Introduction
Osteosarcoma (OS) is the most common primary
malignancy, predominantly arising from metaphysis of the long bones
of adolescents and young adults (1). Almost 80% of the cases of OS occur at
the rapidly growing sites of longitudinal bones, including the
distal femur, proximal tibia and proximal humerus (1–3).
Despite current treatments, combining chemotherapy, surgery and
often radiotherapy, patients with recurrent or metastatic OS
exhibit a poor prognosis, with a 50–60% 5-year-survival rate
worldwide (1). Elucidation of the
fundamental molecular mechanisms underlying the initiation, drug
resistance and development of metastasis is important and urgent
for the development of the next generation of anticancer
therapeutics for OS (2,4).
Our previous study demonstrated that the mRNA
expression of IRX2 is upregulated in OS and acts as an oncogene
(5). IRX2 is a member of the
Iroquois homeobox gene family and its expression has been observed
to increase in several types of cancer, including acute
lymphoblastic leukemia, breast cancer and soft tissue sarcoma
(6–8). Furthermore, a previous study revealed
that the DNA methylation levels of IRX2 are associated with the
aggressiveness of tumor development and the outcome of patients
with lung cancer (9).
The PI3K/Akt pathway is a key regulator of several
physiological and pathological conditions. Previous studies have
revealed that the activation of Akt activates signaling cascades,
which contribute to sustaining tumor outgrowth and metastasis,
including the mTOR and MAPK signaling pathways (10–12).
The activation of Akt has been demonstrated to induce the
transcription of a set of target genes, which ultimately leads to
the regulation of cell growth, motility and apoptosis in OS
(13–16). In addition, the inactivation of Akt
by other genes or by a specific PI3K inhibitor induces G0/G1 cell
cycle arrest, inhibits OS cell proliferation, migration and
invasion, and promotes apoptosis (15,17).
However, whether the activation of Akt is important in IRX2-induced
cell growth and invasion remains to be elucidated.
In the present study, the regulatory mechanism by
which IRX2 potentiates proliferation and invasion through
regulating the PI3K/AKT signal pathway was investigated, which may
provide a potential therapeutic target for better management of
OS.
Materials and methods
Tumor specimens
A total of 68 paired fresh and frozen paired OS
tumor tissues and adjacent normal tissues were collected from
Changzheng Hospital (Shanghai, China) between 2004 and 2009.
Patients were diagnosed with OS based on histopathological
evaluation. The numbers of patients staged I, II and III were 1, 36
and 31, respectively. The details of the patients were as follows:
Age, 7–45 years; gender, 29 female and 39 male; tumor location, 38
femur, 16 tibia, 9 humerus, 5 others. The patients had not received
chemotherapy or radiotherapy prior to surgery. The present study
was performed with the approval of the Medical Ethical Committee of
the Second Military Medical University (Shanghai, China). Written
informed consent was obtained from the patient/patient's family.
All tissue samples were flash-frozen in liquid nitrogen immediately
following collection, and stored at −80°C until use.
Plasmid construction
The coding sequence of human IRX2 (NM_033267.4; NCBI
Reference Sequence, OriGene Technologies, Rockville, MD, USA) was
amplified by performing polymerase chain reaction (PCR) using the
forward primer, 5′-GGATCCATGTCCTACCCGCAGGGC-3′, which introduced a
BamHI site, and the reverse primer,
5′-GAATTCCTATAGGTAGGGCTGGACGC-3′ (Sangon Biotech Co., Ltd.,
Shanghai, China), which introduced an EcoRI site. The PCR
conditions were as follows: Initial denaturation at 98°C for 2 min,
followed by 30 cycles of denaturation at 98°C for 15 sec, primer
annealing at 55°C for 15 sec, and a primer extension step at 72°C
for 2 min, using high-fidelity polymerase PrimeStar (Takara Bio
Inc., Otsu, Japan). The products of the PCR were then cloned into
the BamH1 and EcoRI sites of the pCMV Tag 2B
constitu-tive mammalian expression vector (Stratagene, La Jolla,
CA, USA), according to the manufacturer's instructions. The
sequence of IRX2 was confirmed by sequencing (Sangon Biotech Co.,
Ltd.). This vector was termed pCMV-IRX2 (IRX2). The pCMV Tag 2B
empty vector (termed vector) was used as a negative control under
similar conditions. The overexpression efficiency was assessed 48 h
following transfection by measuring the protein expression levels
in the cell lysates using western blot analysis.
