Introduction
Congenital heart disease is the most common type of
congenital malformation (1). Only
certain types of congenital heart disease are able to recover
naturally, and others present with increased severity and frequency
of complications with increasing age (2). Interventional therapy is one of the
therapeutic methods used for congenital heart disease (2). Blocking cardiac defects with metal
occluders of different shapes is a commonly used surgical
alternative, with the nickel-chromium (Ni-Cr) alloy occluder being
most widely used in the clinical setting (3).
The clinical application of the Ni-Cr alloy occluder
is a result of advances in medical technology and material
sciences, and that of a process of continuous research and
improvement. Use of the Ni-Cr alloy occluder avoids the risks and
trauma of open-heart surgery, allowing short hospital stays and
fast recovery for patients (4).
However, long-term nickel-based alloy implantation has been
increasingly documented to be associated with biological effects,
including cytotoxicity and genotoxicity, eliciting tissue
inflammation and potential sensitization, thus raising questions
over its bio-safety (5). In
previous experiments following-up children receiving an atrial
septal defect occluder implantation, it was observed that the
nickel concentration in the blood was significantly increased 24 h
and one month subsequent to surgery (Zhang et al;
unpublished results). Other studies have also reported that the
Ni-Cr alloy occluder implantation results in the release of the
nickel ion into the chambers of the heart, which may result in cell
damage due to local and systemic toxicity (6) and lead to headaches, dyspnea and
other unpleasant symptoms (7,8).
Being the 'first line of defense', macrophages are
essential determinants of implant biocompatibility (9). Pro-inflammatory studies have observed
that during the first six weeks following implantation, macrophages
interact heavily with the prosthetic device (10). Therefore, the present study aimed
to investigate the cytotoxicity of nickel ions on THP-1, a human
monocytic cell line derived from the peripheral blood, and to
explore the underlying mechanism of the toxicity of the nickel
alloy in the heart during its application for congenital heart
disease. The present study additionally aimed to provide further
insights for physicians and patients on the clinical use and
adverse effects of nickel alloy implantation in congenital heart
disease and various additional associated diseases.
Materials and methods
Materials
The human monoblastic leukemia cell line THP-1 was
obtained from the American Type Culture Collection (Manassas, VA,
USA). Cell culture reagents RPMI 1640 medium and fetal bovine serum
(FBS) were from Gibco-BRL (Invitrogen Life Technologies, Carlsbad,
CA, USA). NiCl2·6H2O, MTT, Hoechst 33342 and
propidium iodide (PI) were obtained from Sigma-Aldrich (St. Louis,
MO, USA). TRIzol reagent was obtained from Invitrogen Life
Technologies. All reagents were of analytical grade.
Cell culture and drug treatment
THP-1 cells were cultured in RPMI 1640 medium
supplemented with 10% FBS in a humidified atmosphere of 5%
CO2 and 95% air at 37°C. Cells were serum-starved for 48
h prior to treatment with NiCl2·6H2O (25, 50,
100, 200, 400 or 800 µM) for 24, 48 or 72 h,
respectively.
Cell growth assay
The MTT assay and cell-counting method were applied
to detect THP-1 cell proliferation subsequent to receiving
different treatment regimens. The apoptosis of THP-1 in response to
nickel ion challenge was detected using flow cytometry (FCM). For
the MTT assay, THP-1 cells were seeded in 96-well plates at a
density of 4×105 cells/well and then exposed to various
concentrations of NiCl2·6H2O for the
different time periods indicated (25, 50, 100, 200, 400 and 800
µM for 24, 48 and 72 h). At the end of the treatments, the
plates were centrifuged at 2,000 × g for 5 min and the supernatants
were discarded. Then cells were further incubated with RPMI 1640
(200 µl/well) supplemented with 0.2% MTT (20 µl/well)
for 4 h at 37°C. Following MTT incubation, 100 µl 100%
dimethyl sulfoxide was added to dissolve the formazan crystals.
Viable cells were counted by reading the absorbance at 570 nm using
a 96-well plate reader (Multiskan MK3; Thermo Fisher Scientific,
Waltham, MA, USA).
Cytotoxicity analysis
Cells were plated on six-well plates and were
allowed to adhere. Following different treatments for 48 h, cells
from each group (5×105 cells/group re-suspended in 0.5
ml ice-cold RPMI 1640) were transferred into a clean centrifuge
tube and incubated with 1.25 µl Hoechst 33342 for 15 min at
room temperature in the dark to stain the nuclei of all cells.
