Introduction
Meningiomas are the second most common type of tumor
of the primary central nervous system and accounts for 24–30% of
all reported brain tumors, with malignant meningiomas (WHO grade
III) accounting for 1–3% of meningiomas (1–3).
Invasive and malignant meningiomas present a significant
therapeutic challenge due to high rates of recurrence and invasion
into the surrounding bone, brain, neural and soft tissues (4,5).
Therefore, understanding the molecular mechanism of invasion may
assist in designing novel therapeutic approaches to reduce the
requirement for repeat surgery, decrease morbidity rates and
improve patient survival rates.
The HER-2 gene is a type of proto-oncogene, also
termed CerbB-2 or neu, is located on chromosome 17, encodes a 185
kDa transmembrane receptor tyrosine kinase (RTK), and is a member
of the epidermal growth factor receptor (EGFR) family, which
contains four homologous members: EGFR/HER-1, HER-2, HER-3 and
HER-4 (6). HER-2 receptors exhibit
functional redundancy with overlapping signaling pathways. HER-2 is
the preferred heterodimerization partner of all EGFR proteins, and
is important in the lateral transmission of signals between other
HER receptors (7). HER receptor
pathway-associated proteins are important in normal cells, as well
as in cancer cells. Activation of 185 kDa RTKs, which are
transmembrane receptors with an intrinsic ability to phosphorylate
tyrosine residues in their cytoplasmic domains, including
phosphatidylinositol 3-kinase (PI3k) and AKT, results in activation
of nuclear transcription factors that induce cell growth and
inhibit apoptosis (8). Therefore,
HER-2 signaling has been reported to induce several phenotypic
changes associated with more aggressive disease, including
increased cell motility, cell proliferation and/or invasive ability
(9).
Since the association between HER-2 gene
amplification and disease prognosis was demonstrated (10,11),
overexpression of the gene has been observed in a variety of
primary human carcinoma, including breast cancer (12,13),
gastric cancer (14,15), gastroesophageal adenocarcinomas
(16) and mucinous epithelial
ovarian cancer (17). Initially,
HER-2 was found to be upregulated in meningioma (18–21).
Subsequently, several studies have provided evidence that Her-2 is
important in meningiomas (19,20).
However, the association and mechanism between meningiomas and the
expression of HER-2 remain to be fully elucidated.
In the present study, the possible role of HER-2 in
cell proliferation, apoptosis and invasion of the IOMM-Lee human
malignant meningioma cell line was investigated. The results may
provide clues for further investigation of the mechanisms
underlying the carcinogenesis of malignant meningioma and provide a
candidate gene for meningioma therapy.
Materials and methods
Cell lines and cell culture
The IOMM-Lee human meningioma cell line was provided
by Dr Jensen and Dr Gillespie of the University of Utah (Salt Lake
City, USA). The cell line was routinely cultured in Dulbecco's
modified Eagle's medium (DMEM; GE Healthcare, Logan, UT, USA),
supplemented with 10% fetal bovine serum (FBS; Gibco-BRL, Carlsbad.
CA, USA), 100 U/ml streptomycin and 100 U/ml penicillin (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China), in a
humidified 37°C incubator containing 5% CO2.
Plasmids and transfection
Fragments of the short hairpin (sh) HER-2 (HER-2-sh)
and HER-2-overexpression sequence were obtained through NCBI
references from HanBio (Shanghai, China) and the NM_004448 GenBank
HER2 NCBI reference sequence, respectively, then the HER-2-sh and
HER-2-overexpression lentiviral vectors were constructed. Nonsense
sequence lentiviral vectors (NC-sh and NC-overexpression) were used
as negative controls. The HER-2-sh lentiviral vectors were
synthesized by HanBio (Shanghai, China) and the
HER-2-overxepression lentiviral vectors were synthesized by
GeneChem (Shanghai, China). The IOMM-Lee cells were transfected
with each group and puromycin was used to screen for stable cell
lines. Cells at 30–50% confluence were transfected with the
lentivirus at a multiplicity of infection of 10 with enhanced
infection solution (7 µg/ml; GeneChem) for 8 h and washed
with fresh medium. Stably transfected cells were selected with 2
µg/ml puromycin (Sigma-Aldrich, St. Louis, MO, USA) for 2
weeks. Stable transformants were identified by fluorescence
microscopy, reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blot analysis.
RT-qPCR analysis
Total RNAs were extracted from the transfected
IOMM-Lee cells using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). The RNA quality and concentration were analyzed
by measuring the absorbance at 260 and 280 nm with the spectrometer
(759S; Shanghai Lengguang Technology Co., Ltd., Shanghai, China),
and the A260/A280 ratios were between 1.8 and 2.0. For
single-stranded cDNA synthesis, the RT reaction was performed using
a Revertaid First Strand cDNA synthesis kit (Transgen, Beijing,
China). qPCR was then performed with 1 µg RNA and 1
µl of the following primers from Invitrogen Life
Technologies: HER-2, forward 5′-CGGACGCCTGATGGGTTAAT-3′ (120 bp)
and reverse 5′-ACAGCAAAGGTTCTACCCCG-3′ and GAPDH, forward
5′-CAGGGCTGCTTTTAACTCTGGT-3′ (203 bp) and reverse
5′-GATTTTGGAGGGATCTCGCT-3′. The qPCR procedure conducted in the ABI
PRISM 7500 system (Applied Biosystems, Waltham, MA, USA) was as
follows: denaturing at 95°C for 2 min and 40 cycles of annealing at
95°C for 15 sec and extension at 58°C for 30 sec.