Cell culture and transfection
The human MG63 and SaOS2 OS cell lines were
purchased from American Type Culture Collection (Rockville, MD,
USA) and cultured in Dulbecco's modified Eagle's medium,
supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100
μg/ml streptomycin (all Invitrogen Life Technologies,
Carlsbad, CA, USA), in a 37°C humidified and 5% CO2
incubator. For the transfection experiments, plasmids
overexpressing IRX2, the coding sequence of human IRX2, without the
stop codon were constructed and were inserted into the pcDNA3.1
vector (Invitrogen Life Technologies). A total of 2×105
cells/well were plated into a 24-well plate 24 h prior to
transfection, and were grown to 30–50% confluence. Effective
transfection reagent (Qiagen Inc., Hilden, Germany) was used to
perform transfection and either 2.0 μg pCMV-IRX2 vector or
2.0 μg pCMV Tag 2B empty vector were used, according to the
manufacturer's instructions. Dimethyl sulfoxide and 10 μM
LY294002 (Sigma-Aldrich) was used to treat the cells for 2 h.
Western blotting
As previously described (5), the total proteins were extracted from
the cells and tissue samples using radioimmunoprecipitation buffer
(Cell Signaling Technology, Inc., Danvers, MA, USA), containing
Protease Inhibitors Cocktail tablets (Roche Diagnostics, Mannheim,
Germany). The proteins were quantified using a bicinchoninic acid
assay (Sangon Biotech, Co, Ltd.), and 50 μg protein from
each sample was separated on 10% SDS-polyacrylamide gels and
transferred onto polyvinylidene fluoride membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membranes were blocked
using 6% not-fat milk in Tris-buffered saline, containing 1%
Tween-20. The membranes were then incubated with the following
antibodies: Mouse polyclonal anti-IRX2 (ab72975) and rabbit
monoclonal anti-VEGF (ab52917) antibodies (1:500; Abcam, Cambridge,
CA, USA), rabbit polyclonal anti-AKT (#9272), rabbit polyclonal
anti-phosphorylated (p)-AKT (#9271) and rabbit polyclonal
anti-MMP-9 (#3852) antibodies (1:1,000; Cell Signaling Technology,
Inc.), and rabbit polyclonal anti-actin antibody (CW0097; 1:5,000;
Cwbiotech, Beijing, China), which was used as an internal control.
Membranes were incubated with horseradish peroxidase (HRP)-labeled
anti-rabbit secondary antibody (CW0103; 1:3000; Cwbiotech) at room
temperate for 1 h. The proteins were detected using an Image Quant™
LAS-4000 (Fujifilm, Tokyo, China) and the chemiluminescence was
directly analyzed and quantified using Image Quant TL version 2005
software (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA).
Cell proliferation assay
Cell viability was assessed using a Cell Counting
kit-8 (CCK8; Dojindo Laboratories, Kumamoto, Japan), according to
the manufacturer's instructions. CCK8 is more sensitive, compared
with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide assay. The MG63-IRX2 and SaOS2-IRX2 cells were treated with
10 μl CCK8 at 37°C for 1 h. The absorbance was then measured
at 450 nm using a microplate reader (Model 680; Bio-Rad
Laboratories, Inc.).