Subsequently, the plates were centrifuged at 2,000 × g for 5 min
and the supernatants were discarded. Cells were then re-suspended
in 0.5 ml ice-cold RPMI 1640 prior to the addition of 10 µl
PI (5 µg/ml) for staining of the dead cells. The samples
were kept on ice in the dark and immediately analyzed using a
FACSCalibur Cell flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA). Cytotoxicity was expressed as the percentage of dead
cells (PI-positive) relative to the total number of cells.
RNA-sequencing assay
THP-1 cells were cultured with different
concentrations of NiCl2·6H2O (25, 50 or 100
µM) for 1 h following starvation for 48 h. Total RNA was
prepared by the acid phenol method using TRIzol reagent in
accordance with the manufacturer's instructions. Areas containing
THP-1 RNAs were sent to Beijing Genomics Institution (Shenzhen,
China) for Illumina sequencing.
Pathway analysis of differentially
expressed genes
Pathways were constructed using the Kyoto
Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/) database using the
Web-based GEne SeT Analysis Toolkit (http://bioinfo.vanderbilt.edu/webgestalt/) to identify
differential networks and core regulators involved in the
nickel-induced cytotoxic response. The analysis completed was
limited to categories with P<0.05.
Quantitative polymerase chain reaction
(qPCR)
PCR was performed using the total RNA (1 µg)
prepared for the RNA-sequencing assay with the primers listed in
Table I under the following
conditions: 40 cycles of 94°C for 2 min, 94°C for 30 sec, 58°C for
30 sec and 72°C for 20 sec. RNA expression levels were detected by
fluorescent qPCR in the presence of SYBR Green on a Bio-Rad iCycler
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
 | Table IPrimer sequences for quantitative
polymerase chain reaction. |
Table I
Primer sequences for quantitative
polymerase chain reaction.
| Genes | Sequence |
|---|
| EPOR | Forward:
GGGCAACTACAGCTTCTCCT |
| Reverse:
ATGGCATGGACTGTGGTCAT |
| RELB | Forward:
TGATCCACATGGAATCGAGA |
| Reverse:
CAGGAAGGGATATGGAAGCA |
| FIGF2 | Forward:
ATGGACCAGTGAAGCGATCAT |