Western blot analysis
The stable cells, which in the exponential growth
phase, were seeded into 6-well plates and were allowed to grow
until 80–90% confluence, following which they were lysed in lysis
buffer (Beyotime Institute of Biotechnology, Beijing, China) on
ice. The cells were harvested, washed twice with 1X
phosphate-buffered saline (PBS) and lysed in 100 µl
radioimmunoprecipitation assay lysis buffer (Vazyme Biotech
(Nanjing) Co., Ltd., Nanjing,China). Protein concentrations were
determined using a bicinchoninic acid kit (Vazyme Biotech (Nanjing)
Co., Ltd.). The proteins were separated using 10% SDS-PAGE and were
then blotted onto nitrocellulose membranes by wet electroblotting
at a constant current 200 mA for 2 h. The membranes were blocked
with 5% non-fat milk powder at room temperature for 1 h and
incubated overnight with the following primary antibodies:
Monoclonal mouse HER-2 (3B5; 1:500; Abcam, Cambridge, MA, USA),
polyclonal rabbit PI3K (C73F8; 1:1,000; Cell Signaling Technology,
Inc., Danvers, MA, USA), polyclonal rabbit phosphorylated (p)-PI3K
(19H8; 1:1,000; Cell Signaling Technology, Inc.), polyclonal rabbit
AKT (193H12; 1:1,000; Cell Signaling Technology, Inc.), polyclonal
rabbit p-AKT (D5G4; 1:1,000; Cell Signaling Technology, Inc.) and
monoclonal mouse β-actin (T0022; 1:5,000; Cell Signaling
Technology, Inc.) at 4°C. Following incubation, the membrane was
rinsed with Tris-buffered saline with Tween 20 (TBST) for 15 min
three times, and incubated with secondary antibody. The membrane
was agitated for 1 h at room temperature, washed again in TBST, and
were developed using an ECL Plus western blotting detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
MTT analysis
To investigate the proliferation ability of the four
cell groups, the present study performed an MTT assay. The stable
cells, in the exponential growth phase, were trypinized,
centrifuged at 400 × g for 5 min at room temperature, resuspend in
complete medium (10% FBS) and were counted. The cells were then
seeded into five 96-well plates (1×103 cells/well), with
five parallel wells for each cell group. The medium was replaced
with 120 µl MTT solution, containing 100 µl medium
and 20 µl MTT (Beijing Solarbio Science & Technology
Co., Ltd.), at different time-points (24, 48, 72, 96 and 120 h).
After 4 h, the medium was aspirated and 150 µl DMSO was
added to each well, then the plate was agitated for 45 sec at 27°C.
The optical density value at a wavelength of 490 nm was determined
using a Multiskan FC Microplate photometer (Thermo Fisher
Scientific, Waltham, MA, USA).
Colony formation assay
The cells (1×103 cells) were plated into
6 cm plates at 37°C. After 2 weeks, the cells were fixed with
methanol and stained using 0.1% crystal violet (Beijing Solarbio
Science & Technology Co., Ltd.). The number of colonies,
defined as ≥50 cells/colony, was then counted using a BX61
microscope (Olympus, Tokyo, Japan). The experiments were performed
in triplicate.
EdU labeling assay
The stable cells, in the exponential growth phase,
were seeded into 6-well plates and were allowed to grow until 50%
confluence at 37°C. EdU (Guangzhou RiboBio Co., Ltd., Guangzhou,
China) was then added to the culture media in concentrations of 50
µmol for 2 h. Following EdU labeling, the cells were washed
twice with PBS and fixed with 4.0% formaldehyde for 30 min at room
temperature. The cells were then fixed in 0.2% Triton X-100
(Beyotime Institute of Biotechnology) for 10 min at 37°C. The
EdU-stained cells were immunostained using the Cell-Light™ EdU
Apollo®643 In Vitro Imaging kit (Guangzhou RiboBio Co.,
Ltd.) and, following washing twice with PBS, the cells were
counter-stained using 1.5 µg/ml Hoechst 33342 (Guangzhou
RiboBio Co., Ltd.), mounted in standard mounting media and imaged
using confocal microscopy (LSM700; Zeiss, Jena, Germany). The EdU
stain was stable indefinitely at ≤4°C.
Cell invasion and migration analysis
Cell invasion was analyzed using a Transwell chamber
containing 24-well plates with an 8 µm membrane. The cells
were added to the upper chambers, respectively, in each group, and
the bottom of the chamber was coated with 1 mg/ml Matrigel for
invasion assays. The cells (3×104) were seeded onto the
Matrigel-coated Transwell chambe. Cell migration analysis was
performed using a wound healing assay, in which stable cells in the
exponential growth phase were trypsinized and seeded onto a 6-well
plate at 1×106 cells/well, with two wells for each cell
group (HER-2-sh-NC, HER-2-sh, HER-2-over-NC and HER-2-over).
Following incubation overnight at 37°C, two parallel wounds of ~400
µm were made using a 10 µl pipette tip. Following
rinsing with PBS three times, the cells were plated in FBS-free
DMEM medium supplement with penicillin/streptomycin (2 ml/well).
Images of the cells were captured at 0 and 24 h, and the distance
migrated was measured. The cell migration rate was obtained by
counting three fields per area, with results presented as the
average of six independent experiments performed over multiple
days.