Invasion assay
The invasive activity of the pCMV-IRX2 transfected
human OS cells was assessed using BD Falcon™ Cell culture inserts
coated with BD Matrigel™ Basement Membrane Matrix (BD Biosciences,
Bedford, MA, USA). Briefly, the transfected MG63 and SaOS2 cells
were resuspended in serum free medium and seeded into the upper
chamber of the assay system. The lower wells of the system were
filled with complete growth medium. Following incubation for 48 h,
the invaded cells were washed twice with ice-cold
phosphate-buffered saline, fixed with 4% paraformaldehyde (Sangon
Biotech Co., Ltd.) for 15 min and stained with methyl violet (0.01%
v/v; Guidechem, Shanghai, China) for 30 min. The numbers of invaded
cells were then counted in more than five randomly selected fields
under a light microscope (IX73-F22FL/PH; Olympus America, Inc.,
Melville, NY, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation and were analyzed using Student's t-test. Statistical
analyses were two-sided and were performed using SPSS 15 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of IRX2 is significantly
upregulated in OS tissue
Our previous study reported that the mRNA expression
levels of IRX2 were significantly higher in OS tissue compared with
adjacent non-tumor tissue (5). Due
to a lack of appropriate antibody against IRX2, semiquantitative
analysis of a tissue array containing small sections of benign and
malignant tumor samples were not assessed using
immunohistochemistry. To further assess the expression of IRX2,
western blotting was performed to determine the protein expression
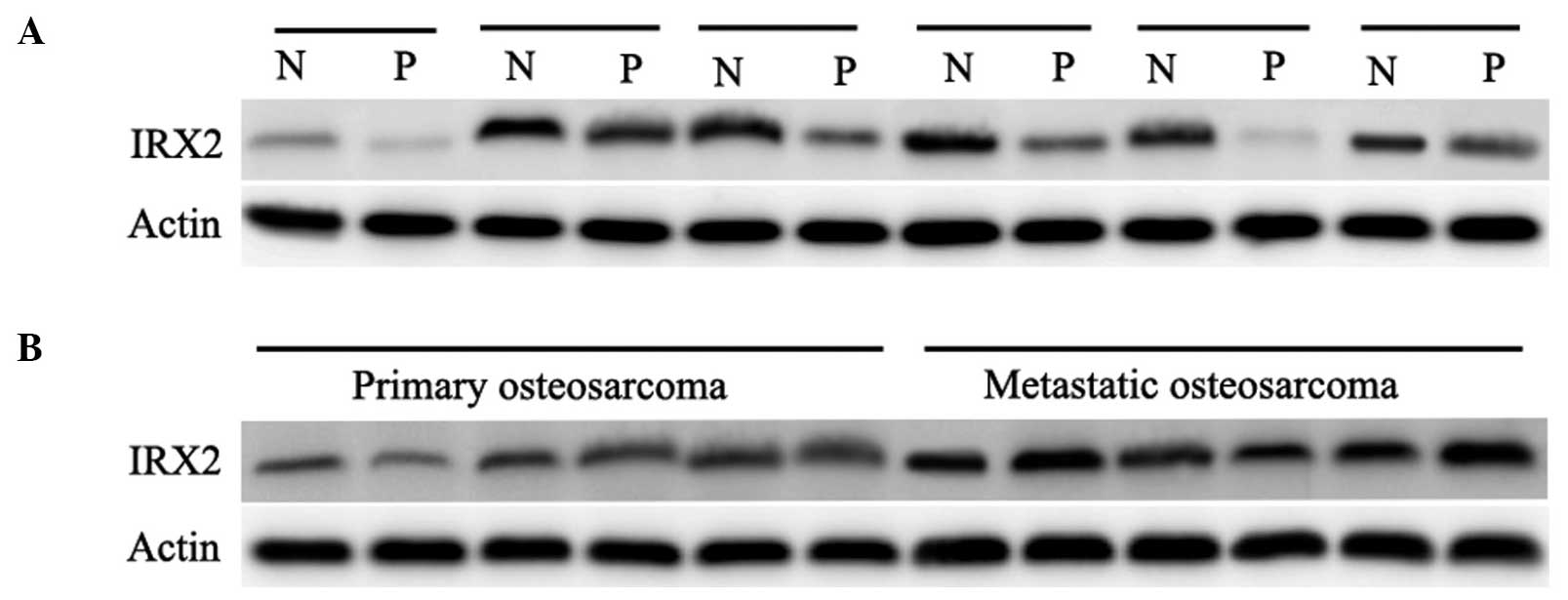
of IRX2. As shown in Fig. 1A, the
protein expression of IRX2 was assessed in individual normal and
malignant tissue samples on the array, and marked overexpression
was observed in the malignant tissue samples, compared with the
normal tissue samples. In addition, the expression levels of IRX2
were compared between primary OS without primary metastasis and OS
with metastasis (Fig. 1B). The
expression of IRX2 was more marked in primary OS with metastasis,
compared with primary OS without metastasis. These results
demonstrated that the expression of IRX2 increased in OS, which
suggested that IRX2 may be involved in the progression of OS.