| Reverse:
GTTCCTCCAAACTAGAAGCAGC |
| SPI-1 | Forward:
GAAAGGTGGGTGAAAGGACCA |
| Reverse:
TGTTGGACTCCTTTGGGCAG |
| TGF-β1 | Forward:
TACAGCACGGTATGCAAGCC |
| Reverse:
GCAACCGATCTAGCTCACAGAG |
| CXCL16 | Forward:
GACATGCTTACTCGGGGATTG |
| Reverse:
GGACAGTGATCCTACTGGGAG |
| CRLF2 | Forward:
AGTGACGGTGACGTGTTCTG |
| Reverse:
CTATGGTGACGTTGCAGGTATT |
| GAPDH | Forward:
TGTTCGTCATGGGTGTGAAC |
| Reverse:
ATGGCATGGACTGTGGTCAT |
Statistical analysis
Values are expressed as the mean ± standard
deviation. The independent-samples t-test was used to compare two
samples and one-way analysis of variance was used for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
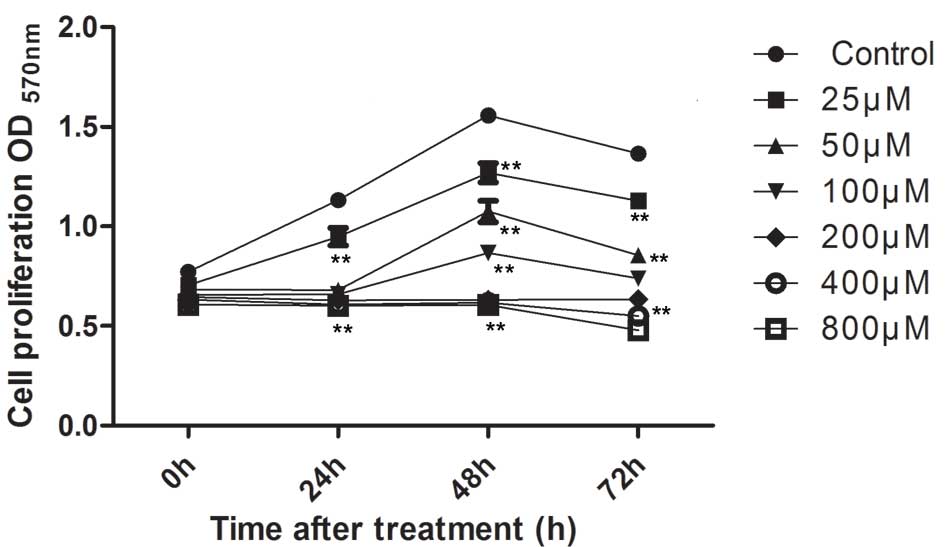
Nickel ions inhibit THP-1 cell
growth
The effect of nickel ions on the proliferation of
THP-1 was investigated. Cells were treated with
NiCl2·6H2O (25, 50, 100, 200, 400 and 800
µM) for 24, 48 and 72 h, and an MTT assay was conducted in
order to examine the growth-inhibitory effect of the treatments. As
presented in Fig. 1, high
concentrations of nickel chloride significantly suppressed cell
proliferation at the three concentrations used (200, 400 and 800
µM; P<0.001). Cell proliferation was completely abolished
following incubation of cells with nickel chloride at these high
concentrations. Lower concentrations of
NiCl2·6H2O (25–100 µM) inhibited cell
proliferation in a time- and dose-dependent manner as compared with
that in the control group.
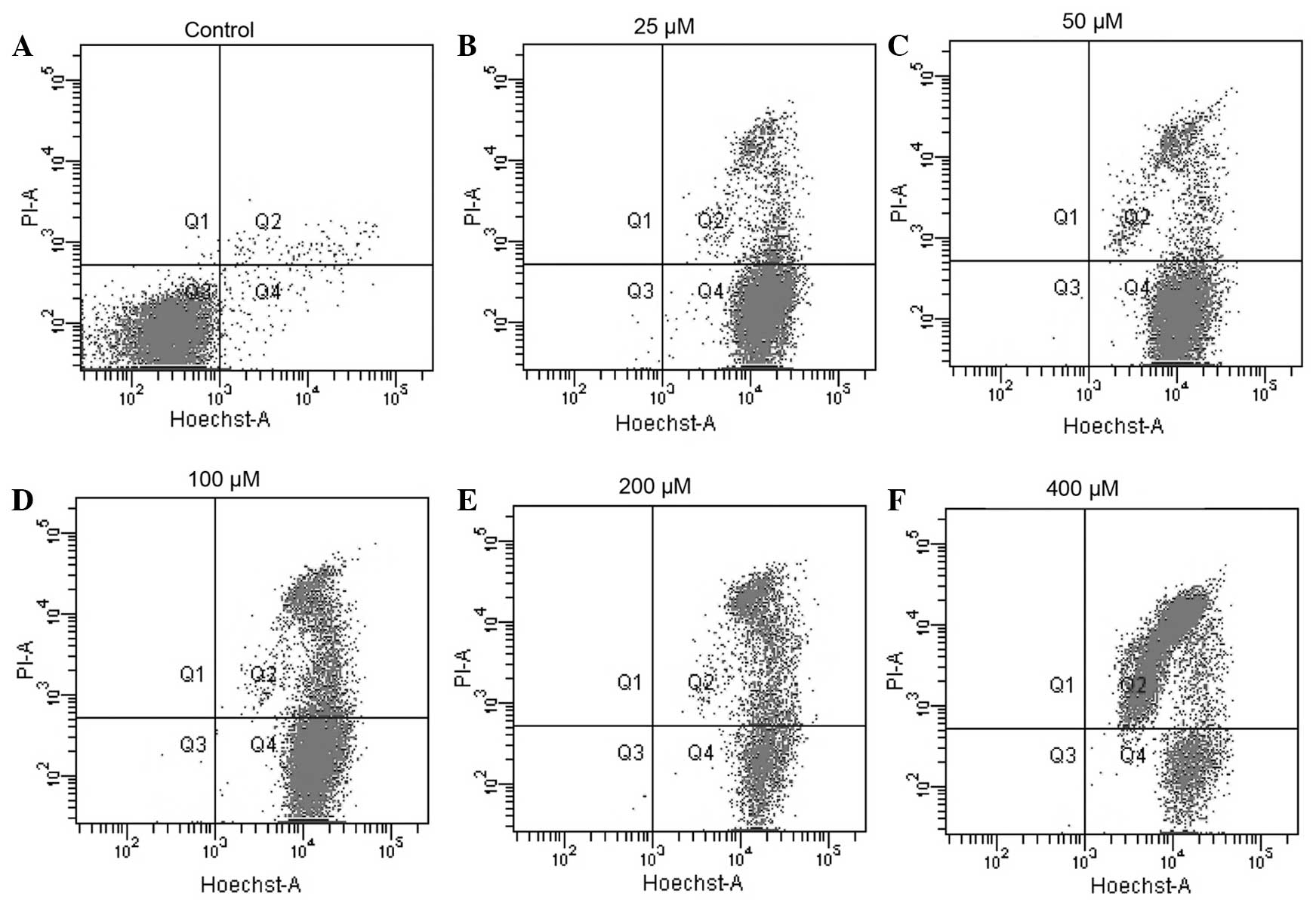
Nickel ions exert toxic effects on THP-1
cells
To investigate the toxicity of the nickel ion on
THP-1 cells, the viability of THP-1 cells treated with nickel
chloride was assessed via FCM. As illustrated in Fig. 2, treatment with nickel chloride
(25–400 µM) for 48 h reduced cell viability in a
dose-dependent manner. The apoptotic rates of the 25, 50, 100, 200
and 400 µM treatment groups were 14.4, 31.4, 45.7, 61.9 and
65.7%, respectively. The apoptotic rate of the cells was then
examined by Hoechst 33342/PI double staining following the various
treatment regimens. In this assay, cytotoxicity of the nickel
