interaction network was
constructed and visualized using Cytoscape (26).
Functional and pathway enrichment
analysis
WebGestalt is used for gene set enrichment pathway
analysis and consists of four modules: Information retrieval; gene
set management; statistics; and organization/visualization
(27,28). Functional and pathway enrichment
analyses were performed for the genes in the interaction network.
P<0.05 was considered to indicate a statistically significant
difference. The raw P-values were adjusted using the Benjamin and
Hochberg method in multtest package.
Results
DE-miRNAs and DEGs
By performing the transformation of probe IDs into
miRNAs or mRNAs, a total of 721 effective human miRNAs (those
converted from corresponding probe IDs) and 29,833 genes were
obtained. In the miRNA and mRNA expression profiles, the following
were screened: 87 DE-miRNAs and 2,058 DEGs in low-grade bladder
cancer samples compared with healthy bladder samples, and 40
DE-miRNAs and 2,477 DEGs in high-grade bladder cancer samples
compared with healthy bladder samples
Comparison of DE-miRNAs and DEGs
DE-miRNAs and DEGs screened from low- and high-grade
bladder cancer samples were compared (Fig. 1). 33 DE-miRNAs identified in both
low- and high-grade bladder cancer samples are listed in Table I, while 1,294 DEGs were identified
in both low- and high-grade bladder cancer samples, compared with
healthy samples. The trends of expression for the 33 DE-miRNAs in
low and high-grade bladder cancer samples were similar, and the
differences in the high-grade bladder cancer samples were more
marked than those in the low-grade bladder cancer samples.
 | Table IDE-miRNAs in low- and high-grade
bladder cancer. |
Table I
DE-miRNAs in low- and high-grade
bladder cancer.
| miR | Low-grade | High-grade |
|---|
| hsa-miR-133b | −10.2175 | −10.2837 |
| hsa-miR-133a | −9.05375 | −10.12 |
| hsa-miR-1 | −8.865 | −9.7912 |
| hsa-miR-490-3p | −8.99875 | −9.095 |
| hsa-miR-139-5p | −8.4075 | −8.6137 |
| hsa-miR-204 | −8.42 | −8.5162 |
| hsa-miR-490-5p | −7.065 | −7.3625 |
| hsa-miR-381 | −6.3325 | −7.3325 |
| hsa-miR-127-3p | −6.7525 | −7.1162 |
| hsa-miR-502-3p | −5.64375 | −6.655 |
| hsa-miR-28-3p | −5.41 | −6.225 |
| hsa-miR-145 | −5.265 | −5.8162 |
| hsa-miR-143 | −4.885 | −5.4862 |
| hsa-miR-125b | −4.4375 | −4.8337 |
| hsa-miR-338-3p | −6.56375 | −4.78 |
|
hsa-miR-199a-5p | −3.1025 | −3.4625 |
| hsa-miR-497 | −2.68 | −3.0137 |
| hsa-miR-30a | −2.325 | −2.4425 |
| hsa-miR-28-5p | −1.8975 | −1.9475 |
| hsa-miR-23b | −2.0875 | −1.9138 |
| hsa-miR-26a | −1.49375 | −1.1363 |
| hsa-miR-106b | 2.37875 | 1.5037 |
| hsa-miR-19a | 3.00625 | 1.6963 |
| hsa-miR-425 | 2.8275 | 2.2575 |
| hsa-miR-494 | 3.20875 | 2.2887 |
| hsa-miR-210 | 3.04 | 3.2862 |
| hsa-miR-200c | 3.97375 | 4.0837 |
| hsa-miR-200b | 4.20375 | 4.415 |
| hsa-miR-200a | 4.15375 | 4.7762 |
| hsa-miR-429 | 4.68 | 4.8925 |
| hsa-miR-141 | 4.6225 | 4.9112 |
| hsa-miR-183 | 9.90375 | 10.3038 |
| hsa-miR-96 | 9.84 | 10.4275 |
DE-miRNA target genes
Predicted target genes were obtained from the miRDB
and microRNA.org databases. In order to improve the
reliability of the predicted target genes, only the target genes
predicted from both databases were selected for further analysis.
