Introduction
Pancreatic cancer (PC) is an aggressive and
devastating disease with a poor prognosis (1). It is the eighth most common cause of
cancer-associated mortality in the world (2,3) and
leads to 227,000 mortalities worldwide every year. The 5-year
survival rate in patients with PC is <5% (4). Cisplatin, a commonly used
chemotherapeutic agent for solid tumors (5), is effective as a single agent or in
combination with other drugs for the treatment of PC (6,7).
Twist, also known as Twist1, belongs to the basic
helix-loop-helix transcription factor family. A high expression of
Twist has been detected in several types of cancer and has been
associated with the initial phase of metastatic progression
(8). A previous study demonstrated
that Twist is upregulated in PC tissues, suggesting that Twist is
involved in the progression of PC (9).
Growth differentiation factor 15 (GDF15), also
termed macrophage inhibitory cytokine-1, is a divergent member of
the transforming growth factor-β superfamily. It has multiple roles
in various pathologies, including inflammation, cancer,
cardiovascular diseases and obesity (10,11).
In cancer, GDF15 has been reported to have tumorigenic and
anti-tumorigenic activities (11,12).
Although the role of GDF15 in tumorigenesis is most likely not
universal in all types of cancer, it is elevated in the serum of PC
patients compared with healthy controls and those with benign
pancreatic neoplasms (13,14). A previous study has demonstrated
that serum GDF15 could be used as a diagnostic biomarker with high
sensitivity and specificity for identifying PC (15).
These previous studies suggest that Twist and GDF15
are involved in PC progression. However, the roles of Twist and
GDF15 in PC remain to be elucidated. The present study examined the
interaction between Twist and GDF15 in PC cell invasion and
chemoresistance to cisplatin.
Materials and methods
Cell lines, plasmids and reagents
The human PC cell lines ASPC-1 (CRL-1682) and BXPC-3
(CRL-1687) were purchased from the American Type Culture Collection
(ATCC, Manassas, VA, USA). Twist (sc-38604-V) and
GDF15 (sc-39798-V) shRNA lentiviral particles, control shRNA
lentiviral particles-A (cat. no. sc-108080) and mouse anti-human
monoclonal Twist antibody (Twist2C1a; cat. no. sc-81417), mouse
anti-human GDF-15 monoclonal antibody (G-5; cat. no. sc-377195) and
mouse anti-human matrix metalloproteinase-2 monoclonal antibody
(MMP-2; cat. no. sc-53630) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The SensoLyte 520 MMP-2
Assay kit (cat. no. AS-71151) was purchased from AnaSpec (Fremont,
CA, USA). The QCM ECMatrix 24-well (8 μM) Fluorimetric Cell
Invasion Assay kit (cat. no. ECM554) was purchased from Chemicon
(Millipore, Billerica, MA, USA). Superfect transfection reagent was
purchased from Qiagen (Valencia, CA, USA). The p38
mitogen-activated protein kinase (MAPK) Assay kit (cat. no. 9820)
was purchased from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Human Twist cDNA was subcloned into a pcDNA 3.1
expression vector (16).
Full-length human GDF15 cDNA (MCG: 4145) vector was purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). The human GDF15
expression vector (pcDNA3-GDF15) was constructed by subcloning the
GDF15 cDNA vector following digestion with EcoRI and
NotI into the pcDNA3.1 expression vector (Invitrogen Life
Technologies). Puromycin, G418, cisplatin, the selective p38 MAPK
inhibitor SB203580 and all chemicals of reagent grade were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Transfection and lentiviral
transduction
The Twist or GDF15 expression vector
was transfected into cells using Superfect transfection reagent
(Qiagen) according to the manufacturer's instructions. Pools of
stable transductants were generated via selection with G418 (600
μg/ml) according to the manufacturer's instructions. The
Twist or GDF15 shRNA lentiviral particles contain
expression constructs encoding target-specific 19–25 nt (plus
hairpin) shRNA designed to specifically knock-down Twist or
GDF15 gene expression. The control shRNA lentiviral
particles contain a scrambled shRNA sequence that does not lead to
specific degradation of any cellular mRNA and was used as a
negative control. Lentiviral transduction was performed in ASPC-1
and BXPC-3 cells. Pools of stable transductants were generated via
selection with puromycin (4 μg/ml) according to the
manufacturer's instructions (Santa Cruz Biotechnology, Inc.).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
RNA was prepared from cells using TRIzol reagent
followed by purification using a TURBO DNA-free kit (Ambion,
Austin, TX, USA). The cDNAs were synthesized using SuperScript II
reverse transcriptase (Invitrogen Life Technologies). qPCR was
performed on the LightCycler thermal cycler system (Roche
Diagnostics, Indianapolis, IN, USA) using an SYBR Green I kit
(Roche Diagnostics) according to the manufacturer's instructions.
The results were normalized against that of the housekeeping gene
glyceral-dehyde-3-phosphate dehydrogenase (GAPDH) in the same
sample. The primers used are as follows: Human GDF15,
forward 5′-CGGTGAATGGCTCTCAGATG-3′ and reverse
5′-CAGGTCCTCGTAGCGTTTCC-3′; human GAPDH, forward
5′-GACTCATGACCACAGTCCATGC-3′ and reverse
5′-AGAGGCAGGGATGATGTTCTG-3′. Each experiment was repeated three
times in duplicate.
Cell invasion assay
In vitro cell invasion assays were performed
with the QCM ECMatrix 24-well (8 μM) Fluorimetric Cell
Invasion Assay kit (Chemicon; Millipore) according to the
manufacturer's instructions (17,18).
The kit used an insert polycarbonate membrane with an 8 μM
pore size. The insert in the invasion kit was coated with a thin
layer of ECMatrix. Cell invasion was determined by fluorescence.
Each experiment was repeated three times in duplicate.
Western blot analysis
Cells were dissolved in 250 μl of 1X SDS
loading buffer (62.5 mm TrisHCl, pH 6.8, 2% SDS, 25% glycerol,
0.01% bromphenol blue, 5% 2-mercaptoethanol) and incubated at 95°C
for 10 min. Equal quantities of proteins for each sample were
separated by 10% SDS-polyacrylamide gel and blotted onto a
polyvinylidene difluoride microporous membrane (Millipore).