Cell cycle and apoptotic assays using
flow cytometry
The stable cells, in the logarithmic growth phase,
were seeded into a 6-well plate (2×105 cells/well), and
were trypsinized and resuspended the following day at 37°C
overnight. The cells were washed with PBS twice at 400 × g for 5
min. For cell cycle analysis, propidium iodide was attributable to
the cell cycle and the distribution of cells was analyzed using
flow cytometry (FC500; Beckman Coulter, Inc., Brea, CA, USA). For
analysis of apoptosis, an Annexin-V-PE/7-AAD apoptosis detection
kit (Nanjing KeyGen Biotech. Co., Ltd., Nanjing, China) was used,
according to the manufacturer's instructions. Apoptosis was
analyzed using flow cytometry. The cells undergoing apoptosis were
annexin V-PE-positive and 7-AAD-negative.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS Inc., Chicago, IL, USA). The data are presented as
the mean ± standard deviation. Statistical analyses were performed
using analysis of variance or Student's t-test. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Expression of HER-2 in human meningioma
cell lines
In order to investigate the function of HER-2 in the
IOMM-Lee cells, the present study examined the expression of HER-2
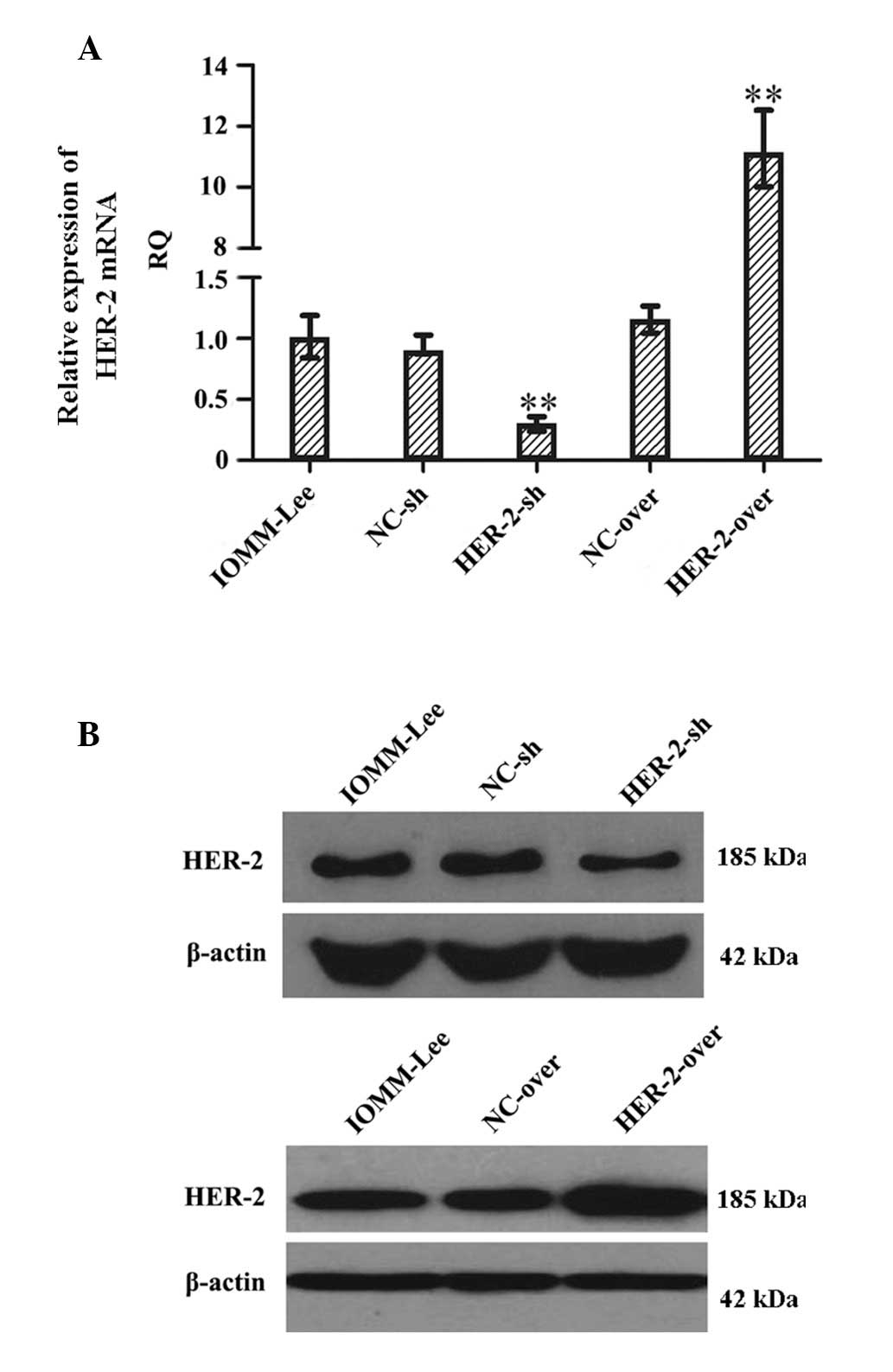
in the IOMM-Lee cell lines using RT-qPCR following transfection for
96 h (Fig. 1A). The expression of
HER-2 in the IOMM-Lee cells transfected with HER-2-sh decreased
67.2%, compared with the negative control cells or the cells
transfected with NC-sh (P<0.01). The expression of HER-2 in the
HER-2-overexpression IOMM-Lee cells was significantly increased
(8.7-fold), compared with the mock or NC cells (P<0.01). Western
blot analysis also revealed similar effects on the protein levels
of HER-2 72 h post-infection (Fig.
1B), suggesting that the protein level of HER-2 in the HER-2-sh
group decreased and that in the HER-2-overexpression group
increased, compared with the mock or NC cells.
HER-2 increases cell proliferation in
IOMM-Lee cells
The present study observed a significant decrease in
proliferation following transfection with the silence lentiviral
vector in the MTT assay. On the fifth day, the HER-2-sh cell
resistance was 1.66-fold higher than that of the NC-sh cells
(P<0.05; Fig. 2A). In the
colony formation assay, the number of colonies in the HER-2-sh cell
group decreased by 69.1%, compared with that in the NC-sh cell
group (P<0.05; Fig. 2B). In the
EdU labeling assay, a decrease in cell proliferation was observed
in the HER-2-sh cells (1.88-fold), compared with the NC-sh cells
(P<0.05; Fig. 2C). By contrast,
the overexpression lentiviral vector exhibited significantly
increased cell proliferation in the MTT assay, and the results of
the colony formation assay and EdU labeling assay were
comparatively higher, at 24.4, 49 and 39.5%, respectively
(P<0.05; Fig. 2A–C). These
results indicated that the proliferative ability of the IOMM-Lee
cells was significantly enhanced by increasing the expression of
HER-2.
