Introduction
Kidney stone disease is major health concern and an
economic burden on health systems (1). Kidney stones, which can cause kidney
injury, are common and frequent problems. A previous study revealed
that kidney stones are associated with a significant loss of kidney
function and contribute to the risk of end-stage kidney disease
(2). Shoag et al (3) suggested that a history of kidney
stones is associated with an increased risk of chronic kidney
disease (CKD) and requirement for dialysis treatment among females,
when adjusted for co-morbidities.
Although several forms of surgery, including
extracorporeal shock wave lithotripsy, are performed to remove
kidney stones, stone recurrence and kidney injury remain serious
issues. Therefore, preventing and treating kidney stones is
critical. Calcium oxalate stones remain the most common type of
nephrolithiasis, accounting for ~80% of kidney stones (4). The exact mechanism of kidney stone
formation remains to be fully elucidated. Currently, investigations
are focusing on crystal formation in the kidney, which is the early
stage of stone development. Reactive oxygen species (ROS) are
considered to be important in kidney crystal formation. Huang et
al (5) reported that ROS
activation of nicotinamide adenine dinucleotide phosphate (NADPH)
contribute to renal tubular cell injury, however, the inhibitor of
NADPH decreases the expression of kidney injury molecular-1
(KIM-1). In addition, other previous studies have revealed that
oxalate-mediated oxidative stress in erythrocytes may contribute to
the tubular damage and stone accumulation in patients with
hyperoxaluria (6).
Several previous studies have suggested epigenetic
modulation in kidney diseases. Inhibition of histone deacetylase
(HDAC) activity attenuates renal injury via anti-inflammatory and
anti-fibrotic effects (7,8). Advani et al (9) demonstrated that long-term
administration of vorinostat, also termed suberanilohydroxamic acid
(SAHA) attenuates renal injury ina mouse model of diabetes by
reducing oxidative nitrosative stress (9). The transcription factor, Nrf2, is a
key regulator of antioxidant-responsive genes and is bound to its
inhibitor, Kelch-like ECH-associated protein 1 (Keap1). Wang et
al (10) demonstrated that the
HDAC inhibitor, trichostatin A (TSA), suppresses the expression of
Keap1, activates Nrf2 and enhances Nrf2-ARE binding. Furthermore,
TSA contributes to neuroprotection following cerebral ischemia, the
effects of which are not observed in Nrf2-deficient mice (10). Taken together, these findings
suggest that HDAC inhibitors exert renoprotective and
anti-oxidative functions, and may be a potential drug target for
kidney stones. According to previous experimental methods (11), the renal calcium oxalate animal
model was established in the present study to investigate whether
SAHA improves kidney injury and to determine the possible mechanism
of kidney stone development.
Materials and methods
Animals
A total of 24 healthy wild type male C57BL/6 mice,
aged 8 weeks, were obtained from the Shanghai SLAC Lab Animal Co,
Ltd. (Shanghai, China) and were raised in the SPF Animal Facility
of the Second Military Medical University (Shanghai, China). All
animals had free access to standard laboratory food and drinking
water, and were maintained under a controlled 12 h light/day cycle
at 20–25°C with a relative humidity of 55–65%. Animal care and
procedures followed the recommendations of the NIH Guide for the
Care and Use of Laboratory Animals. The present study was approved
by the Committee on Ethics of Biomedicine Research, Second Military
Medical University.
Drugs and chemicals
Vorinostat was purchased from Selleck Chemicals
(Houston,. TX, USA) and was dissolved in DMSO (Sigma-Aldrich, St.
Louis, MO, USA) to a final concentration of 50 mg/2 ml. Glyoxylate
was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo,
Japan), the Oxidative Stress kit was purchased from Jiancheng
Institute of Biotechnology (Nanjing, China) and the von Kossa
Staining Commercial kit was purchased from Shanghai Shunbai
Biotechnology, Co., Ltd. (Shanghai, China). A mouse monoclonal
antibody against osteopontin (OPN; cat. no. sc-73631; 1:50) and the
appropriate secondary antibody were purchased from Santa-Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA), and a rabbit polyclonal
antibody against CD44 (cat. no. 15675-1-AP; 1:100) was purchased
from Proteintech Group (Chicago, IL, USA). The TUNEL commercial kit
was purchased from EMD Millipore (Billerica, MA, USA).
Study design and procedure
A total of 24 mice were acclimatized for 1 week
prior to the experiments. The animals were assigned randomly into
four groups (n=6 per group): Control group (no treatment); saline
group, (intraperitoneal injection of 50 mg/kg/day saline); DMSO
group (an intra-abdominal injection of 50 mg/kg/day DMSO); and SAHA
group (intraperitoneal injection of 50 mg/kg/day SAHA). Following
treatment for 6 h, all groups, with the exception of the control
group, received an intraperitoneal injection of 100 mg/kg/day
glyoxylate. The procedures were performed daily for 7 days.