Subsequently, DEGs were mapped to the target genes. The DE-target
genes and their corresponding DE-miRNAs are listed in Table II.
 | Table IIDEGs targeted by DE-miRNAs. |
Table II
DEGs targeted by DE-miRNAs.
| miR | Target gene(s) |
|---|
| hsa-miR-1 | NETO2, AXL, BDNF,
LASP1
GNPDA2, TDP1, POLR2K, POGK
OAT, CHST11, CNN3, TPM4
ANKRD29, TIMP3, FBLN2
RABGAP1L |
| hsa-miR-106b | E2F1 |
| hsa-miR-125b | ERBB2, ERBB3,
CDKN2A |
| hsa-miR-133a | KRT7 |
| hsa-miR-143 | DNMT3A |
| hsa-miR-145 | KRT7 |
| hsa-miR-19a | ESR1 |
| hsa-miR-19a | NR4A2 |
| hsa-miR-200a | ZEB1, ZEB2 |
| hsa-miR-200b | ZEB2, ZEB1 |
| hsa-miR-200c | ZEB1 |
| hsa-miR-26a | EZH2 |
| hsa-miR-338-3p | UBE2Q1 |
Interaction network construction
Using the STRING online software, the associations
between DEGs were analyzed, and an interaction network was
constructed. The regulatory associations between miRNAs and target
genes combined with interactions of the DE-target genes were used
for network construction using Cytoscape software (Fig. 2).
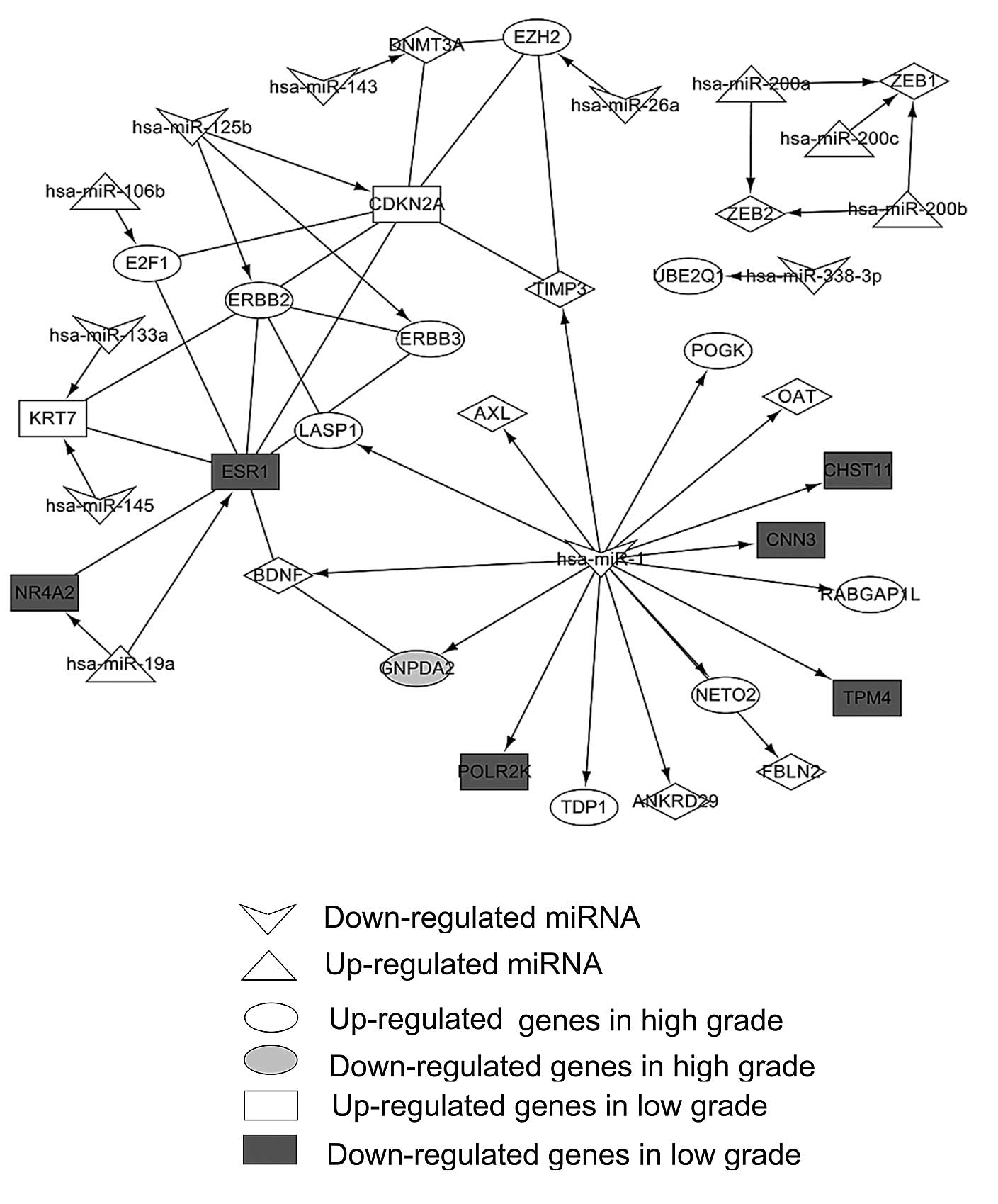 | Figure 2Interaction network of differentially
expressed miRNAs and their differentially expressed target genes.
Diamonds represent common differential genes from the low- and
high-grade bladder cancer groups. miRNA, microRNA; NETO2,
neuropilin and tolloid (TLL)-like 2; BDNF, brain-derived
neurotrophic factor; LASP1, LIM and SH3 Protein 1; GNPDA2,
glucosamine-6-phosphate deaminase 2; TDP1, tyrosyl-DNA
phosphodiesterase 1; POLR2K, polymerase (RNA) II (DNA directed)
polypeptide K; POGK; pogo transposable element with KRAB domain;