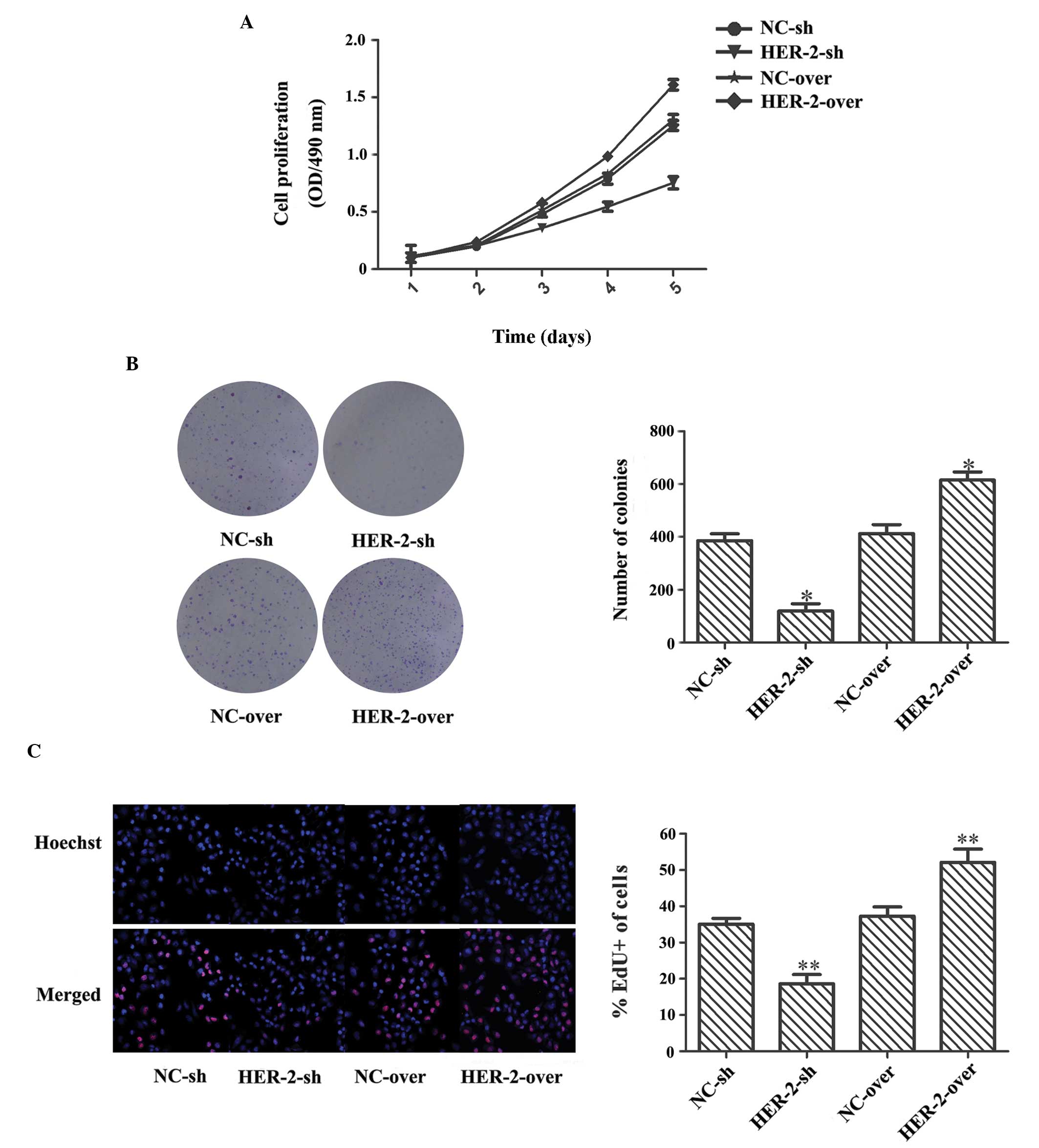 | Figure 2HER-2 increases cell proliferation,
colony formation and DNA duplication in IOMM-Lee cells. (A) MTT
assay revealed that, on the fifth day, HER-2-sh cell resistance was
1.66-fold higher than that of the NC-sh cells, whereas the
resistance of the HER-2-over cells was reduced 24.4%, compared with
the NC-over cells (P<0.05). (B) In the colony formation assay,
the number of colonies in the HER-2-sh cells decreased 69.1%,
compared with the NC-sh cells, whereas the number of colonies in
the HER-2-over cells increased (*P<0.05).
Magnification, ×1. (C) In the EdU labeling assay, compared with the
NC controls, positive labeling of the HER-2-sh cells was decreased
and that of the HER-2-over cells was increased
(**P<0.05). Newly synthesized DNA is stained red by
EdU and nuclei are stained blue by Hoechst 33342; magnification,
×200. The data are expressed as the mean standard deviation from
three independent experiments. NC, negative control; sh, short
hairpin; over, overexpression; OD, optical density. |
Effect of HER-2 on IOMM-Lee cells
invasion
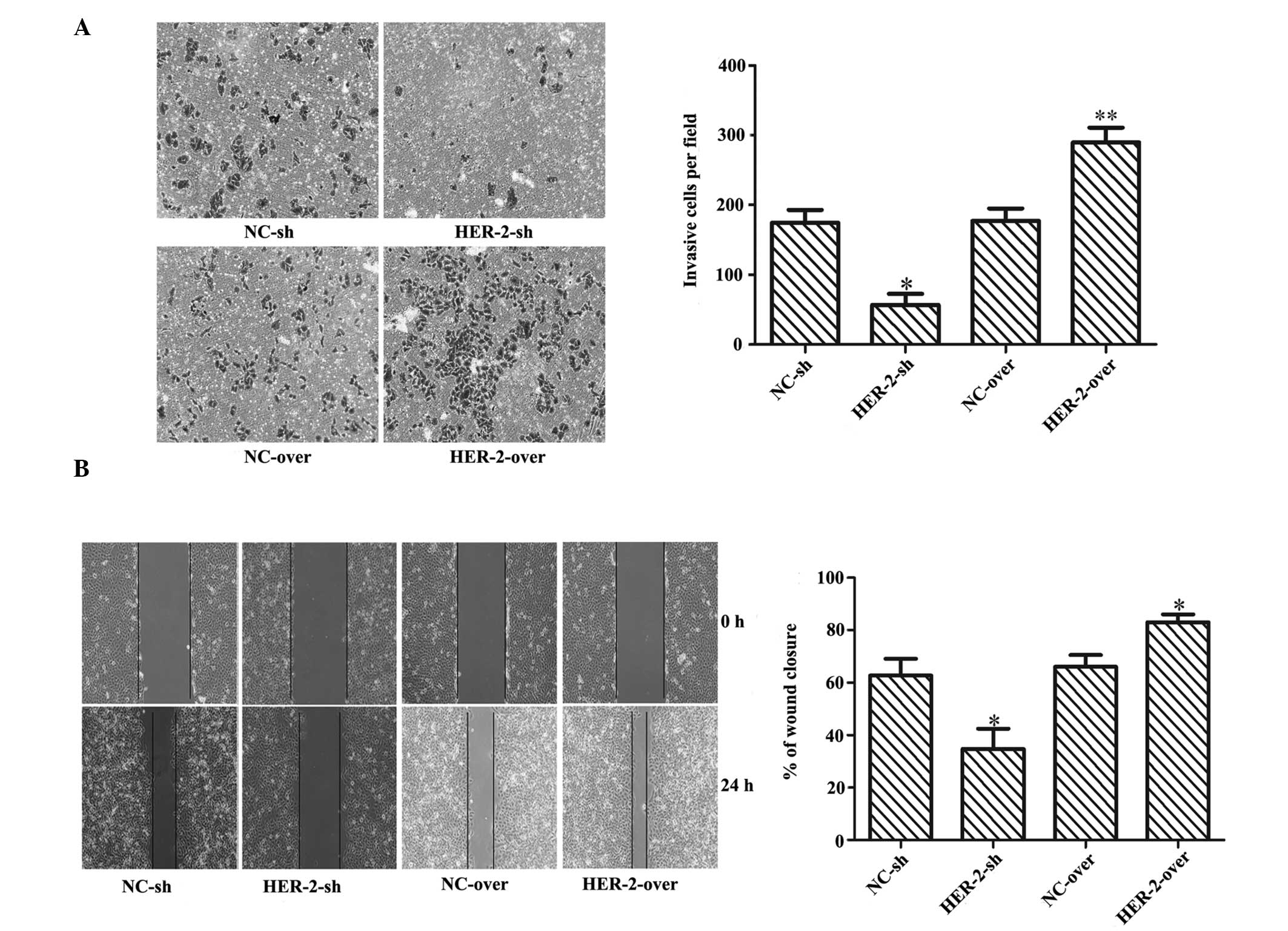
To determine whether HER-2 affected the invasion and