Biochemical indicators
The mice were placed in metabolic cages for 24 h for
urine collection. The blood was collected from all animals prior to
sacrifice under 3% pentobarbital sodium anesthesia. The levels of
serum creatinine (Scr) and blood urea nitrogen (BUN) were measured
with the assitance of the University of Shanghai Hospital Clinical
Laboratory (Shanghai, China) using a BC-2800 Vet Animal Auto
Biochemistry Analyzer (Guangzhou Shihai Medical Equipment Co.,
Ltd., Guangdong, China). The ratios of urinary concentration of
calcium to creatinine were also calculated with the assistance of
the University of Shanghai Hospital Clinical Laboratory using a
BC-2800 Vet Animal Auto Biochemistry Analyzer (Guangzhou Shihai
Medical Equipment Co., Ltd.).
Measurement of urinary KIM-1
The protein levels of KIM-1 were quantified using a
modified enzyme-linked immunosorbent assay (ELISA) system. The
wells of an ELISA plate (USCN Life Science Inc., Wuhan, China) were
coated with 200 µl anti-KIM-1 polyclonal antibody (1.0
µg/ml) and incubated overnight at 4°C in 50 mM carbonate
solution. The wells were blocked with a 1% bovine serum albumin
solution in phosphate-buffered saline (PBS) and were washed four
times with PBS containing 0.05% Tween-20 (PBST). The serum samples
(100 µl) and urine samples (200 µl) were then added
to each well at room temperature for 3 h. Following incubation, the
samples were washed four times with PBST prior to incubation with
biotinylated anti-KIM-1 monoclonal antibody. Following incubation
with primary antibody, the samples were incubated with horseradish
peroxidase-conjugated streptavidin, with tetramethylbenzidine as
the substrate. The levels were quantified using a microplate reader
to detect the concentration and a standard curve was created.
Kidney sample collection and pathological
analysis
All animals underwent heart perfusion, and the left
kidneys were removed and placed into eppendorf tubes with 4%
paraformaldehyde. Heart perfusion enables the preservation of the
kidneys for morphological observations. The kidneys were embedded
in paraffin wax (Hubei Laike Medicine Instrument Co., Ltd.,
Xiangfan, China) and cross-sections were sliced at 3 µm
(Leica RM2235; Hubei Lai Ke Medicine Equipment Co., Ltd.). These
slices were then prepared for von Kossa immunohistochemistry and
TUNEL staining. The right kidneys were stored in eppendorf tubes in
a refrigerator at −80°C for determining the calcium oxalate