Membranes were incubated for 1 h at room temperature with a 1:500
dilution of the following primary antibodies: Anti-Twist,
anti-GDF-15, and anti-MMP-2, and then washed and revealed using
horseradish peroxidase-conjugated bovine anti-mouse secondary
antibodies (cat. no. sc-2371; Santa Cruz Biotechnology, Inc.,
1:5,000, 1 h). Peroxidase was revealed with a GE Healthcare ECL kit
(Shanghai, China). Three independent experiments were performed for
each western blot analysis.
MMP-2 activity assay
MMP-2 activity was measured with the SensoLyte 520
MMP-2 Assay kit (AnaSpec) according to the manufacturer's
instructions (19,20). The supernatants were collected and
then incubated with 4-aminophenylmercuric acetate and MMP-2
substrate. The fluorescence intensity at Ex/Em wavelengths of 490
nm/520 nm were used as a measure of MMP-2 activity. Each experiment
was repeated three times in duplicate.
Cisplatin chemosensitivity assay
Cells were plated in triplicate in 96-well plates at
a density of 5,000 cells. After 24 h of incubation, the medium was
replaced by fresh medium with or without various concentrations of
cisplatin (0.1, 0.25, 0.5, 1.0, 1.5, 3.0, 6.0, 15.0, 30.0, or 55.0
mM) (Sigma-Aldrich). Subsequently, cell viability was assayed 48 h
later using a modified MTT assay as previously described (21). The half maximal inhibitory
concentration (IC50) values were defined as the concentrations
resulting in a 50% reduction in growth compared with control cell
growth.
p38 MAPK activity assay
p38 MAPK activity was measured using the p38 MAPK
Assay kit (Cell Signaling Technology, Inc.) according to the
manufacturer's instructions (22).
Briefly, cells were directly lysed in the culture dishes. Cell
lysates were sonicated and centrifuged at 20,000 x g for 10 min at
4°C. The supernatant containing equivalent quantities of protein
(200 μg) was incubated by gentle rocking with 20 μl
of immobilized mouse anti-human phospho-p38-MAPK monoclonal
antibody (28B10; cat. no. 9216; Cell Signaling Technology, Inc.;
1:500) for 16 h at 4°C. The immunoprecipitates were washed twice
with the lysing buffer and pelleted by centrifu-gation at 20,000 ×
g for 10 min at 4°C. The p38 MAPK assay was performed using
activating transcription factor 2 (ATF2) fusion protein (2
μg) as a substrate in the presence of 200 μM ATP and
1X kinase buffer according to the manufacturer's instructions.
Samples were resolved on a 12% SDS-PAGE gel and visualized by
autoradiography.
Statistical analysis
Statistical analyses were performed with SPSS for
Windows 19.0 (SPSS, Inc., Chicago, IL, USA). All data values are
expressed as the mean ± standard deviation. Comparisons of means
among multiple groups were performed with one-way analysis of
variance followed by post hoc pairwise comparisons using
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Overexpression and knockdown of Twist and
GDF15 in human PC cells
To investigate the functional interaction between
Twist and GDF15 in PC cells, Twist and GDF15 were stably
overexpressed in ASPC-1 and BXPC-3 human PC cells by stable
transfection. By contrast, the cells were also stably transduced
with lentiviral shRNAs to knock down Twist and GDF15, respectively.
As shown in Fig. 1, Twist and
GDF15 were constitutively expressed in ASPC-1 and BXPC-3 cells.
Compared with the controls, Twist was overexpressed >4.8 fold
and knocked down >80% in ASPC-1 and BXPC-3 cells, respectively;
GDF was overexpressed >4.2 fold and knocked down >80% in
ASPC-1 and BXPC-3 cells, respectively. GDF15 expression in the
cells increased (by >3.3 fold) and decreased (>60%) in
parallel with Twist overexpression and knockdown, respectively. By
contrast, overexpression and knockdown of GDF15 had no significant
effect on Twist expression (Fig.
1). Our pilot study suggested that Twist regulates GDF15
expression in PC cells by a p38 MAPK-dependent mechanism (data not
shown). Therefore, a selective p38 MAPK inhibitor SB203580 (10
μM) was included in all experiments in the present study
(23). As shown in Fig. 1, the p38 MAPK inhibitor had no
significant effect on the constitutive expression level of Twist,
whereas it eradicated Twist-induced GDF15 expression in PC cells.
RT-qPCR assays revealed a similar data trend (Fig. 2), suggesting that Twist regulates
GDF15 expression at the mRNA level.