migration of IOMM-Lee cells, Matrigel invasion assays and wound
healing assays were performed. The results suggested that
increasing the expression of HER-2 enhanced the motility of the
IOMM-Lee cells, while downregulation of HER-2 significantly
inhibited the invasion and migration of the IOMM-Lee cells. As
shown in Fig. 3A, compared with
the NC cells, the invading ability of the HER-2-sh-transfected
IOMM-Lee cells was significantly lower (67.6%; P<0.01), whereas
the invasion of the HER-2-overexpression cells increased by 63.6%
(P<0.05). The migrating ability of the HER-2-sh IOMM-Lee cells
was significantly reduced by 44.7%, compared with the NC cells, but
was increased in the HER-2-overexpression cells by 25.8%
(P<0.05; Fig. 3B).
Effect of HER-2 on IOMM-Lee cell
cycle
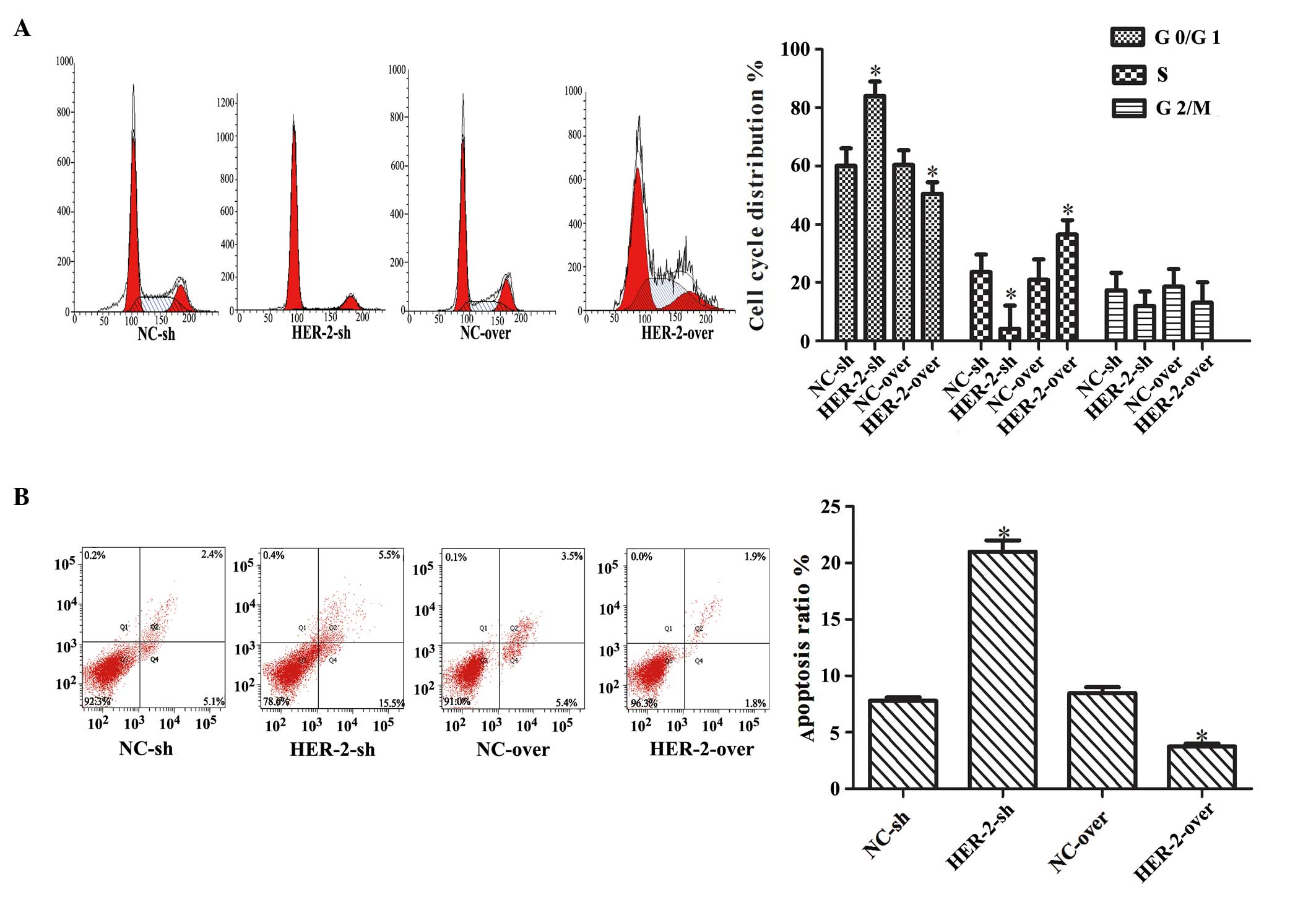
The present study further investigated the effect of
HER-2 on the cell cycle using flow cytometry. Downregulation of the
HER-2 gene resulted in a significantly higher percentage of cells
in the G1/G0 phase and decreased percentage of cells in the S
phase. By contrast, overexpression of HER-2 had the opposite effect
(P<0.05; Fig. 4A).