challenge was indicated as the percentage of dead cells
(PI-positive) relative to the total number of cells
(Hoechst-positive). The extent of THP-1 cell apoptosis was
identified to be proportional to the nickel ion concentration.
Incubation of THP-1 for 48 h with low concentrations of nickel (25
and 50 µM) resulted in low apoptotic rates (Fig. 2B and C). By contrast, incubation
with high concentrations of nickel ions (>100 µM) was
observed to have a negative effect on cell viability as indicated
by the increased percentage of dead cells (25.2, 60.7 and 85.7% for
100, 200 and 400 µM nickel, respectively) (Fig. 2D, E and F).
Differential gene expression of THP-1
cells following treatment with nickel ions
To elucidate the transcriptional events associated
with nickel ion-induced THP-1 cytotoxicity, a high-throughput
RNA-sequencing assay was conducted using RNA samples extracted from
the nickel-challenged cells. Electrophoresis and ethidium bromide
staining indicated the successful extraction of RNA products from
treated cells. A total of 1 µg total RNA was then pooled
from each treatment group (control, 25, 50 and 100 µM
NiCl2·6H2O) for comparison of the gene
expression profiles via Illumina sequencing. The differentially
expressed genes were then filtered. To characterize the functional
consequences of alterations in gene expression associated with
nickel cytotoxicity, pathway analysis of DEGs was conducted using
the web-based KEGG pathway database. The top four enriched KEGG
pathway categories of upregulated genes were cytokine-cytokine
receptor interaction, osteoclast differentiation, steroid
biosynthesis and the chemokine signaling pathway (Table II). The top four enriched KEGG
pathway categories of downregulated genes were olfactory
transduction, taste transduction, RNA transport and the mRNA
surveillance pathway (Table
III).
 | Table IITop four enriched Kyoto Encyclopedia
of Genes and Genomes pathway categories of upregulated genes. |
Table II
Top four enriched Kyoto Encyclopedia
of Genes and Genomes pathway categories of upregulated genes.