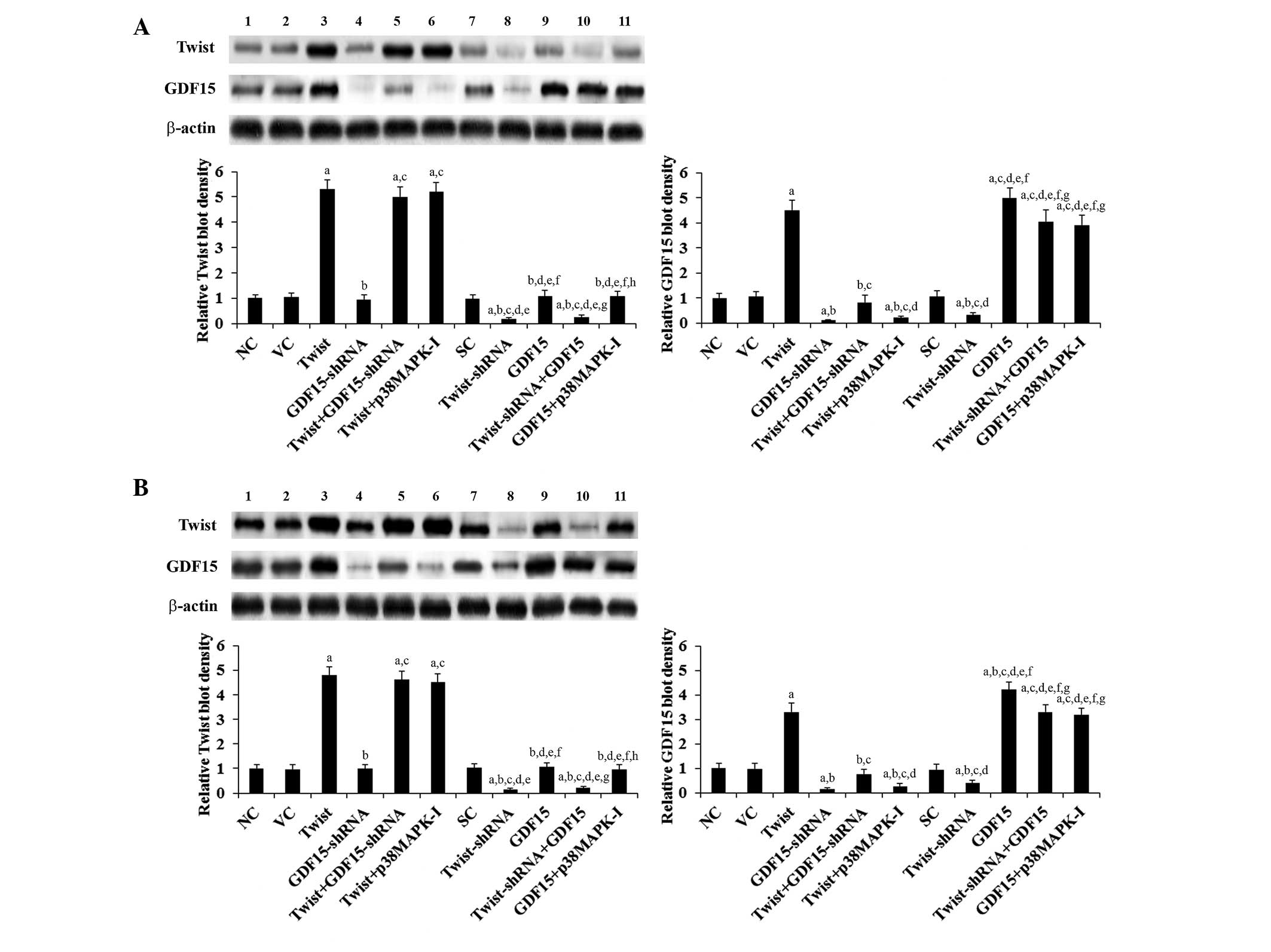 | Figure 1Protein levels of Twist and GDF15 in
human PC cells. In (A) ASPC-1 and (B) BXPC-3 human PC cells, the
protein levels of Twist and GDF15 were determined using western
blot analysis in NC cells (NC, lane 1), cells stably transfected
with the empty pcDNA3.1 vector (VC, lane 2), cells stably
transfected with the pcDNA3-Twist expression vector (Twist, lane
3), cells stably transduced with GDF15-shRNA (lane 4), cells stably
transfected with Twist and transduced with GDF15-shRNA (Twist +
GDF15-shRNA, lane 5), cells stably transfected with Twist and
treated with the selective p38 MAPK inhibitor SB203580 (10
μM) for 30 min (Twist + p38MAPK-I, lane 6), cells stably
transduced with SC shRNA (SC, lane 7), cells stably transduced with
Twist-shRNA (lane 8), cells stably transfected with the
pcDNA3-GDF15 expression vector (GDF15, lane 9), cells stably
transduced with Twist-shRNA and transfected with GDF15 (Twist-shRNA
+ GDF15, lane 10) and cells stably transfected with GDF15 and
treated with SB203580 (10 μM) for 30 min (GDF15 + p38MAPK-I,
lane 11). β-actin was used as a loading control. The density of
Twist and the GDF15 blots was normalized against that of the
β-actin blot to obtain a relative blot density, which is expressed
as fold changes to that of NC (designated as 1). Three independent
experiments were performed for each western blot analysis. Data
values are expressed as the mean + standard deviation.
aP<0.05 vs. controls (NC, VC and SC);
bP<0.05 vs. Twist; cP<0.05 vs.
GDF15-shRNA; dP<0.05 vs. Twist + GDF15-shRNA;
eP<0.05 vs. Twist + p38MAPK-I; fP<0.05
vs. Twist-shRNA; gP<0.05 vs. GDF15;
hP<0.05 vs. Twist-shRNA + GDF15. GD15, growth
differentiation factor 15; PC, pancreatic cancer; NC, normal
control; SC, scrambled control; MAPK, mitogen-activated protein
kinase. |
 | Figure 2mRNA levels of Twist and GDF15 in PC
cells. In (A) ASPC-1 and (B) BXPC-3 PC cells, the mRNA levels of
Twist and GDF15 were determined by reverse transcription
quantitative polymerase chain reaction in NC cells, cells stably
transfected with the empty pcDNA3.1 vector (VC), cells stably
transfected with Twist, cells stably transduced with GDF15-shRNA,
cells stably transfected with Twist and transduced with GDF15-shRNA
(Twist + GDF15-shRNA), cells stably transfected with Twist and
treated with the selective p38 MAPK inhibitor SB203580 (10
μM) for 30 min (Twist + p38MAPK-I), cells stably transduced
with SC shRNA, cells stably transduced with Twist-shRNA, cells
stably transfected with GDF15, cells stably transduced with
Twist-shRNA and transfected with GDF15 (Twist-shRNA + GDF15) and
cells stably transfected with GDF15 and treated with SB203580 (10
μM) for 30 min (GDF15 + p38MAPK-I). The Twist and the GDF15
mRNA levels are shown as fold changes to those of NC (designated as
1). Each experiment was repeated three times in duplicate. Data
values are expressed as the mean + standard deviation.
aP<0.05 vs. controls (NC, VC and SC);
bP<0.05 vs. Twist; cP<0.05 vs.