concentration and levels of malondialdehyde (MDA), superoxide
dismutase (SOD) and glutathione reductase (GSH). The slices
underwent deparrafinization and hydration using a series of
dilutions of xylene and alcohol, followed by staining using the von
Kossa kit and subsequent eosin counterstaining (Beyotime Institute
of Biotechnology, Haimen, China). The stained slices were then
assessed using microscopy (Nikon Eclipse 50i; Shanghai Henghao
Instruments Co. Ltd., Shanghai, China) for the distribution of
calcium oxalate crystals, characterized by black calcium oxalate
crystal deposits. The number of crystals in a total cross-sectional
tissue area was determined using Adobe Photoshop software version
7.0 (Adobe Systems, Inc., San Jose, CA, USA) in 20 randomly
selected fields (magnification, x200). For determination of the
levels of OPN and CD44,paraffin sections were high pressure treated
for 2 min and blocked with 0.5% H2O2 in
methanol for 15 min, washed in 0.01 M PBST and further treated with
non-fat milk in PBS for 30 min at room temperature. The slides were
then incubated overnight at 4°C with primary antibodies against OPN
and CD44, followed by incubation with the secondary antibody. The
positive staining of OPN and CD44 were measured as the ratio of
integral optical density/field of kidney cross-sections, using
Image-pro Plus software version 6.0. Using the TUNEL kit and methyl
green counterstaining, the number of TUNEL-positive cells were
counted using Image-Pro Plus software. A total of six
randomly-selected fields were used under a magnification of
x400.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago., IL, USA). The data are expressed as
the mean ± standard deviation. Statistical significance was
determined using one-way analysis of variance and Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
SAHA ameliorates kidney injury induced by
CaOx and reduces crystal formation
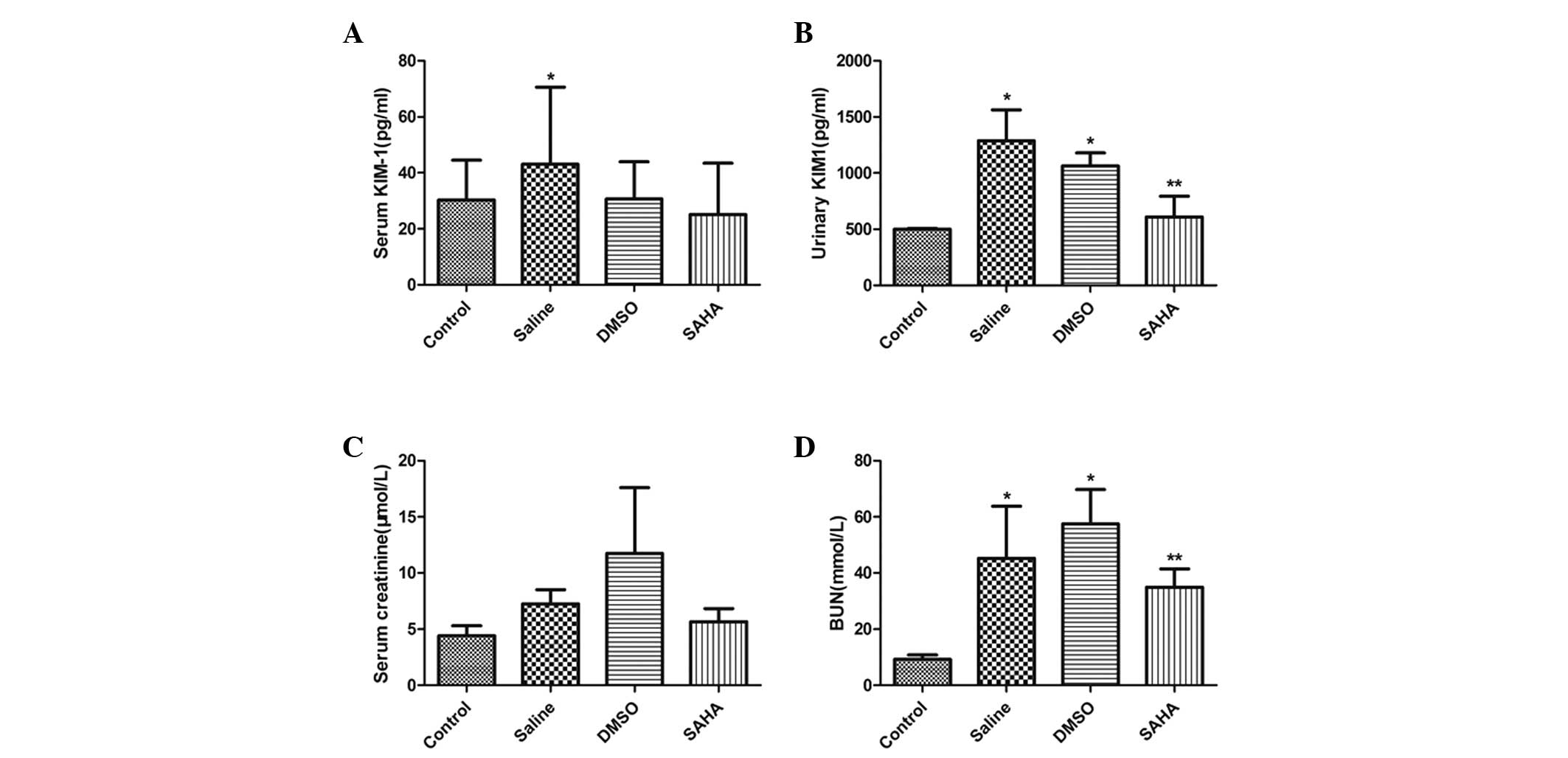
The results of the ELISA demonstrated a significant
increase in the urinary and serum levels of KIM-1 in the saline and
DMSO-treated groups. The SAHA group exhibited a significant
reduction in urinary KIM-1 excretion (Fig. 1A and B). The levels of BUN and Scr
were higher in the saline and DMSO groups, compared with the
control group. The level of BUN in the SAHA group decreased
significantly, however, this group exhibited no significant change
in levels serum creatinine (Fig. 1C
and D).