| Pathway | P-value | Upregulated
genes |
|---|
| Cytokine-cytokine
receptor interaction |
6.77×10−8 | TGFB1, CXCR4,
TNFRSF1B, CD70, CCL5, EGFA, VEGFB, TNFRSF10B, TNF, IL10RA, IL3RA,
TNFSF9, CSF2RA, EPOR, TNFSF13B, FLT3LG, CD40, IL2RG, IL23A, CSF1,
CXCL16, PDGFA, TNFRSF12A, CRLF2, CCL2, CCL24, CTF1, CCL3, FIGF,
CCL13, INHBE, LTA, CCL4, CCL21, XCR1, LEP, TNFSF18, CCL3L3, CL3L1,
GDF6, CCL4L2, IL17B, GH1, CXCL11, TNFRSF4, INHBA, CCR7, CCR5,
CCL26, IFNB1, GDF5, TNFRSF18, CCL8 |
| Osteoclast
differentiation |
1.07×10−4 | SPI1, CYBA, TGFB1,
NCF4, JUND, RAC1, STAT1, GAB2, NFKBIA, FCGR1A, JUN, PPARG, IKBKG,
LILRB5, TNF, NFKB2, LILRA5, RELB, LILRB2, OSCAR, NCF1, SOCS3, CSF1,
SOCS1, FCGR1B, BLNK, LILRB3, LILRA6, IFNB1 |
| Steroid
biosynthesis |
1.13×10−4 | FDFT1, SQLE, DHCR7,
EBP, SDHL, TM7SF2, HSD17B7, CYP51A1, SOAT2 |
| Chemokine signaling
pathway |
2.41×10−4 | GNB2, CXCR4, RAC1,
STAT1, FOXO3, CCL5, HCK, NFKBIA, NFKBIB, HRAS, IKBKG, SHC2, FGR,
ARRB2, NCF1, ADCY2, CXCL16, AC092535.1, CCL2, GRK7, CCL24, CCL3,
CCL13, CCL4, CCL21, CXCR1, ADCY8, CCL3L3, GNGT2, CCL3L1, CCL4L2,
CXCL11, CCR7, CCR5, CCL26, CCL8 |
 | Table IIITop four enriched Kyoto Encyclopedia
of Genes and Genomes pathway categories of downregulated genes. |
Table III
Top four enriched Kyoto Encyclopedia
of Genes and Genomes pathway categories of downregulated genes.
| Pathway | P-value | Downregulated
genes |
|---|
| Olfactory
transduction |
2.91×10−4 | PRKACB, CAMK2D,
PDC, CNGA4, CNGB1, OR2V1, CYorf17, OR11L1, OR10A2, OR52L1, OR52K1,
OR6V1, OR2T33, DAPL1, OR6K3, OR13G1 |
| Taste
transduction |
1.22×10−3 | PRKACB, TAS2R10,
TAS2R50, TAS2R4, TAS2R46, GRM4, TAS2R43, TAS2R13, TAS2R60, TAS2R3,
GNG13, TAS2R39 |
| RNA transport |
1.61×10−3 | EIF4G2, XPO1, TPR,
TMEM48, PNN, NUP205, DDX39B, PRMT5, NUP155, NUP160, NCBP1, POP1,
NUP50, GEMIN5, NUP107, NUP88, NUP54, NXT2, NXF3, NUPL1, THOC1,
SUMO1, EIF4E, NUP43, TRNT1, RPP40, PABPC1L, SUMO4, PABPC4L,
NXF2B |
| mRNA surveillance
pathway |
2.13×10−3 | GSPT1, NUDT21, PNN,
PAPOLA, DDX39B, CPSF3, NCBP1, CSTF3, RNGTT, HBS1L, NXT2, NXF3,