GDF15-shRNA; dP<0.05 vs. Twist + GDF15-shRNA;
eP<0.05 vs. Twist + p38MAPK-I; fP<0.05
vs. Twist-shRNA; gP<0.05 vs. GDF15. GDF15, growth
differentiation factor 15; PC, pancreatic cancer; NC, normal
control; MAPK, mitogen-activated protein kinase; SC, scrambled
control. |
Effects of overexpression and knockdown
of Twist and GDF15 on PC cell invasion and MMP-2
expression/activity
To examine the individual effect of and interaction
between Twist and GDF15 on PC cell invasion, in vitro cell
invasion assays were performed. Compared with the controls,
overexpression of Twist increased cell invasion by 3.4 and 2.1 fold
in ASPC-1 and BXPC-3 cells, respectively, which was eradicated by
knockdown of GDF15 or SB203580 (10 μM; Fig. 3). By contrast, knockdown of Twist
decreased cell invasion by ~60% in ASPC-1 and BXPC-3 cells,
respectively, which was completely reversed by overexpression of
GDF15 (Fig. 3). Compared with the
controls, overexpression of GDF15 increased cell invasion by 3.7
fold in ASPC-1 cells and 2.3 fold in BXPC-3 cells, whereas
knockdown of GDF15 decreased cell invasion by ~85% in the two cell
lines (Fig. 3).
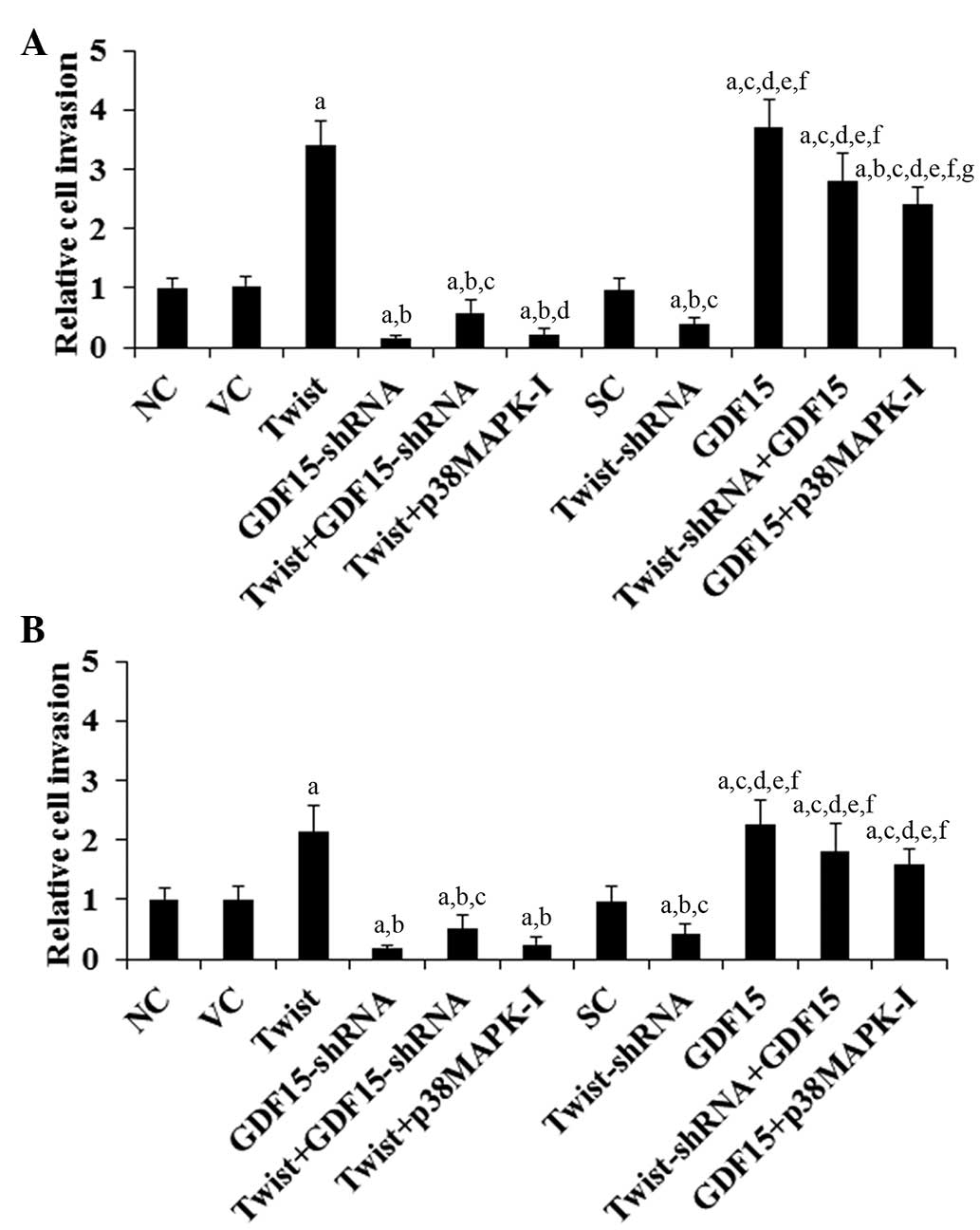 | Figure 3Effect of overexpression and knockdown
of Twist and GDF15 on the invasion of PC cells. In vitro
cell invasion assays were performed in (A) ASPC-1 and (B) BXPC-3 PC
cells. Cell invasion in NC cells, cells stably transfected with the
empty pcDNA3.1 vector (VC), cells stably transfected with Twist,
cells stably transduced with GDF15-shRNA, cells stably transfected
with Twist and transduced with GDF15-shRNA (Twist + GDF15-shRNA),
cells stably transfected with Twist and treated with the selective
p38 MAPK inhibitor SB203580 (10 μM) for 30 min (Twist +
p38MAPK-I), cells stably transduced with SC shRNA, cells stably
transduced with Twist-shRNA, cells stably transfected with GDF15,
cells stably transduced with Twist-shRNA and transfected with GDF15
(Twist-shRNA + GDF15) and cells stably transfected with GDF15 and
treated with SB203580 (10 μM) for 30 min (GDF15 + p38MAPK-I)
determined by fluorescence and shown as fold changes to that of NC
(designated as 1). Each experiment was repeated three times in
duplicate. Data values are expressed as the mean + standard
deviation. aP<0.05 vs. controls (NC, VC and SC);
bP<0.05 vs. Twist; cP<0.05 vs.