OAT, ornithine aminotransferase; CHST11, carbohydrate (chondroitin
4) culfotransferase 11; CNN3, calponin 3, acidic; TPM4, tropomyosin
4; ANKRD29, ankyrin repeat domain 29; TIMP3, tissue inhibitor of
metalloproteinases 3; FBLN2, fibulin 2; RABGAP1L, RAB GTPase
activating protein 1-like; E2F1, E2F transcription factor 1; ERBB,
V-Erb-B2 avian erythroblastic leukemia viral oncogene homolog;
CDKN2A, cyclin-dependent kinase inhibitor 2A; KRT7, keratin 7;
ESR1, estrogen receptor 1; NR4A2, nuclear receptor subfamily 4,
group A, member 2; ZEB, zinc finger E-box binding homeobox; UBE2Q1,
ubiquitin-conjugating enzyme E2Q family member 1; EZH2, enhancer of
zeste homolog 2. |
Functional and pathway enrichment
analysis of genes in the interaction network
WebGestalt was used in order to conduct functional
and enrichment pathway enrichment analysis of DEGs in the
interaction network. The 10 most significantly enriched functions
are listed in Table III, which
includes function of negative regulation of apoptosis [adjusted
P-value (adj p)=4.12E-04], negative regulation of programmed cell
death (adj p=4.39E-04) and negative regulation of cell death (adj
p=4.44E-04). Three Kyoto encyclopedia of genes and genomes
biological pathways were enriched: hsa05219 bladder cancer (adj
p=0.003494), hsa05223 non-small cell lung cancer (adj p=0.005726)
and hsa05212 pancreatic cancer (adj p=0.010013), which included
three DE-target genes [E2F transcription factor 1 (E2F1), CDKN2A
and V-Erb-B2 avian erythroblastic leukemia viral oncogene homolog 2
(ERBB2); Table IV]. E2F1 was
targeted by hsa-miR-106b, and CDKN2A and ERBB2 were targeted by
hsa-miR-125b.