PAPOLG, CSTF2T, PPP2R3A, PABPC1L, PABPC4L, PAPOLB, NXF2B |
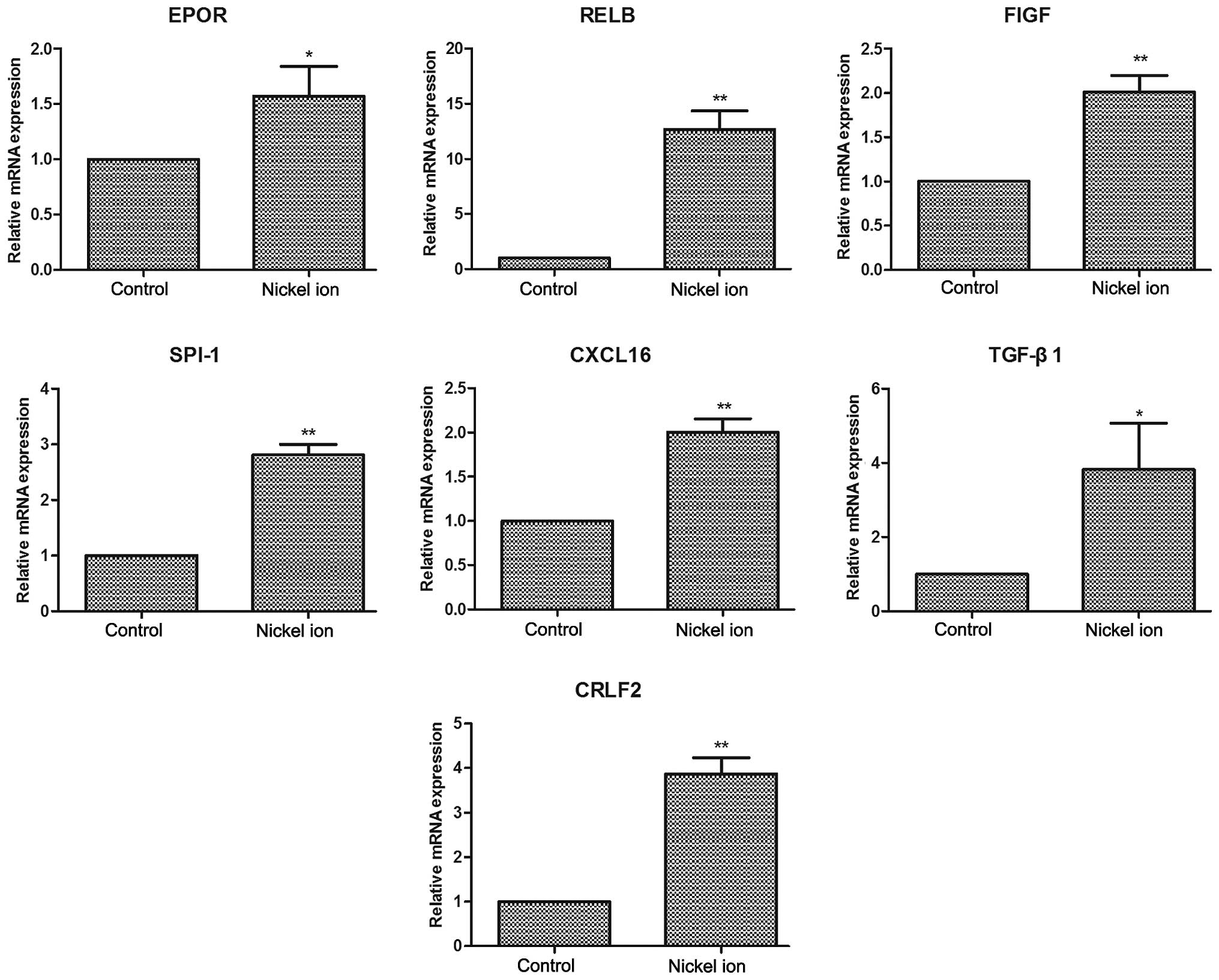
Confirmation of cytotoxicity-associated
genes
The authenticity and reliability of the
cytotoxicity-associated genes was then verified by specifically
examining the expression levels of ten congenital heart
disease-associated genes, including EPOR, As presented in Fig. 3, RELB, FIGF, SPI-1, TGF-β1, CXCL16
and CRLF2 through qPCR analysis. RELB, FIGF, SPI-1, CXCL16 and
CRLF2 mRNA expression levels of nickel-treated cells presented were
significantly increased compared with those in the control group
(P<0.01). TGF-β1 and EPOR mRNA expression levels were also
significantly increased following nickel challenge (P<0.05).
Discussion
The present study investigated the cytotoxic effects
of nickel ions on THP-1 cells and the possible mechanisms involved.
The results suggested that nickel ions exerted a growth-inhibitory
effect on THP-1 cells and in particular, it was demonstrated that
the toxicity of nickel ions to THP-1 cells may be associated with
the differential expression of certain genes, including EPOR, RELB,
FIGF, SPI-1, TGF-β1, CXCL16 and CRLF2.
In the present study, it was observed that the
proliferation of THP-1 cells gradually increased following
stimulation for a short period of time with low concentrations
(25–100 µM) of nickel ions, which may be associated with the
incompletely compromised ability of the cells to resist and
metabolize the insult. However, with the increases in the nickel
ion concentration and incubation time, a rapid decline in the
number of live cells was observed. The cells began to die when
incubated with high concentrations of nickel ions (200–800
µM). Cell viability analysis suggested that the
concentration and incubation time with the nickel ions may be two
important indicators of the toxic effect of nickel. Thus, correct
assessment of nickel ion toxicity requires to comprehensively take
the concentration and time period of its use into account.