GDF15-shRNA; dP<0.05 vs. Twist + GDF15-shRNA;
eP<0.05 vs. Twist + p38MAPK-I; fP<0.05
vs. Twist-shRNA; gP<0.05 vs. GDF15. GDF15, growth
differentiation factor 15; PC, pancreatic cancer; NC, normal
control; MAPK, mitogen-activated protein kinase; SC, scramble
control. |
MMPs have a critical role in cancer cell invasion
(24). Among the different MMPs
assessed, MMP-2 expression was found to be significantly altered by
Twist and GDF15 in PC cells (data not shown). Compared with the
controls, overexpression of Twist increased MMP-2 expression by 3.9
and 2.5 fold in ASPC-1 and BXPC-3 cells, respectively, which was
eradicated by knockdown of GDF15 or SB203580 (10 μM;
Fig. 4). By contrast, knockdown of
Twist decreased MMP-2 expression by 51% in ASPC-1 and 43% in BXPC-3
cells, which was completely reversed by overexpression of GDF15
(Fig. 4). Compared with the
controls, overexpression of GDF15 increased MMP-2 expression by 4.1
fold in ASPC-1 cells and 2.5 fold in BXPC-3 cells, whereas
knockdown of GDF15 decreased MMP-2 expression by ~80% in the two
cell lines (Fig. 4). A similar
data trend was observed with MMP-2 activity (Fig. 5).
 | Figure 4Effect of overexpression and knockdown
of Twist and GDF15 on MMP-2 expression in PC cells. In (A) ASPC-1
and (B) BXPC-3 PC cells, the expression of MMP-2 was determined by
western blot analysis in NC cells (NC, lane 1), cells stably
transfected with the empty pcDNA3.1 vector (VC, lane 2), cells
stably transfected with Twist (lane 3), cells stably transduced
with GDF15-shRNA (lane 4), cells stably transfected with Twist and
transduced with GDF15-shRNA (Twist + GDF15-shRNA, lane 5), cells
stably transfected with Twist and treated with the selective p38
MAPK inhibitor SB203580 (10 μM) for 30 min (Twist +
p38MAPK-I, lane 6), cells stably transduced with SC shRNA (SC, lane
7), cells stably transduced with Twist-shRNA (lane 8), cells stably
transfected with GDF15 (lane 9), cells stably transduced with
Twist-shRNA and transfected with GDF15 (Twist-shRNA + GDF15, lane
10) and cells stably transfected with GDF15 and treated with
SB203580 (10 μM) for 30 min (GDF15 + p38MAPK-I, lane 11).
β-actin was used as a loading control. The density of the MMP-2
blot was normalized against that of the β-actin blot to obtain a
relative blot density, which is expressed as fold changes to that
of NC (designated as 1). Three independent experiments were
performed for each western blot analysis. Data values are expressed
as the mean + standard deviation. aP<0.05 vs.
controls (NC, VC and SC); bP<0.05 vs. Twist;
cP<0.05 vs. GDF15-shRNA; dP<0.05 vs.
Twist + GDF15-shRNA; eP<0.05 vs. Twist + p38MAPK-I;
fP<0.05 vs. Twist-shRNA; gP<0.05 vs.
GDF15. GDF15, growth differentiation factor 15; MMP-2, matrix
metalloproteinase-2; PC, pancreatic cancer; NC, normal control;
MAPK, mitogen-activated protein kinase; SC, scramble control. |
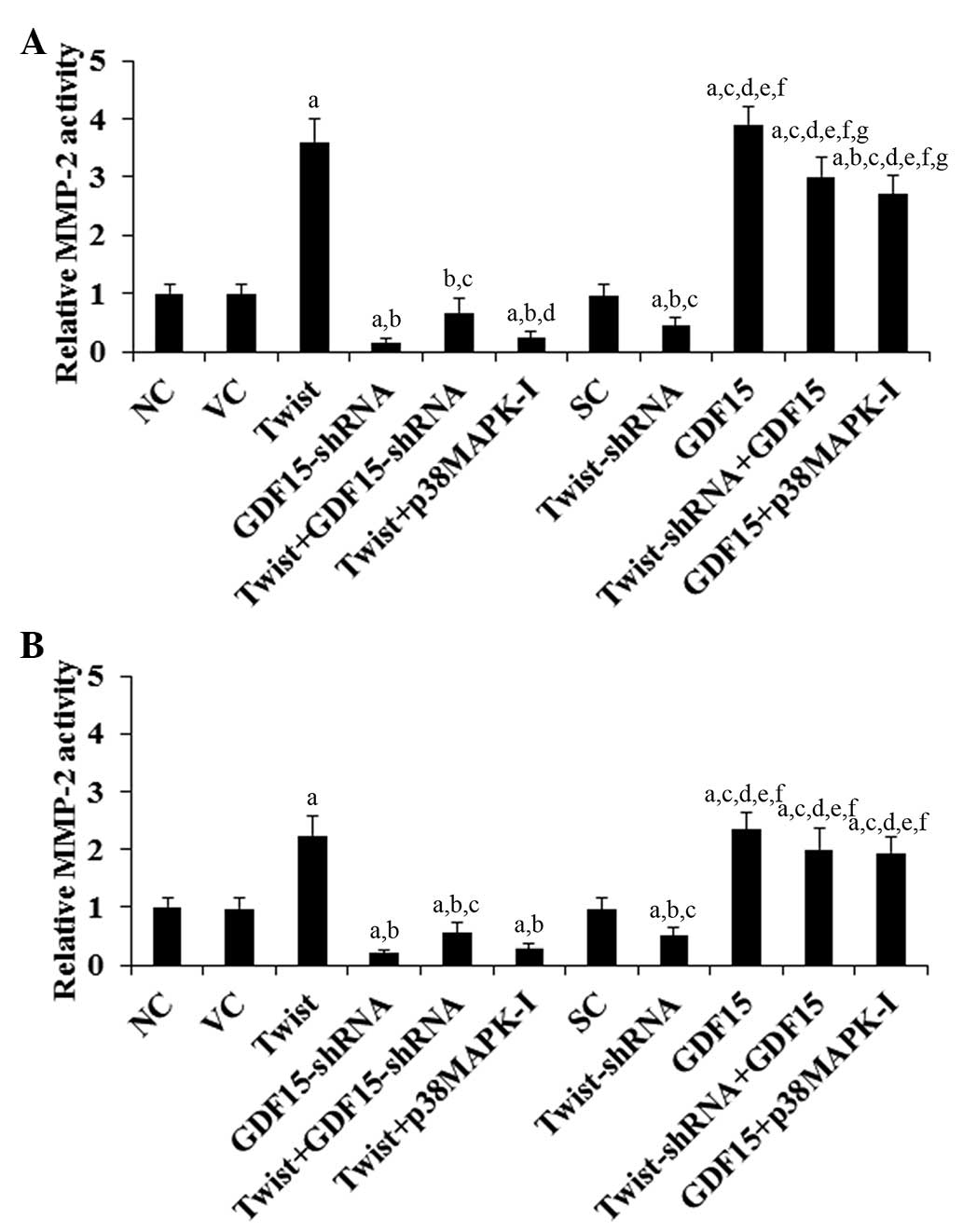 | Figure 5Effect of overexpression and
knockdown of Twist and GDF15 on MMP-2 activity in PC cells. In (A)
ASPC-1 and (B) BXPC-3 PC cells, the MMP-2 activity was determined
in NC cells, cells stably transfected with the empty pcDNA3.1
vector (VC), cells stably transfected with Twist, cells stably
transduced with GDF15-shRNA, cells stably transfected with Twist
and transduced with GDF15-shRNA (Twist + GDF15-shRNA), cells stably
transfected with Twist and treated with the selective p38 MAPK
inhibitor SB203580 (10 μM) for 30 min (Twist + p38MAPK-I),
cells stably transduced with SC shRNA (SC), cells stably transduced
with Twist-shRNA, cells stably transfected with GDF15, cells stably