 | Table IIITop 10 significantly enriched
functions for the differentially expressed target genes in the
interaction network. |
Table III
Top 10 significantly enriched
functions for the differentially expressed target genes in the
interaction network.
| ID | Function | Count | Adjusted
P-value | Genes |
|---|
| GO:0043066 | Negative regulation
of apoptosis | 6 | 4.12E-04 | BDNF, ERBB3, ERBB2,
CHST11, NR4A2, ESR1 |
| GO:0043069 | Negative regulation
of programmed cell death | 6 | 4.39E-04 | BDNF, ERBB3, ERBB2,
CHST11, NR4A2, ESR1 |
| GO:0060548 | Negative regulation
of cell death | 6 | 4.44E-04 | BDNF, ERBB3, ERBB2,
CHST11, NR4A2, ESR1 |
| GO:0042981 | Regulation of
apoptosis | 8 | 4.77E-04 | BDNF, CDKN2A,
ERBB3, ERBB2, CHST11, NR4A2, ESR1, TIMP3 |
| GO:0043067 | Regulation of
programmed cell death | 8 | 5.06E-04 | BDNF, CDKN2A,
ERBB3, ERBB2, CHST11, NR4A2, ESR1, TIMP3 |
| GO:0010941 | Regulation of cell
death | 8 | 5.18E-04 | BDNF, CDKN2A,
ERBB3, ERBB2, CHST11, NR4A2, ESR1, TIMP3 |
| GO:0043523 | Regulation of
neuron apoptosis | 4 | 5.89E-04 | BDNF, ERBB3, NR4A2,
ESR1 |
| GO:0030155 | Regulation of cell
adhesion | 4 | 0.001985 | CDKN2A, ERBB3,
FBLN2, ERBB2 |
| GO:0006355 | Regulation of
transcription, DNA-dependent | 10 | 0.003174 | E2F1, DNMT3A,
CDKN2A, POGK, POLR2K, EZH2, NR4A2, ESR1, ZEB2, ZEB1 |
| GO:0033084 | Regulation of
immature T cell proliferation in the thymus | 2 | 0.003693 | CDKN2A, ERBB2 |
 | Table IVEnriched KEGG pathways for
differentially expressed target genes in the interaction
network. |
Table IV
Enriched KEGG pathways for
differentially expressed target genes in the interaction
network.
| ID | KEGG | Adjusted
P-value | Genes |
|---|
| hsa05219 | Bladder cancer | 0.003494 | E2F1, CDKN2A,
ERBB2 |
| hsa05223 | Non-small cell lung
cancer | 0.005726 | E2F1, CDKN2A,
ERBB2 |
| hsa05212 | Pancreatic
cancer | 0.010013 | E2F1, CDKN2A,
ERBB2 |
Discussion
In the present study, using bioinformatic methods,
significant DE-miRNAs in bladder cancer tissue samples and
DE-target genes from mRNA expression profiles were screened.
According to the functional analysis, the DEGs were most
significantly associated with cell apoptosis. Results of pathway
analysis suggested that E2F1, which is targeted by hsa-miR-106b,
and CDKN2A and ERBB2, which are targeted by hsa-miR-125b, were
shown to be involved in the bladder cancer pathway.
Neely et al (29) investigated the expression levels of
343 miRNAs in bladder cancer cells, and demonstrated that there
were 9 DE-miRNAs (miR-21, -31, -200a, -200c, -205, -373, -487b,
-498 and -503) between invasive bladder cancer and noninvasive
bladder cancer. Similarly, 94 DE-miRNAs were screened in the
present study, of which 33 were differentially expressed in low- as
well as high-grade bladder cancer samples. These included miR-200a
and -200c. Furthermore, studies have suggested that miR-200c
expression is associated with early stage T1 bladder tumor
progression. Furthermore, miR-200 and -205 loci are associated with
coordinated epigenetic repression in bladder cell lines and bladder
tumors (30). Therefore, miR-200a
and-200c may be involved in bladder cancer.