IRX2 promotes OS cell growth and invasion
in vitro
The effect of IRX2 on the proliferation and
migration of OS cancer cells was assessed by knocking down IRX2
(5). To further assess the role of
IRX2 in OS cancer cells, a mammalian expression vector was used to
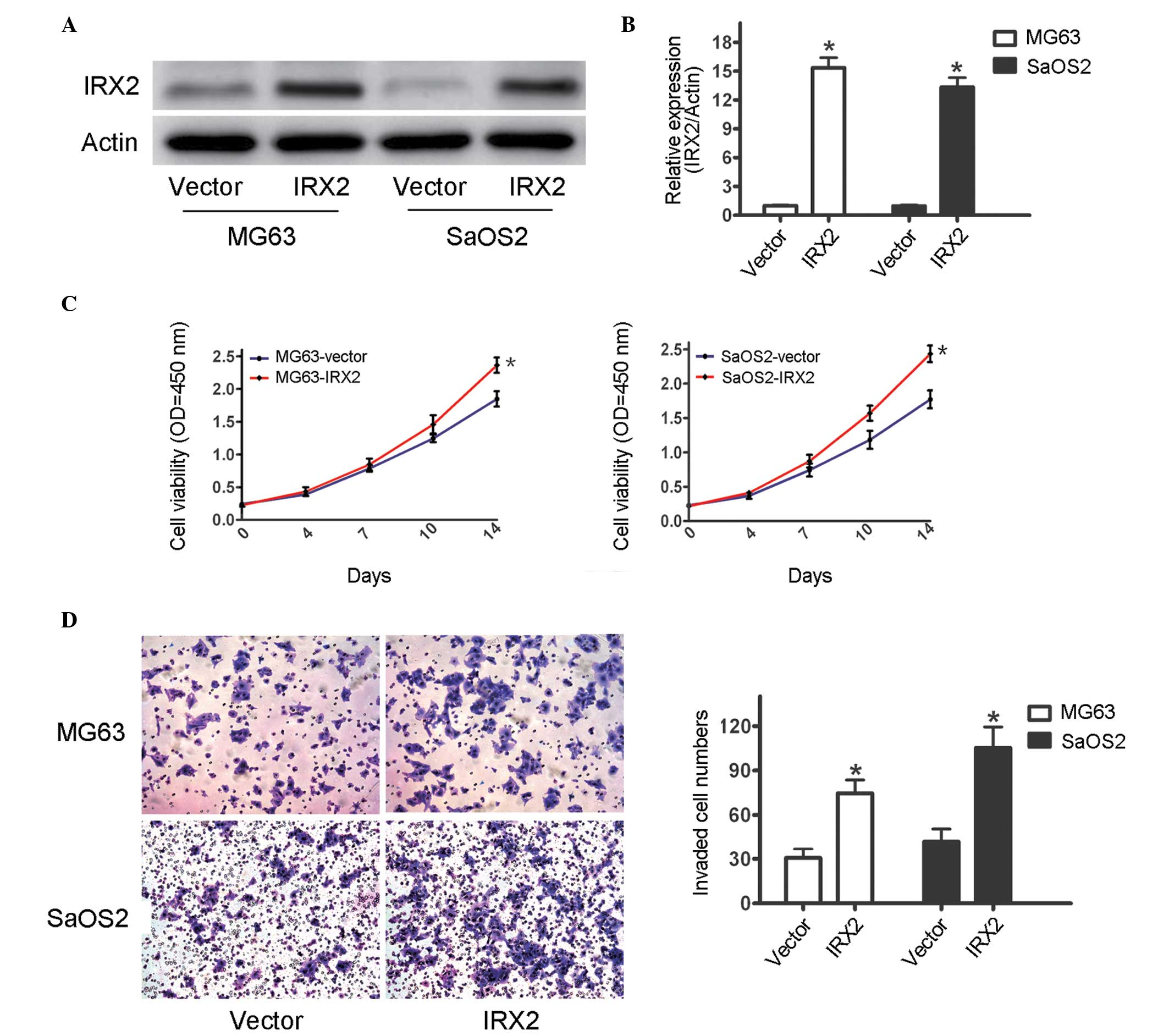
force the expression of IRX2. It was observed that IRX2 cDNA
transfection increased the mRNA and protein expression levels of
IRX2 in the MG63 and SaOS2 cells (Fig.