transduced with Twist-shRNA and transfected with GDF15 (Twist-shRNA
+ GDF15) and cells stably transfected with GDF15 and treated with
SB203580 (10 μM) for 30 min (GDF15 + p38MAPK-I). The MMP-2
activity is shown as fold changes to that of NC (designated as 1).
Each experiment was repeated three times in duplicate. Data values
are expressed as the mean + standard deviation. Each experiment was
repeated three times in duplicate. Data values are expressed as the
mean + standard deviation. aP<0.05 vs. controls (NC,
VC and SC); bP<0.05 vs. Twist; cP<0.05
vs. GDF15-shRNA; dP<0.05 vs. Twist+GDF15-shRNA;
eP<0.05 vs. Twist+p38MAPK-I; fP<0.05
vs. Twist-shRNA; gP<0.05 vs. GDF15. GDF15, growth
differentiation factor 15; MMP-2, matrix metalloproteinase-2; PC,
pancreatic cancer; NC, normal control; MAPK, mitogen-activated
protein kinase; SC, scramble control. |
Effects of overexpression and knockdown
of Twist and GDF15 on PC cell chemoresistance to cisplatin
To examine the individual effect of and interaction
between Twist and GDF15 on PC chemoresistance, cisplatin IC50
values were examined in PC cells. A higher IC50 value was
considered to correspond with clinical chemoresistance to
cisplatin. As shown in Fig. 6,
after 48 h of cisplatin treatment, the cisplatin IC50 values for
ASPC-1 and BXPC-3 cells were 5.6 and 6.1 μM, respectively.
Overexpression of Twist significantly increased the IC50 values to
20.5 and 12.7 μM, respectively, which was eradicated by
knockdown of GDF15 or SB203580 (10 μM; Fig. 6). By contrast, knockdown of Twist
decreased the IC50 values to 2.3 and 3.4 μM, which was
completely reversed by overexpression of GDF15 (Fig. 6). Overexpression of GDF15 increased
the IC50 values of ASPC-1 and BXPC-3 cells to 23.2 and 15.4
μM, respectively, while knockdown of GDF15 decreased the
IC50 values to 1.3 and 2.1 μM, respectively (Fig. 6).
 | Figure 6Effect of overexpression and
knockdown of Twist and GDF15 on chemoresistance to cisplatin in PC
cells. (A) ASPC-1 and (B) BXPC-3 PC cells were treated with or
without various concentrations of cisplatin for 48 h. The half
maximal inhibitory concentration (IC50) values were determined in
NC cells, cells stably transfected with the empty pcDNA3.1 vector
(VC), cells stably transfected with Twist, cells stably transduced
with GDF15-shRNA, cells stably transfected with Twist and
transduced with GDF15-shRNA (Twist + GDF15-shRNA), cells stably
transfected with Twist and treated with the selective p38 MAPK
inhibitor SB203580 (10 μM) for 30 min (Twist + p38MAPK-I),
cells stably transduced with SC shRNA (SC), cells stably transduced
with Twist-shRNA, cells stably transfected with GDF15, cells stably
transduced with Twist-shRNA and transfected with GDF15 (Twist-shRNA
+ GDF15) and cells stably transfected with GDF15 and treated with
SB203580 (10 μM) for 30 min (GDF15 + p38MAPK-I). Each
experiment was repeated three times in duplicate. Data values are
expressed as the mean + standard deviation. aP<0.05
vs. controls (NC, VC and SC); bP<0.05 vs. Twist;
cP<0.05 vs. GDF15-shRNA; dP<0.05 vs.
Twist+GDF15-shRNA; eP<0.05 vs. Twist+p38MAPK-I;
fP<0.05 vs. Twist-shRNA; gP<0.05 vs.
GDF15. GDF15, growth differentiation factor 15; PC, pancreatic
cancer; NC, normal control; MAPK, mitogen-activated protein kinase;
SC, scramble control. |
Effects of overexpression and knockdown
of Twist and GDF15 on p38 MAPK activity in PC cells
The above results suggested that Twist promotes PC
cell invasion and chemoresistance to cisplatin largely through
regulating GDF15 expression by a p38 MAPK-dependent mechanism.