Multiple studies have examined the association
between nickel exposure and blood and heart diseases (11,12).
The toxicity of the nickel ion to the heart has become a focus of
research in recent years since nickel-associated products are being
increasingly used in heart disease therapy (13). Accumulating evidence has
demonstrated that nickel exposure negatively regulates cardiac
function (14,15). The results from a previous study
indicated that heart rate variability (a predictor for arrhythmias,
mortality risk and severity of illness) was regulated by
nickel-induced oxidative-inflammatory responses (14). Thus, it is suggested that nickel
may cause certain cardio-regulatory responses and may induce
varying responses during systolic and diastolic phases (15).
Although the phenomenon of nickel-induced
cardiotoxicity has raised increasing concerns, only few studies are
available on the mechanism of nickel ion-induced toxicity to the
heart during its use in congenital heart disease (16). Previous studies have demonstrated
that congenital heart disease is regulated and affected by multiple
genes and pathways, including EPOR, SPI-1, p38/MAPK and TGF-β1
(17–20). It remains to be fully elucidated
whether there is an association between nickel toxicity and
congenital heart disease, and if so, what the associated genes and
pathways are. In the present study, the expression levels of EPOR,
RELB, FIGF, SPI-1, TGF-β1, CXCL16 and CRLF2 were observed to be
significantly increased subsequent to nickel ion stimulation in
THP-1 cells. Nickel can also generate cytotoxic reactive oxygen
species through TGF-β1 activation (20). CRLF2 gene alterations have been
observed to correlate with poor prognosis in Japanese
BCR-ABL1-negative high-risk B-cell precursor acute lymphoblastic
leukemia (21), which suggests
that the downregulation of CRLF2 may block the inflammatory effect
of nickel ions. FIGF was detected in adult lung and heart tissues
and was observed to exhibit mitogenic activity on fibroblasts
(22,23). CXCL16 appears to have a role in the
homing of CD4(+) T cells in acute and chronic rejection models of
heart allotransplantation (24).
Nickel ion stimulation exerted differential effects on these heart
disease-associated genes, which may indicate that the toxicity
mechanism of the nickel ion may be regulated by these genes.
Further studies, including loss-of-function analysis by RNA
inference and in vivo experiments, are required to fully
elucidate the mechanistic significance of these genes and pathways
in the observed nickel cytotoxicity.
In conclusion, the present study demonstrated that
the toxicity of the nickel ion to THP-1 cells may be controlled by
or is associated with certain genes, including EPOR, RELB, FIGF,
SPI-1, TGF-β1, CXCL16 and CRLF2. These observations provided
insight and advance the understanding of the genetic basis of
nickel ion-induced toxicity in its therapeutic use for congenital
heart disease.
References
|
1
|
Stan MN, Ammash NM, Warnes CA, Brennan MD,
Thapa P, et al: Body mass index and the development of
amiodarone-induced thyrotoxicosis in adults with congenital heart
disease - a cohort study. Int J Cardiol. 167:821–826. 2013.
View Article : Google Scholar
|
|
2
|
Schranz D and Michel-Behnke I: Advances in
interventional and hybrid therapy in neonatal congenital heart
disease. Semin Fetal Neonatal Med. 18:311–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mori Y, Takahashi K and Nakanishi T:
Complications of cardiac catheterization in adults and children
with congenital heart disease in the current era. Heart Vessels.
28:352–359. 2013. View Article : Google Scholar
|
|
4
|
Dancea A, Justino H and Martucci G:
Catheter intervention for congenital heart disease at risk of
circulatory failure. Can J Cardiol. 29:786–795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Braga M, Quecchia C, Perotta C, Timpini A,
Maccarinelli K, et al: Systemic nickel allergy syndrome: Nosologic
framework and usefulness of diet regimen for diagnosis. Int J
Immunopathol Pharmacol. 26:707–716. 2013.PubMed/NCBI
|
|
6
|
Sutton NJ, Greenberg MA, Menegus MA, Lui G
and Pass RH: Caring for the adult with congenital heart disease in
an adult catheterization laboratory by pediatric interventionalists
- safety and efficacy. Congenit Heart Dis. 8:111–116. 2013.
View Article : Google Scholar
|
|
7
|
Karaś Z and Bładek J: Nickel in the
environment and morbid symptoms. Przegl Lek. 61(Suppl 3): 55–57.