According to the present study, hsa-miR-125b targets
CDKN2A and ERBB2, which were differentially expressed in low- and
high-grade bladder cancer samples. The CDKN2A locus encodes the
tumor suppressor gene p16 and p14ARF, which induce cell
cycle arrest (31,32). Using RT-qPCR in order to detect
homozygous deletions, Berggren et al (33) demonstrated that CDKN2A/ARF
inactivation occurs in the early stages of TCC. ERBB2 is closely
associated with bladder cancer (34). In ~40% studies, membranous ERBB2
expression was found to be greater in bladder cancer samples
compared with healthy samples (35). miRNA-10a is overexpressed in
noninvasive bladder cancer, and miRNA-222 and-125b are
overexpressed in invasive bladder cancer (36). Therefore, hsa-miR-125b may be used
as a biomarker for the diagnosis of bladder cancer (36). Huang et al (37) found that miRNA-125b inhibits the
E2F3-cyclinA2 signaling pathway and suppresses bladder tumor cells
growth during G phase via the reduction of E2F3 expression.
Therefore, hsa-miR-125b downregulation may lead to bladder cancer
via the upregulation of CDKN2A and ERBB2.
In the present study, E2F1 targeted by hsa-miR-106b
was shown to participate in the bladder cancer pathway. E2F1 and
E2F3 are members of E2F transcription factors family, and they
combine with the retinoblastoma protein (pRB) tumor suppressor
(36). Additionally, miR-106a is
involved in the regulation of a number of genes, such as TGFBR2 and
RB, and controls cell cycle regulation, migration, angiogenesis and
apoptosis via a number of different pathways (38). Ivanovska et al (39) demonstrated that miR-106b promotes
cell cycle progression via the TP53-p21 pathway, via p21
suppression. Hershko et al (40) found that E2F1 may regulate the
expression of the pro-apoptotic BH3-only proteins, Noxa, Hrk/DP5,
p53-upregulated modulator of apoptosis and bcl-2 interacting
mediator of cell death. Furthermore, increased PUMA and Noxa
expression is associated with E2F1-induced apoptosis. E2F1
expression may induce oncogenic stress and promote premalignant
cell apoptosis, thereby inhibiting tumor development (41). The majority of the functions
enriched in the present study were associated with cell apoptosis.
Therefore, overexpression of E2F1, which is targeted by
hsa-miR-106b, may be one of the possible mechanisms underlying the
development of bladder cancer.
In conclusion, a number of DE-miRNAs and target DEGs
involved in bladder cancer were identified. In particular,
hsa-miR-106b and 125b may be involved in bladder cancer by
targeting E2F1, CDKN2A and ERBB2. Therefore, these miRNAs and genes
may be useful biomarkers of bladder cancer.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shariat SF, Casella R, Khoddami SM,
Hernandez G, Sulser T, Gasser TC and Lerner SP: Urine detection of
survivin is a sensitive marker for the noninvasive diagnosis of
bladder cancer. J Urol. 171:626–630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM: The global burden of urinary
bladder cancer. Scand J Urol Nephrol Suppl. 218:12–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kadlubar FF and Badawi AF: Genetic
susceptibility and carcinogen-DNA adduct formation in human urinary
bladder carcinogenesis. Toxicol Lett. 82–83:627–632. 1995.