Therefore, the individual effect of and interaction between Twist
and GDF15 on p38 MAPK activity was next examined, which was
measured by phosphorylation of ATF2, a substrate of activated p38
MAPK (22). As evidenced by
increased levels of phosphorylated ATF2, overexpression of Twist
induced p38 MAPK activity by 4.2 and 3.9 fold in ASPC-1 and BXPC-3
cells, respectively, which was eradicated by SB203580 (10
μM) but not knockdown of GDF15 (Fig. 7). By contrast, knockdown of Twist
decreased p38 MAPK activity by ~70% in ASPC-1 and BXPC-3 cells,
which was not significantly affected by overexpression of GDF15
(Fig. 7). Compared with the
controls, overexpression and knockdown of GDF15 demonstrated no
significant effect on p38 MAPK activity (Fig. 7).
 | Figure 7Effect of overexpression and
knockdown of Twist and GDF15 on p38 MAPK activity in PC cells. In
(A) ASPC-1 and (B) BXPC-3 PC cells, the p38 MAPK activity was
determined by measuring the phosphorylation of ATF2, a substrate of
activated p38 MAPK. The levels of p-ATF2 were determined by western
blot analysis in NC cells (NC, lane 1), cells stably transfected
with the empty pcDNA3.1 vector (VC, lane 2), cells stably
transfected with Twist (lane 3), cells stably transduced with
GDF15-shRNA (lane 4), cells stably transfected with Twist and
transduced with GDF15-shRNA (Twist + GDF15-shRNA, lane 5), cells
stably transfected with Twist and treated with the selective p38
MAPK inhibitor SB203580 (10 μM) for 30 min (Twist +
p38MAPK-I, lane 6), cells stably transduced with SC shRNA (SC, lane
7), cells stably transduced with Twist-shRNA (lane 8), cells stably
transfected with GDF15 (lane 9), cells stably transduced with
Twist-shRNA and transfected with GDF15 (Twist-shRNA + GDF15, lane
10) and cells stably transfected with GDF15 and treated with
SB203580 (10 μM) for 30 min (GDF15 + p38MAPK-I, lane 11).
The p38 MAPK activity is shown as fold changes to that of NC
(designated as 1). Each experiment was repeated three times in
duplicate. Data are expressed as the mean + standard deviation.
aP<0.05 vs. controls (NC, VC and SC);
bP<0.05 vs. Twist; cP<0.05 vs.
GDF15-shRNA; dP<0.05 vs. Twist + GDF15-shRNA;
eP<0.05 vs. Twist + p38MAPK-I; fP<0.05
vs. Twist-shRNA; gP<0.05 vs. GDF15;
hP<0.05 vs. Twist-shRNA + GDF15. GDF15, growth
differentiation factor 15; MAPK, mitogen-activated protein kinase;
PC, pancreatic cancer; NC, normal control; MAPK, mitogen-activated
protein kinase; ATF2, activating transcription factor 2; p-ATF2,
phosphorylated ATF2; SC, scramble control. |
Discussion
The present study demonstrated that Twist promotes
PC cell invasion and cisplatin chemoresistance largely through
GDF15. Overexpression and knockdown of Twist in PC cells increased
and decreased the expression of GDF15, respectively, at the mRNA
and the protein levels, but not vice versa. The findings suggest
that Twist induces GDF15 expression in PC cells at the gene
transcription/mRNA level. In addition, a selective p38 MAPK
inhibitor readily eliminated Twist-induced GDF15 expression in PC
cells without significantly altering the expression of Twist,
indicating that Twist induces GDF15 expression in a p38
MPAK-dependent manner in PC cells. How Twist transcriptionally
regulates the expression of GDF15 through p38 MAPK in PC cells will
be examined in future studies.
As evidenced by gene overexpression and knockdown
experiments, Twist and GDF15 individually promotes PC cell invasion
and cisplatin resistance. In addition, knockdown of GDF15
eradicated the stimulatory effects of overexpressing Twist, while
overexpression of GDF15 completely reversed the inhibitory effects
of knocking down Twist. The findings indicate that GDF15 is
functionally downstream of Twist and largely mediates the promoting
effects of Twist on PC cell invasion and cisplatin resistance,
which corroborates our finding that Twist induces GDF15 expression
in PC cells.
While the selective p38 MAPK inhibitor SB203580
abrogated the promoting effects of Twist overexpression on PC cell
invasion and cisplatin resistance, overexpression of GDF15
significantly augmented PC cell invasion and cisplatin resistance
in the presence of SB203580. The results suggest that Twist and
GDF15 act functionally upstream and downstream of p38 MAPK,
respectively. This is in agreement with our findings that while
overexpression and knockdown of Twist increased and decreased p38
MAPK activity, respectively, GDF15 demonstrated no significant
effect on p38 MAPK activity in PC cells. Previous studies have
suggested an important role of p38 MAPK in PC cell invasion
(25,26). Our findings indicate that p38 MAPK
mediates Twist-induced GDF15 expression in PC cells, which markedly
promotes PC cell invasion. Thus, the importance of p38 MAPK
signaling in PC progression is at least partially fulfilled through
Twist/GDF15 signaling.
MMPs are critical for cancer cell invasion (17,18).
Previous studies have suggested that MMP-2 is important for PC cell
invasion in vitro (26). In
the present study, it was found that Twist markedly increased MMP-2
expression/activity through GDF15, suggesting that the Twist/GDF15
signaling axis is important for PC progression. GDF15 has been
reported to have tumorigenic and anti-tumorigenic activities
(11,12). While Twist has been widely
associated with the initial phase of metastatic progression
(8), a previous study demonstrated
that Twist decreases cisplatin resistance in osteosarcoma cells,
suggesting that Twist, like GDF15, has a dual role in cancer cell
malignancy and chemoresistance, depending on tissue specificity
(27). Since Twist and GDF15 have
been found to be overexpressed in various types of cancer (8,28),
it would be of significance to define the role of the Twist/GDF15
signaling axis in other types of cancer besides PC in future
studies.
In conclusion, the present study for the first time,
to the best of our knowledge, demonstrated that Twist promotes PC
cell invasion and cisplatin chemoresistance through inducing GDF15
expression by a p38 MAPK-dependent mechanism. This adds new
insights into the molecular mechanisms underlying PC progression
and chemoresistance.
References
|
1
|
Jiang H, Duan B, He C, et al: Cytoplasmic
HSP90α expression is associated with perineural invasion in
pancreatic cancer. Int J Clin Exp Pathol. 7:3305–3311. 2014.
|
|
2
|
Spano JP, Chodkiewicz C, Maurel J, et al:
Efficacy of gemcitabine plus axitinib compared with gemcitabine
alone in patients with advanced pancreatic cancer: An open-label
randomised phase II study. Lancet. 371:2101–2108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
López R, Méndez CM, Fernández MJ, et al:
Phase II trial of erlotinib plus capecitabine as first-line
treatment for metastatic pancreatic cancer (XELTA study).