Collectively, these data suggested that HER-2 enhanced the
proliferation of the IOMM-Lee cells by promoting the G1-S cell
cycle transition. The effect of HER-2 on cell apoptosis was further
investigated and the results revealed that decreasing the
expression of HER-2 increased apoptosis significantly. By contrast,
overexpres-sion of HER-2 decreased apoptosis of the IOMM-Lee cells
(P<0.05; Fig. 4B).
HER-2 affects protein expression levels
of PI3K and AKT in IOMM-Lee cells
In order to investigate the association between
HER-2 and the activity of the PI3K/AKT signaling pathway, the
present study measured the protein levels of PI3K, p-PI3K, AKT and
p-AKT in the IOMM-Lee cells following transfection. The results
revealed that, compared with NC group, the protein levels of PI3K,
p-PI3K and p-AKT in the downregulated HER-2 group were
significantly reduced by 51.6, 40.5 and 76%, respectively
(P<0.05; Fig. 5A and B). By
contrast, in the upregulated HER-2 cells, the protein levels of
PI3K, p-PI3K and p-AKT increased by 41.9, 30.6 and 99.4%,
respectively (P<0.05; Fig. 5A and
B). However, no difference was observed in the protein
expression of AKT in these two groups, compared with the NC
group.
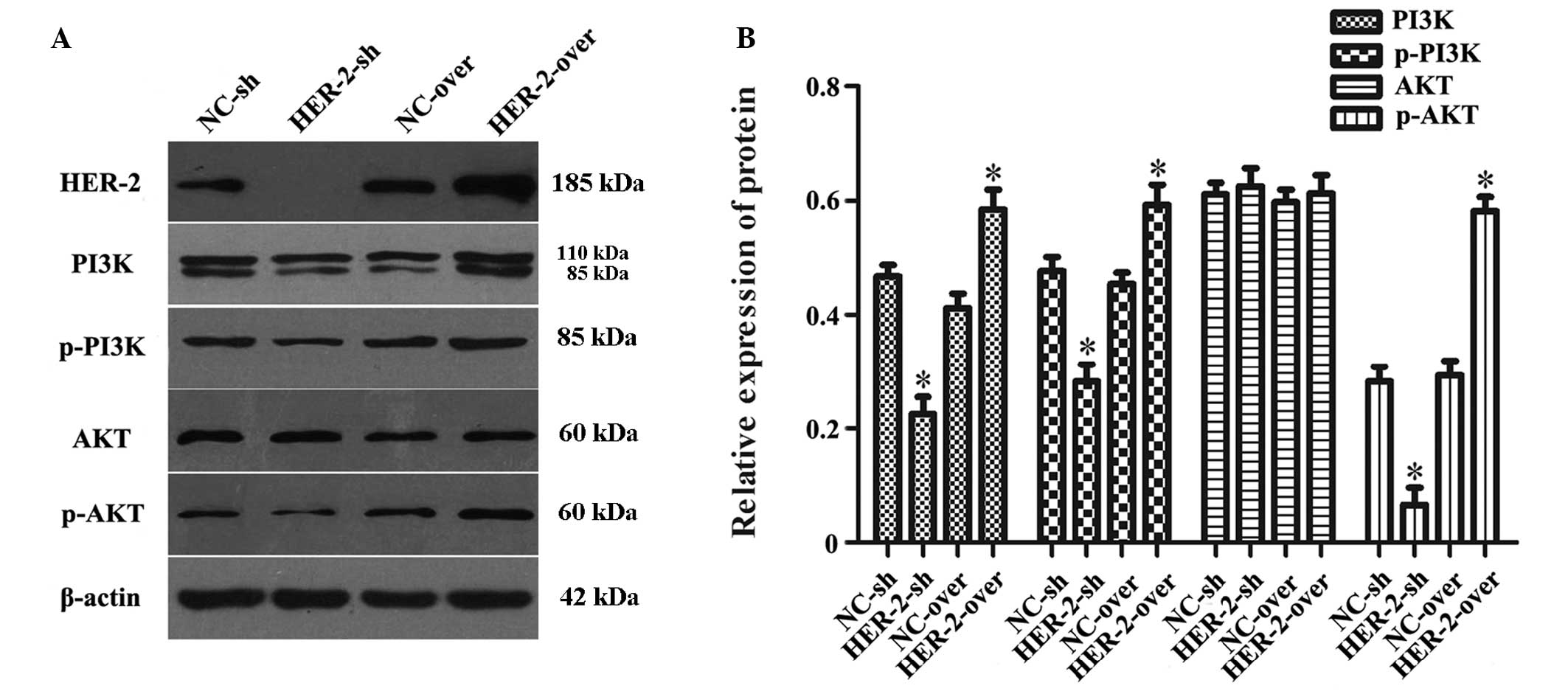 | Figure 5HER-2 affects the protein expression
levels of PI3K and AKT in IOMM-Lee cells. (A) Protein levels of
PI3K, p-PI3K, AKT and p-AKT in the IOMM-Lee cells were determined
using western blot analysis 72 h post-transfection. β-actin was
used as an internal loading control. (B) Protein levels of PI3K,
p-PI3K and p-AKT in the HER-2-sh group were markedly decreased. In
the HER-2-over group, the protein levels of PI3K, p-PI3K and p-AKT
were increased, compared with the NC control and following
normalization against β-actin (*P<0.05). No
difference was observed between the protein expression of AKT in
the HER-2-sh and HER-2-over cells and that in the NC control was no
difference. The data are expressed as the mean standard deviation
from three independent experiments. NC, negative control; sh, short
hairpin; over, overexpression; PI3K, phosphatidylinositol 3-kinase;
p-, phosphorylated. |
Discussion
HER-2 is an oncogene in human carcinoma, and a
number studies have demonstrated that it is significantly
upregulated in several types of human malignancy, with studies
indicating that HER-2 is important in tumor cell proliferation,
invasion and apoptosis (22,23).