2A and B). To confirm whether IRX2 regulated OS cell growth, an
MTT assay was performed, the results of which demonstrated that
overexpression of IRX2 significantly promoted cell growth in the
two OS cell lines (Fig. 2C). The
invasion assays demonstrated that the forced expression of IRX2 led
to an increase in invasive ability of the cells transfected with
IRX2, compared with the cells transfected with the control vector
(Fig. 2D). These results further
confirmed IRX2 as an oncogene and that it was important in the
progression of OS.
IRX2 upregulates the expression of
p-AKT/MMP-9 and VEGF signaling pathways
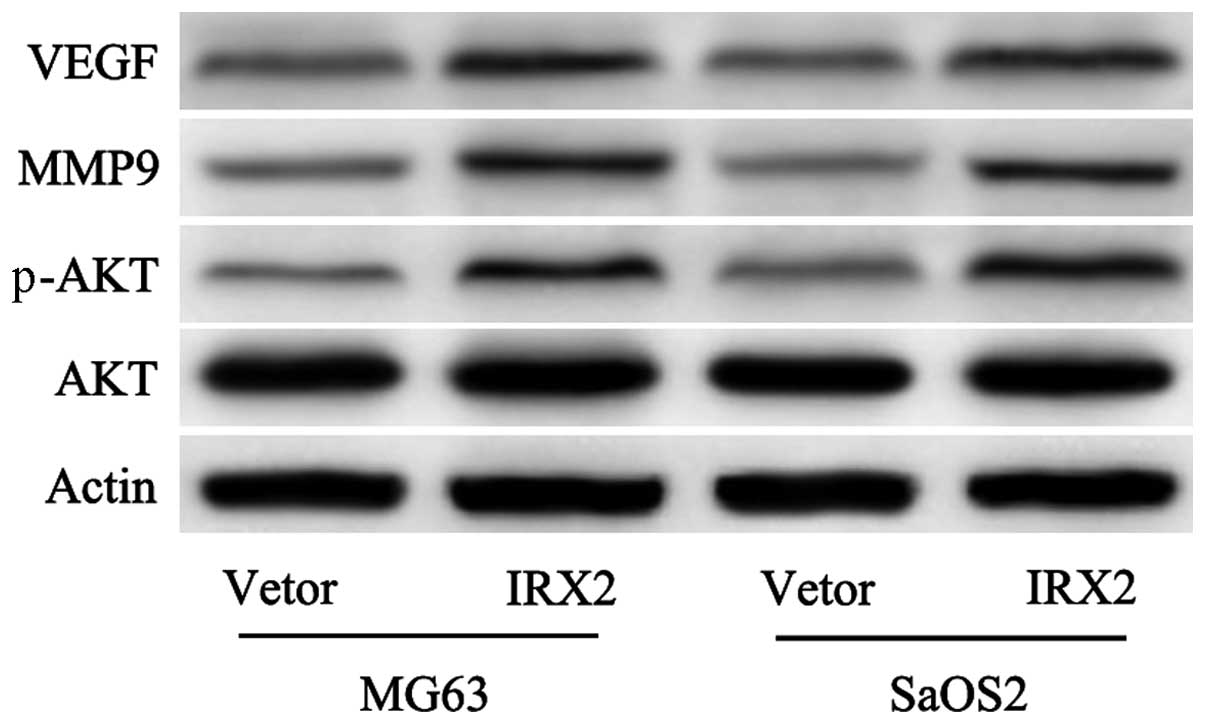
Our previous study demonstrated that inhibition of
the expression of IRX2 inactivated the PI3K/Akt signaling pathway
and reduced the expression of MMP-9 (5). The present study proceeded to
determine whether the overexpression of IRX2 activated the PI3K/Akt
signaling pathway and increased the expression of MMP-9. In
agreement with the results observed from knocking out IRX2,
overexpression of IRX2 in the MG63 and SaOS2 cells led to a
significant increase in the expression levels of p-AKT and MMP-9
(Fig. 3). In addition, VEGF, which
is an important mediator of vascularization and the expression of
which is associated with increased aggressive behavior (18,19),
was markedly upregulated in the cells transfected with IRX2
(Fig. 3). These finding suggested
a mechanism, whereby the PI3K/Akt signaling pathway may be a
required medium in IRX2-induced cell proliferation and invasion in
OS cells.