View Article : Google Scholar
|
|
5
|
Jung I and Messing E: Molecular mechanisms
and pathways in bladder cancer development and progression. Cancer
Control. 7:325–334. 2000.PubMed/NCBI
|
|
6
|
Kaderlik KR and Kadlubar FF: Metabolic
polymorphisms and carcinogen-DNA adduct formation in human
populations. Pharmacogenetics. 5:S108–S117. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bell DA, Taylor JA, Paulson DF, Robertson
CN, Mohler JL and Lucier GW: Genetic risk and carcinogen exposure:
A common inherited defect of the carcinogen-metabolism gene
glutathione S-transferase M1 (GSTM1) that increases susceptibility
to bladder cancer. J Natl Cancer Inst. 85:1159–1164. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
9
|
Chiyomaru T, Enokida H, Tatarano S,
Kawahara K, Uchida Y, Nishiyama K, Fujimura L, Kikkawa N, Seki N
and Nakagawa M: miR-145 and miR-133a function as tumour suppressors
and directly regulate FSCN1 expression in bladder cancer. Br J
Cancer. 102:883–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ichimi T, Enokida H, Okuno Y, Kunimoto R,
Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama
K and Seki N: Identification of novel microRNA targets based on
microRNA signatures in bladder cancer. Int J Cancer. 125:345–352.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanke M, Hoefig K, Merz H, et al: A robust
methodology to study urine microRNA as tumor marker: microRNA 126
and microRNA 182 are related to urinary bladder cancer. Urol Oncol.
28:655–661. 2010. View Article : Google Scholar
|
|
12
|
Suzuki-Takahashi I, Kitagawa M, Saijo M,
Higashi H, Ogino H, Matsumoto H, Taya Y, Nishimura S and Okuyama A:
The interactions of E2F with pRB and with p107 are regulated via
the phosphorylation of pRB and p107 by a cyclin-dependent kinase.
Oncogene. 10:1691–1698. 1995.PubMed/NCBI
|
|
13
|
Cordon-Cardo C, Wartinger D, Petrylak D,
Dalbagni G, Fair WR, Fuks Z and Reuter VE: Altered expression of
the retinoblastoma gene product: Prognostic indicator in bladder
cancer. J Natl Cancer Inst. 84:1251–1256. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Habuchi T, Ogawa O, Kakehi Y, Ogura K,
Koshiba M, Sugiyama T and Yoshida O: Allelic loss of chromosome 17p
in urothelial cancer: Strong association with invasive phenotype. J
Urol. 148:1595–1599. 1992.PubMed/NCBI
|
|
15
|
Esrig D, Elmajian D, Groshen S, Freeman
JA, Stein JP, Chen SC, Nichols PW, Skinner DG, Jones PA and Cote
RJ: Accumulation of nuclear p53 and tumor progression in bladder
cancer. N Engl J Med. 331:1259–1264. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyao N, Tsai YC, Lerner SP, Olumi AF,
Spruck CH III, Gonzalez-Zulueta M, Nichols PW, Skinner DG and Jones
PA: Role of chromosome 9 in human bladder cancer. Cancer Res.
53:4066–4070. 1993.PubMed/NCBI
|
|
17
|
Kamb A, Gruis NA, Weaver-Feldhaus J, Liu
Q, Harshman K, Tavtigian SV, Stockert E, Day RS III, Johnson BE and
Skolnick MH: A cell cycle regulator potentially involved in genesis
of many tumor types. Science. 264:436–440. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hecker N, Stephan C, Mollenkopf HJ, Jung
K, Preissner R and Meyer HA: A new algorithm for integrated
analysis of miRNA-mRNA interactions based on individual
classification reveals insights into bladder cancer. PloS One.
8:e645432013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujita A, Sato JR, Rodrigues LO, Ferreira
CE and Sogayar MC: Evaluating different methods of microarray data
normalization. BMC Bioinformatics. 7:4692006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smyth GK: Limma: Linear models for
microarray data. Bioinformatics and computational biology solutions
using R and Bioconductor. Gentleman R, Carey V, Dudoit S, Irizarry
R and Huber W: Springer; New York: pp. 397–420. 2005
|
|
21
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. J R Static Soc B Stat Methodol. 1:289–300.
1995.
|
|
22
|
Wang X: miRDB: A microRNA target
prediction and functional annotation database with a wiki
interface. RNA. 14:1012–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X and El Naqa IM: Prediction of both
conserved and nonconserved microRNA targets in animals.