2004.In Polish.
|
|
8
|
Sunderman FW Jr, Dingle B, Hopfer SM and
Swift T: Acute nickel toxicity in electroplating workers who
accidently ingested a solution of nickel sulfate and nickel
chloride. Am J Ind Med. 14:257–266. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lavin Y and Merad M: Macrophages:
Gatekeepers of tissue integrity. Cancer Immunol Res. 1:201–209.
2013. View Article : Google Scholar
|
|
10
|
Röstlund T, Thomsen P, Bjursten LM and
Ericson LE: Difference in tissue response to nitrogen-ion-implanted
titanium and c.p. titanium in the abdominal wall of the rat. J
Biomed Mater Res. 24:847–860. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim Y, Wang X, Zhang XS, Grigoriu S, Page
R, et al: Escherichia coli toxin/antitoxin pair MqsR/MqsA regulate
toxin CspD. Environ Microbiol. 12:1105–1121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou YP, Gu JY, Shao YF, Song YF, Jing YH,
et al: The characteristics of placental transfer and tissue
concentrations of nickel in late gestational rats and fetuses.
Placenta. 32:277–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ries MW, Kampmann C, Rupprecht HJ,
Hintereder G, Hafner G and Mayer J: Nickel release after
implantation of the Amplatzer occluder. Am Heart J. 145:737–741.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chuang HC, Hsueh TW, Chang CC, Hwang JS,
Chuang KJ, et al: Nickel-regulated heart rate variability: The
roles of oxidative stress and inflammation. Toxicol Appl Pharmacol.
266:298–306. 2013. View Article : Google Scholar
|
|
15
|
Creutzenberg O: Biological interactions
and toxicity of nanoma-terials in the respiratory tract and various
approaches of aerosol generation for toxicity testing. Arch
Toxicol. 86:1117–1122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wertman B, Azarbal B, Riedl M and Tobis J:
Adverse events associated with nickel allergy in patients
undergoing percu-taneous atrial septal defect or patent foramen
ovale closure. J Am Coll Cardiol. 47:1226–1227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Essafi-Benkhadir K, Refai A, Riahi I,
Fattouch S, Karoui H and Essafi M: Quince (Cydonia oblonga Miller)
peel polyphenols modulate LPS-induced inflammation in human
THP-1-derived macrophages through NF-κB, p38MAPK and Akt
inhibition. Biochem Biophys Res Commun. 418:180–185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Azakie A, Fineman J and He Y: Differential
responses of the right ventricle to abnormal loading conditions in
vivo: Possible pathophysiologic mechanisms. J Thorac Cardiovasc
Surg. 145:1335–1344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin C, Zhou S, Xiao Y and Chen L:
Erythropoietin enhances mitochondrial biogenesis in cardiomyocytes
exposed to chronic hypoxia through Akt/eNOS signalling pathway.
Cell Biol Int. 38:335–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yi X, Li X, Zhou Y, Ren S, Wan W, Feng G
and Jiang X: Hepatocyte growth factor regulates the TGF-β1-induced
proliferation, differentiation and secretory function of cardiac
fibroblasts. Int J Mol Med. 34:381–390. 2014.PubMed/NCBI
|
|
21
|
Yamashita Y, Shimada A, Yamada T, et al:
IKZF1 and CRLF2 gene alterations correlate with poor prognosis in
Japanese BCR-ABL1-negative high-risk B-cell precursor acute
lympho-blastic leukemia. Pediatr Blood Cancer. 60:1587–1592. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rocchigiani M, Lestingi M, Luddi A,
Orlandini M, Franco B, et al: Human FIGF: Cloning, gene structure
and mapping to chromosome Xp22.1 between the PIGA and the GRPR
genes. Genomics. 47:207–216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avantaggiato V, Orlandini M, Acampora D,
Oliviero S and Simeone A: Embryonic expression pattern of the
murine figf gene, a growth factor belonging to platelet-derived
growth factor/vascular endothelial growth factor family. Mech Dev.
73:221–224. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mitsuhashi N, Wu GD, Zhu H, Kearns-Jonker
M, Cramer DV, et al: Rat chemokine CXCL11: Structure, tissue
distribution, function and expression in cardiac transplantation
models. Mol Cell Biochem. 296:1–9. 2007. View Article : Google Scholar : PubMed/NCBI
|