PI3K/AKT is required for IRX2-enhanced
cell proliferation and invasion, and for the activation of MMP-9
and VEGF in OS cells
Activation of the PI3K/AKT signaling pathway appears
to be involved in various aspects of tumor development, including
cell proliferation, differentiation, migration, invasion, apoptosis
and angiogenesis, in several types of cancer (20,21).
Previous studies have demonstrated that the PI3K/AKT signaling
pathway is important in the proliferation and metastasis of OS
cells by regulating the expression levels of MMP-9 and VEGF
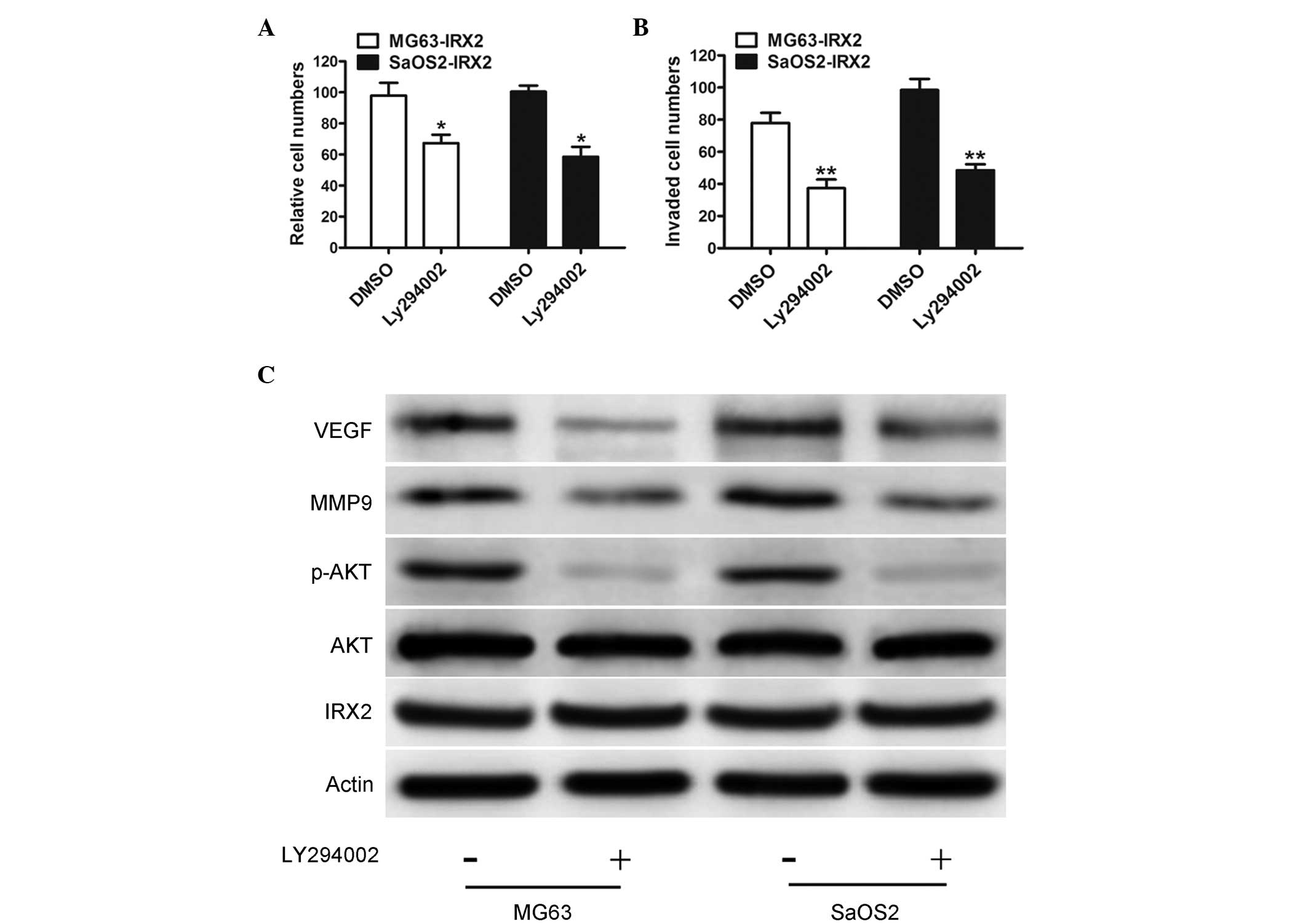
(22,23). To examine whether IRX2-induced cell
proliferation and invasion is dependent on increased PI3K/AKT
signaling pathway activity, the PI3K/AKT pathway was inhibited
using Ly294002, a PI3K inhibitor. Inhibition of PI3K markedly
reduced the invasive abilities of the IRX-transfected MG63 and
SaOS2 cells, compared with the control vector-transfected cells
(Fig. 4A). Furthermore,
IRX2-mediated OS cell invasion was markedly inhibited by Ly294002
(Fig. 4B). To further investigate
whether the activation of the PI3K/AKT pathway is involved in
IRX2-mediated upregulation of the expression levels of MMP-9 and
VEGF, western blotting was performed. The results revealed that the
protein expression levels of MMP-9 and VEGF were significantly
decreased (Fig. 4C). These data
suggested that activation of the PI3K/Akt survival pathway
contributed to IRX2-induced growth and migration in the OS
cells.