Our previous study demonstrated that overexpression of HER-2 in
patients with meningioma was associated with poor prognosis
(24). The exact nature of the
contribution made by increased protein levels of HER-2 to human
meningioma cell proliferation and motility during the progression
of carcinoma, however, remains to be fully elucidated.
In the present study, HER-2 was demonstrated to
regulate the biochemical characteristics of human malignant
meningioma cells. When the gene expression of HER-2 was
downregulated, the proliferative ability of the cells declined,
determined using MTT, colony formation and Edu labeling assays, and
the invasive ability was decreased, observed using a Matrigel
invasion assay. In terms of the cell cycle, these cells were
arrested at the G0/G1-phase and apoptosis was increased. When the
gene expresion of HER-2 was upregulated, the proliferate ability of
the cells increased, and the invasive and migration abilities
increased significantly. The cell cycle was promoted at the
G1/S-phase and apoptosis was decreased. Therefore, the present
study demonstrated that HER-2 promoted cell proliferation and
invasion and inhibited apoptosis in the human malignant meningioma
IOMM-Lee cells.
It has been reported that the oncogenic effects of
HER-2 depend predominantly on preservation of the 'lipogenic
phenotype' (25). Ligand
stimulation induces dimerization of the HER receptor, either to a
homodimer or heterodimer, which leads to self-phosphorylation on
tyrosine residues localized to the C-terminal domain of the HER
receptors (26). HER-2 is the
preferred heterodimerization partner of all EGFR proteins, and is
important in the lateral transmission of signals between other EGFR
receptors (7). The activated
phosphorylated HER receptors then activate a variety of downstream
signaling modules, includeing the PI3K/Akt/mammalian target of
rapamycin (mTOR) pathway, the mitogen-activated protein kinase
pathway and the phospholipase C pathway (27).
The PI3K/AKT pathway is an essential pathway for
various cellular processes, including cell growth, cell survival,
motility, angiogenesis and metabolism (28,29).
Therefore, the present study hypothesized that HER-2 can affect the
protein synthesis or activities of PI3K/AKT, leading to the
facilitation of cell motility and invasion. To assess this
hypothesis, the present study used western blot analysis to detect
the expression levels of PI3K/AKT. Upregulation of the expression
of HER-2 led to increased levels of PI3K. PI3K is pivotal in
further signaling of the pathway, as it is the substrate of various
protein kinases containing a pleckstrin homology domain, including
the serine-threonine kinase AKT (30). AKT kinases belong to the AGC kinase
family, associated with AMP/GMP kinases and protein kinase C
(31). Activated AKT can
phosphorylate a number of downstream effectors, regulating a
variety of essential processes, including protein synthesis, cell
metabolism, cell growth/proliferation, cell survival and resistance
to various exogenous stresses (32). Full AKT activation depends on the
concomitant phosphorylation of two distinct sites, which can be
activated independently. PDK-1-catalyzed T308 phosphorylation
inside the activation loop serves as a readout of PI3K activation.
By contrast, phosphorylation of S473 in the hydrophobic motif of
the C-terminal tail indicates mTORC2 to AKT feedback signaling
activity or induction by the PI3K-related kinase superfamily or
DNA-dependent protein kinase (33). There are also other signals, which
regulate AKT, including the extracellular signal-regulated kinase
(ERK)/MAPK signaling pathway and the Janus kinase-signal
transducers and activators of transctition pathway (34,35).
Therefore, in the present study, the levels of p-AKT increased with
the increase in p-PI3K, however, AKT may not be changed completely.
Further investigations are required to clarify the exact molecular
mechanisms.
In conclusion, the results of the present study
indicated that overexpression of HER-2 stimulated human meningioma
cell proliferation and invasion, which may contribute to meningioma
development and progression. These results may explain, in part,
the observation that the overexpression of HER-2 during the
progression of meningioma is clinically associated with a high
metastatic potential and poor prognosis. Furthermore, variation in
the expression of HER-2 affected the protein levels of PI3K and AKT
in the IOMM-Lee cells, which provided evidence for a functional
linkage between HER-2 signaling and the activity of PI3K/AKT in
cell invasion. These results suggested that the HER-2/PI3K/AKT
pathway may be a valuable target for the development of novel
therapeutic approaches to prevent or suppress cell invasion and
metastasis in meningioma.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant. no. 81260372) and the
Natural Science Foundation of Jiangxi Province (grant. no.
20114BAB205047).
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen L, Zou X, Wang Y, Mao Y and Zhou L:
Central nervous system tumors: a single center pathology review of
34, 140 cases over 60 years. BMC Clin Pathol. 13:142013. View Article : Google Scholar
|
|
3
|
Mawrin C and Perry A: Pathological
classification and molecular genetics of meningiomas. J Neurooncol.
99:379–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andrae N, Kirches E, Hartig R, et al:
Sunitinib targets PDGF-receptor and Flt3 and reduces survival and
migration of human meningioma cells. Eur J Cancer. 48:1831–1841.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vranic A: Antigen expression on recurrent
meningioma cells. Radiology and oncology. 44:107–112. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sahlberg KK, Hongisto V, Edgren H, et al:
The HER2 amplicon includes several genes required for the growth
and survival of HER2 positive breast cancer cells. Mol Oncol.