Anticancer Res. 33:717–723. 2013.PubMed/NCBI
|
|
4
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung TW, Choi HJ, Kim SJ, et al: The
ganglioside GM3 is associated with cisplatin-induced apoptosis in
human colon cancer cells. PLoS One. 9:e927862014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gresham GK, Wells GA, Gill S, Cameron C
and Jonker DJ: Chemotherapy regimens for advanced pancreatic
cancer: A systematic review and network meta-analysis. BMC Cancer.
14:4712014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramfidis VS, Psyrri A, Syrigos KN and Saif
MW: First line treatment for metastatic pancreatic adenocarcinoma:
Looking for the step forward. JOP. 15:286–288. 2014.PubMed/NCBI
|
|
8
|
Entz-Werlé N, Choquet P, Neuville A, et
al: Targeted apc;twist double-mutant mice: A new model of
spontaneous osteosarcoma that mimics the human disease. Transl
Oncol. 3:344–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohuchida K, Mizumoto K, Ohhashi S, et al:
Twist, a novel oncogene, is upregulated in pancreatic cancer:
Clinical implication of Twist expression in pancreatic juice. Int J
Cancer. 120:1634–1640. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Breit SN, Johnen H, Cook AD, et al: The
TGF-β superfamily cytokine, MIC-1/GDF15: A pleotrophic cytokine
with roles in inflammation, cancer and metabolism. Growth Factors.
29:187–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mimeault M and Batra SK: Divergent
molecular mechanisms underlying the pleiotropic functions of
macrophage inhibitory cytokine-1 in cancer. J Cell Physiol.
224:626–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khaled YS, Elkord E and Ammori BJ:
Macrophage inhibitory cytokine-1: A review of its pleiotropic
actions in cancer. Cancer Biomark. 11:183–190. 2012.PubMed/NCBI
|
|
13
|
Koopmann J, Buckhaults P, Brown DA, et al:
Serum macrophage inhibitory cytokine 1 as a marker of pancreatic
and other periampullary cancers. Clin Cancer Res. 10:2386–2392.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ozkan H, Demirbaş S, Ibiş M, Akbal E and
Köklü S: Diagnostic validity of serum macrophage inhibitor cytokine
and tissue polypeptide-specific antigen in pancreatobiliary
diseases. Pancreatology. 11:295–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen YZ, Liu D, Zhao YX, Wang HT, Gao Y
and Chen Y: Diagnostic performance of serum macrophage inhibitory
cytokine-1 in pancreatic cancer: A meta-analysis and
meta-regression analysis. DNA Cell Biol. 33:370–377. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuo N, Shiraha H, Fujik T, et al: Twist
expression promotes migration and invasion in hepatocellular
carcinoma. BMC Cancer. 9:2402009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang B, Feng P, Xiao Z and Ren EC: LIM and
SH3 protein 1 (Lasp1) is a novel p53 transcriptional target
involved in hepatocellular carcinoma. J Hepatol. 50:528–537. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng Y, Hu J, Ma J, et al: RNAi-mediated
silencing of VEGF-C inhibits non-small cell lung cancer progression
by simultaneously down-regulating the CXCR4, CCR7, VEGFR-2 and
VEGFR-3-dependent axes-induced ERK, p38 and AKT signalling
pathways. Eur J Cancer. 47:2353–2363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jo YK, Park SJ, Shin JH, et al: ARP101, a
selective MMP-2 inhibitor, induces autophagy-associated cell death
in cancer cells. Biochem Biophys Res Commun. 404:1039–1043. 2011.
View Article : Google Scholar
|
|
20
|
Qazi H, Shi ZD and Tarbell JM: Fluid shear
stress regulates the invasive potential of glioma cells via
modulation of migratory activity and matrix metalloproteinase
expression. PLoS One. 6:e203482011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding X, Zhang Z, Li S and Wang A:
Combretastatin A4 phosphate induces programmed cell death in
vascular endothelial cells. Oncol Res. 19:303–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Wu H and Miller AH: Interleukin
1alpha (IL-1alpha) induced activation of p38 mitogen-activated
protein kinase inhibits glucocorticoid receptor function. Mol
Psychiatry. 9:65–75. 2004. View Article : Google Scholar
|
|
23
|
Leelahavanichkul K, Amornphimoltham P,
Molinolo AA, Basile JR, Koontongkaew S and Gutkind JS: A role for
p38 MAPK in head and neck cancer cell growth and tumor-induced
angiogenesis and lymphangiogenesis. Mol Oncol. 8:105–118. 2014.
View Article : Google Scholar :
|
|
24
|
Li Y, Liao Q, Li K, Zhong D, Weng X and Mi
M: Knockdown of endothelin A receptor expression inhibits
osteosarcoma pulmonary metastasis in an orthotopic xenograft mouse
model. Mol Med Rep. 5:1391–1395. 2012.PubMed/NCBI
|
|
25
|
Cui XP, Qin CK, Zhang ZH, et al: HOXA10
promotes cell invasion and MMP-3 expression via TGFβ2-mediated
activation of the p38 MAPK pathway in pancreatic cancer cells. Dig
Dis Sci. 59:1442–1451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan F, Ma S, Cao W, et al: SDF-1α
upregulation of MMP-2 is mediated by p38 MAPK signaling in
pancreatic cancer cell lines. Mol Biol Rep. 40:4139–4146. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu J, Liao Q, He H, Zhong D and Yin K:
TWIST interacts with β-catenin signaling on osteosarcoma cell
survival against cisplatin. Mol Carcinog. 53:440–446. 2014.
View Article : Google Scholar
|
|
28
|
Bauskin AR, Brown DA, Kuffner T, et al:
Role of macrophage inhibitory cytokine-1 in tumorigenesis and
diagnosis of cancer. Cancer Res. 66:4983–4986. 2006. View Article : Google Scholar : PubMed/NCBI
|





