Discussion
OS is the most common primary malignant tumor of
bone, generally following an aggressive clinical course, and
presents a major therapeutic challenge (24). Despite progress in understanding
the molecular biology of OS, elucidation of the underlying
mechanisms associated with the development of OS is important for
effective treatment strategies.
The present study established an IRX2-overexpression
model and observed that IRX2 promoted cell growth and invasion
in vitro. There were several lines of evidence to support
this. The expression of IRX2 was first confirmed to be upregulated
in OS tissue, particularly in patients with tumor metastasis.
Secondly, overexpression of IRX2 was observed to promote the
viability and invasion of the OS cells. Thirdly, IRX2 activated the
PI3K/Akt signaling pathway and induced the upregulation of the
expression levels of MMP-9 and VEGF. In addition, the present study
provided evidence demonstrating that activation of the PI3K/Akt
signaling pathway was involved in IRX2-induced cell viability and
invasion, and in upregulation of MMP-9 and VEGF in the OS cells.
These results indicated for the first time, to the best of our
knowledge, that IRX2 promoted OS cell growth and invasion through
the PI3K/Akt signaling-mediated activation of MMP-9 and VEGF.
Although a number of previous studies have indicated
that IRX2 genes are usually amplified in cancer progression
(6–8), the detail mechanisms involved in
tumor progression remained to be elucidated. Our previous studies
demonstrated that IRX2 silencing significantly reduces the
proliferation and inhibits the invasiveness of U2OS and SaOS2 OS
cells (5). The PI3K/Akt signaling
pathway is involved in development and tumorigenesis by regulating
several aspects of cell proliferation and invasiveness (12,13,21).
Angiogenesis is required to sustain primary tumor growth and
metastasis (25). The present
study revealed that IRX2 transfection increased the phosphorylation
of AKT and upregulation of MMP-9 and VEGF. To confirm that the
PI3K/AKT pathway was involved in the IRX2-mediated cell
proliferation and invasion of the OS cells, the PI3K/AKT pathway
was inhibited using Ly294002, a PI3K inhibitor. The results
demonstrated that the phosphorylation of AKT was inhibited by the
specific inhibitors independently. IRX2-induced proliferation and
invasion were reversed and the protein expression levels of MMP-9
and VEGF were significantly reduced by Ly294002, suggesting that
IRX2 affected cell proliferation and invasion, and the expression
levels of MMP-9 and VEGF via the PI3K/AKT signaling pathways.
In conclusion, the results of the present study
provided further evidence that the expression of IRX2 was
frequently increased in OS tissue samples, and that a novel
molecular mechanism responsible for the IRX2-induced OS cell growth
involved the PI3K/AKT signaling-mediated production of MMP-9 and
VEGF.
References
|
1
|
Picci P, Mercuri M, Ferrari S, et al:
Survival in high-grade osteosarcoma: Improvement over 21 years at a
single institution. Ann Oncol. 21:1366–1373. 2010. View Article : Google Scholar
|
|
2
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: A
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cesari M, Alberghini M, Vanel D, et al:
Periosteal osteosarcoma: A single-institution experience. Cancer.