7:392–401. 2013. View Article : Google Scholar
|
|
7
|
Liu X, Zhang Y, Ren W and Rao G: ErbB2
gene silencing and its effect on PTEN in SACC-83 salivary adenoid
cystic carcinoma cells. Oncol Rep. 24:1291–1296. 2010.PubMed/NCBI
|
|
8
|
Way TD, Kao MC and Lin JK: Apigenin
induces apoptosis through proteasomal degradation of HER2/neu in
HER2/neu-overexpressing breast cancer cells via the
phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem.
279:4479–4489. 2004. View Article : Google Scholar
|
|
9
|
Park HK, Kim IH, Kim J and Nam TJ:
Induction of apoptosis and the regulation of ErbB signaling by
laminarin in HT-29 human colon cancer cells. Int J Mol Med.
32:291–295. 2013.PubMed/NCBI
|
|
10
|
Paik S, Bryant J, Tan-Chiu E, et al: HER2
and choice of adjuvant chemotherapy for invasive breast cancer:
National Surgical Adjuvant Breast and Bowel Project Protocol B-15.
J Natl Cancer Inst. 92:1991–1998. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pegram MD: Treating the HER2 pathway in
early and advanced breast cancer. Hematol Oncol Clin North Am.
27:751–765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Cosimo S, Arpino G and Generali D:
Neoadjuvant treatment of HER2 and hormone-receptor positive breast
cancer-moving beyond pathological complete response. Breast.
23:188–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding H, Helguera G, Rodriguez JA, et al:
Polymalic acid nanobioconjugate for simultaneous immunostimulation
and inhibition of tumor growth in HER2/neu-positive breast cancer.
J Control Release. 171:322–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boku N: HER2-positive gastric cancer.
Gastric Cancer. 17:1–12. 2014. View Article : Google Scholar :
|
|
15
|
Qi W, Li X, Zhang Y, et al: Overexpression
of Her-2 upregulates FoxM1 in gastric cancer. Int J Mol Med.
33:1531–1538. 2014.PubMed/NCBI
|
|
16
|
Jeung J, Patel R, Vila L, et al:
Quantitation of HER2/neu expression in primary gastroesophageal
adenocarcinomas using conventional light microscopy and
quantitative image analysis. Arch Pathol Lab Med. 136:610–617.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chao WR, Lee MY, Lin WL, et al: HER2
amplification and overexpression are significantly correlated in
mucinous epithelial ovarian cancer. Hum Pathol. 45:810–816. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Waage IS, Vreim I and Torp SH:
C-erbB2/HER2 in Human Gliomas, Medulloblastomas and Meningiomas: A
Minireview. Int J Surg Pathol. 21:573–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahzouni P and Movahedipour M: An
immunohistochemical study of HER2 expression in meningioma and its
correlation with tumor grade. Pathol Res Pract. 208:221–224. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loussouarn D, Brunon J, Avet-Loiseau H, et
al: Prognostic value of HER2 expression in meningiomas: an
immunohistochemical and fluorescence in situ hybridization study.
Hum Pathol. 37:415–421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdelzaher E, El-Gendi SM, Yehya A and
Gowil AG: Recurrence of benign meningiomas: predictive value of
proliferative index, BCL2, p53, hormonal receptors and HER2
expression. Br J Neurosurg. 25:707–713. 2011. View Article : Google Scholar
|
|
22
|
Fu J, Tian C, Xing M, et al: KU004 induces
G1 cell cycle arrest in human breast cancer SKBR-3 cells by
modulating PI3K/Akt pathway. Biomed Pharmacother. 68:625–630. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan S, Shukla S, Sinha S, Lakra AD, Bora
HK and Meeran SM: Centchroman suppresses breast cancer metastasis
by reversing epithelial-mesenchymal transition via downregulation
of HER2/ERK1/2/MMP-9 signaling. Int J Biochem Cell Biol. 58:1–16.
2015. View Article : Google Scholar
|
|
24
|
Wang CL, Mei JH, Wang SS, et al:
Expression of HER2/neu in meningiomas: an immunohistochemistry and
fluorescence in situ hybridization study. Zhonghua Bing Li Xue Za
Zhi. 39:156–160. 2010.In Chinese. PubMed/NCBI
|
|
25
|
Lin VC, Chou CH, Lin YC, et al: Osthole
suppresses fatty acid synthase expression in HER2-overexpressing
breast cancer cells through modulating Akt/mTOR pathway. J Agr Food
Chem. 58:4786–4793. 2010. View Article : Google Scholar
|
|
26
|
Kuo HP, Hsu SC, Ou CC, et al: Ganoderma
tsugae extract inhibits growth of HER2-overexpressing cancer cells
via modulation of HER2/PI3K/Akt signaling pathway. Evid Based
Complement Alternat Med. 2013:2194722013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roskoski R Jr: The ErbB/HER family of
protein-tyrosine kinases and cancer. Pharmacol Res. 79:34–74. 2014.
View Article : Google Scholar
|
|
28
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bartholomeusz C and Gonzalez-Angulo AM:
Targeting the PI3K signaling pathway in cancer therapy. Expert Opin
Ther Tar. 16:121–130. 2012. View Article : Google Scholar
|
|
30
|
Steelman LS, Stadelman KM, Chappell WH, et
al: Akt as a therapeutic target in cancer. Expert Opin Ther
Targets. 12:1139–1165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR signaling in cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lauring J, Park BH and Wolff AC: The
Phosphoinositide-3-kina se-Akt-mTOR pathway as a therapeutic target
in breast cancer. J Natl Compr Canc Netw. 11:670–678.
2013.PubMed/NCBI
|
|
33
|
Briest F and Grabowski P:
PI3K-AKT-mTOR-signaling and beyond: the complex network in
gastroenteropancreatic neuroendocrine neoplasms. Theranostics.
4:336–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jang YN and Baik EJ: JAK-STAT pathway and
myogenic differentiation. Jak-Stat. 2:e232822013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kavitha K, Kowshik J, Kishore TK, et al:
Astaxanthin inhibits NF-kappaB and Wnt/beta-catenin signaling
pathways via inactivation of Erk/MAPK and PI3K/Akt to induce
intrinsic apoptosis in a hamster model of oral cancer. Biochim
Biophys Acta. 1830:4433–4444. 2013. View Article : Google Scholar : PubMed/NCBI
|