Bioinformatics. 24:325–332. 2008. View Article : Google Scholar
|
|
24
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource: Targets and expression.
Nucleic Acids Res. 36(Database): D149–D153. 2008. View Article : Google Scholar :
|
|
25
|
Szklarczyk D, Franceschini A, Kuhn M,
Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork
P, et al: The STRING database in 2011: Functional interaction
networks of proteins, globally integrated and scored. Nucleic Acids
Res. 39(Database): D561–D568. 2011. View Article : Google Scholar :
|
|
26
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar :
|
|
27
|
Zhang B, Kirov S and Snoddy J: WebGestalt:
An integrated system for exploring gene sets in various biological
contexts. Nucleic Acids Res. 33(Web Server): W741–W748. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duncan D, Prodduturi N and Zhang B:
WebGestalt2: An updated and expanded version of the Web-based Gene
Set Analysis Toolkit. BMC Bioinformatics. 11(Suppl 4): 102010.
View Article : Google Scholar
|
|
29
|
Neely LA, Rieger Christ KM, Neto BS, et
al: A microRNA expression ratio defining the invasive phenotype in
bladder tumors. Urol Oncol. 28:39–48. 2010. View Article : Google Scholar
|
|
30
|
Wiklund ED, Bramsen JB, Hulf T, Dyrskjøt
L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS,
Borre M, et al: Coordinated epigenetic repression of the miR-200
family and miR-205 in invasive bladder cancer. Int J Cancer.
128:1327–1334. 2011. View Article : Google Scholar
|
|
31
|
Haber DA: Splicing into senescence: The
curious case of p16 and p19ARF. Cell. 91:555–558. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lloyd AC: p53: Only ARF the story. Nat
Cell Biol. 2:E48–E50. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Berggren P, Kumar R, Sakano S, et al:
Detecting homozygous deletions in the
CDKN2A(p16(INK4a))/ARF(p14(ARF)) gene in urinary bladder cancer
using real-time quantitative PCR. Clin Cancer Res. 9:235–242.
2003.PubMed/NCBI
|
|
34
|
Cordon-Cardo C: Molecular alterations
associated with bladder cancer initiation and progression. Scand J
Urol Nephrol Suppl. 42(s218): 154–165. 2008. View Article : Google Scholar
|
|
35
|
Røtterud R, Nesland JM, Berner A and Fosså
SD: Expression of the epidermal growth factor receptor family in
normal and malignant urothelium. BJU Int. 95:1344–1350. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Veerla S, Lindgren D, Kvist A, Frigyesi A,
Staaf J, Persson H, Liedberg F, Chebil G, Gudjonsson S, Borg A, et
al: MiRNA expression in urothelial carcinomas: Important roles of
miR-10a, miR-222, miR-125b, miR-7 and miR-452 for tumor stage and
metastasis, and frequent homozygous losses of miR-31. Int J Cancer.
124:2236–2242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang L, Luo J, Cai Q, Pan Q, Zeng H, Guo
Z, Dong W, Huang J and Lin T: MicroRNA-125b suppresses the
development of bladder cancer by targeting E2F3. Int J Cancer.
128:1758–1769. 2011. View Article : Google Scholar
|
|
38
|
Blenkiron C and Miska EA: miRNAs in
cancer: Approaches, aetiology, diagnostics and therapy. Hum Mol
Genet. 16:R106–R113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ivanovska I, Ball AS, Diaz RL, et al:
MicroRNAs in the miR-106b family regulate p21/CDKN1A and promote
cell cycle progression. Mol Cell Biol. 28:2167–2174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hershko T and Ginsberg D: Up-regulation of
Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J
Biol Chem. 279:8627–8634. 2004. View Article : Google Scholar
|
|
41
|
Ginsberg D: E2F1 pathways to apoptosis.
FEBS Lett. 529:122–125. 2002. View Article : Google Scholar : PubMed/NCBI
|