117:1731–1735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas DM: Wnts, bone and cancer. J
Pathol. 220:1–4. 2010. View Article : Google Scholar
|
|
5
|
Liu T, Zhou W, Zhang F, et al: Knockdown
of IRX2 inhibits osteosarcoma cell proliferation and invasion by
the AKT/MMP9 signaling pathway. Mol Med Rep. 10:169–174.
2014.PubMed/NCBI
|
|
6
|
Kadota M, Sato M, Duncan B, et al:
Identification of novel gene amplifications in breast cancer and
coexistence of gene amplification with an activating mutation of
PIK3CA. Cancer Res. 69:7357–7365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adamowicz M, Radlwimmer B, Rieker RJ, et
al: Frequent amplifications and abundant expression of TRIO, NKD2
and IRX2 in soft tissue sarcomas. Genes Chromosomes Cancer.
45:829–838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang H, Wilson CS, Harvey RC, et al: Gene
expression profiles predictive of outcome and age in infant acute
lymphoblastic leukemia: A Children's Oncology Group study. Blood.
119:1872–1881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sato T, Arai E, Kohno T, et al: Epigenetic
clustering of lung adenocarcinomas based on DNA methylation
profiles in adjacent lung tissue: Its correlation with smoking
history and chronic obstructive pulmonary disease. Int J Cancer.
135:319–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Francipane MG and Lagasse E: mTOR pathway
in colorectal cancer: An update. Oncotarget. 5:49–66.
2014.PubMed/NCBI
|
|
11
|
Pandurangan AK and Esa NM: Signal
transducer and activator of transcription 3-a promising target in
colitis-associated cancer. Asian Pac J Cancer Prev. 15:551–560.
2014. View Article : Google Scholar
|
|
12
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: an
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo YS, Zhao R, Ma J, et al: βig-h3
promotes human osteosarcoma cells metastasis by interacting with
integrin alpha2beta1 and activating PI3K signaling pathway. PLoS
One. 9:e902202014. View Article : Google Scholar
|
|
15
|
Gong C, Liao H, Wang J, et al: LY294002
induces G0/G1 cell cycle arrest and apoptosis of cancer stem-like
cells from human osteosarcoma via down-regulation of PI3K activity.
Asian Pac J Cancer Prev. 13:3103–3107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang A, He S, Sun X, et al: Wnt5a
promotes migration of human osteosarcoma cells by triggering a
phosphatidylinositol-3 kinase/Akt signals. Cancer Cell Int.
14:152014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao H, Li M, Li L, et al: MiR-133b is
down-regulated in human osteosarcoma and inhibits osteosarcoma
cells proliferation, migration and invasion and promotes apoptosis.
PLoS One. 8:e835712013. View Article : Google Scholar
|
|
18
|
Kang J, Rychahou PG, Ishola TA, et al:
N-myc is a novel regulator of PI3K-mediated VEGF expression in
neuroblastoma. Oncogene. 27:3999–4007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garcia V, Garcia JM, Silva J, et al:
Levels of VEGF-A mRNA in plasma from patients with colorectal
carcinoma as possible surrogate marker of angiogenesis. J Cancer
Res Clin Oncol. 134:1165–1171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dillon RL and Muller WJ: Distinct
biological roles for the akt family in mammary tumor progression.
Cancer Res. 70:4260–4264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hassan B, Akcakanat A, Holder AM and
Meric-Bernstam F: Targeting the PI3-kinase/Akt/mTOR signaling
pathway. Surg Oncol Clin N Am. 22:641–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao CL, Lai KC, Huang AC, et al: Gallic
acid inhibits migration and invasion in human osteosarcoma U-2 OS
cells through suppressing the matrix metalloproteinase -2/-9,
protein kinase B (PKB) and PKC signaling pathways. Food Chem
Toxicol. 50:1734–1740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu J, Wu X, Zhong D, et al: Short Hairpin
RNA (shRNA) Ether à go-go 1 (Eag1) inhibition of human osteosarcoma
angiogenesis via VEGF/PI3K/AKT signaling. Int J Mol Sci.
13:12573–12583. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Maldegem AM, Bhosale A, Gelderblom HJ,
Hogendoorn PC and Hassan AB: Comprehensive analysis of published
phase I/II clinical trials between 1990–2010 in osteosarcoma and
Ewing sarcoma confirms limited outcomes and need for translational
investment. Clin Sarcoma Res. 2:52012. View Article : Google Scholar
|
|
25
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|