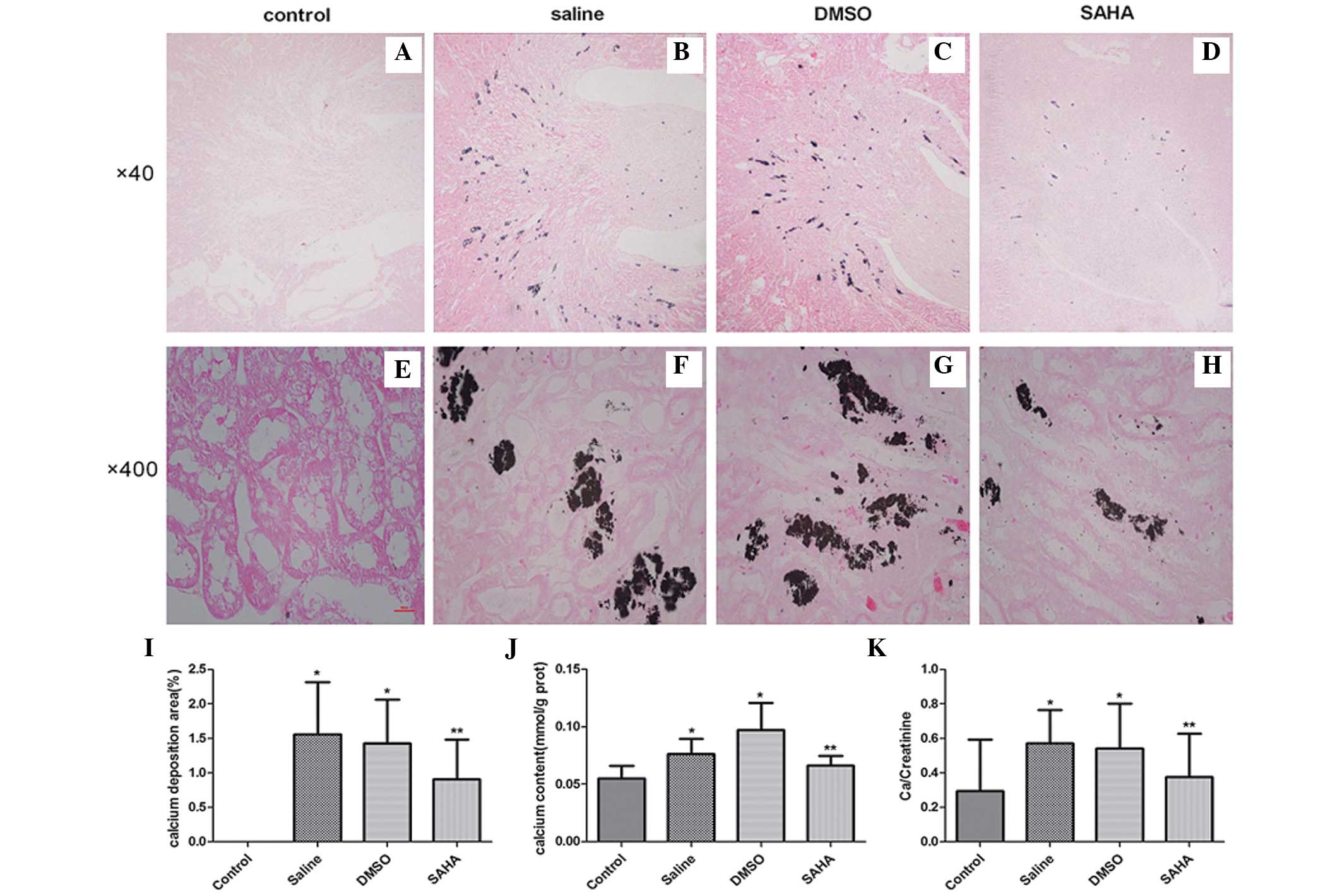
Following intraperitoneal injection of glyoxylate
for 7 days, the deposition of calcium oxalate crystals was assessed
using microscopy (Nikon Eclipse 50i) in the experimental groups, in
the region between the renal cortex and the medulla, particularly
in the corticomedullary junction. Treatment with SAHA ameliorated
crystal aggregation (Fig. 2A–H).
The semi-quantitative assessment of kidney crystal formation
revealed that the numbers of crystals in the saline and DMSO groups
were significantly higher, compared with that in the control group
(P<0.001). However, the SAHA-treated kidneys exhibited fewer
crystals (P<0.05; Fig. 2I;
Table I) than these two groups. In
addition, the calcium concentrations in the kidney tissues of the
SAHA group were significantly lower, compared with the other
experimental groups (Fig. 2J). The
presence of crystals led to a rise in urinary concentrations of
Ca/creatinine, as observed in the saline and DMSO groups. The
Ca/creatinine concentrations were significantly decreased following
treatment with SAHA (Fig. 2K). No
significant difference was observed between the DMSO and saline
groups.
 | Table ISemi-quantification of calcium oxalate
crystal formation and immunohistochemical staining for CD44 and
OPN. |
Table I
Semi-quantification of calcium oxalate
crystal formation and immunohistochemical staining for CD44 and
OPN.
| Group | Crystal deposition
(%) | Average CD44
(IOD) | Average OPN
(IOD) |
|---|
| Control | 0 | 0.05±0.01 | 0.42±0.04 |
| Saline | 1.56±0.76a | 0.09±0.03a | 0.49±0.07a |
| DMSO | 1.43±0.63a | 0.08±0.01a | 0.49±0.06a |
| SAHA | 0.91±0.58b | 0.05±0.02b | 0.43±0.05b |
SAHA decreases the level of CaOx-induced
oxidative stress
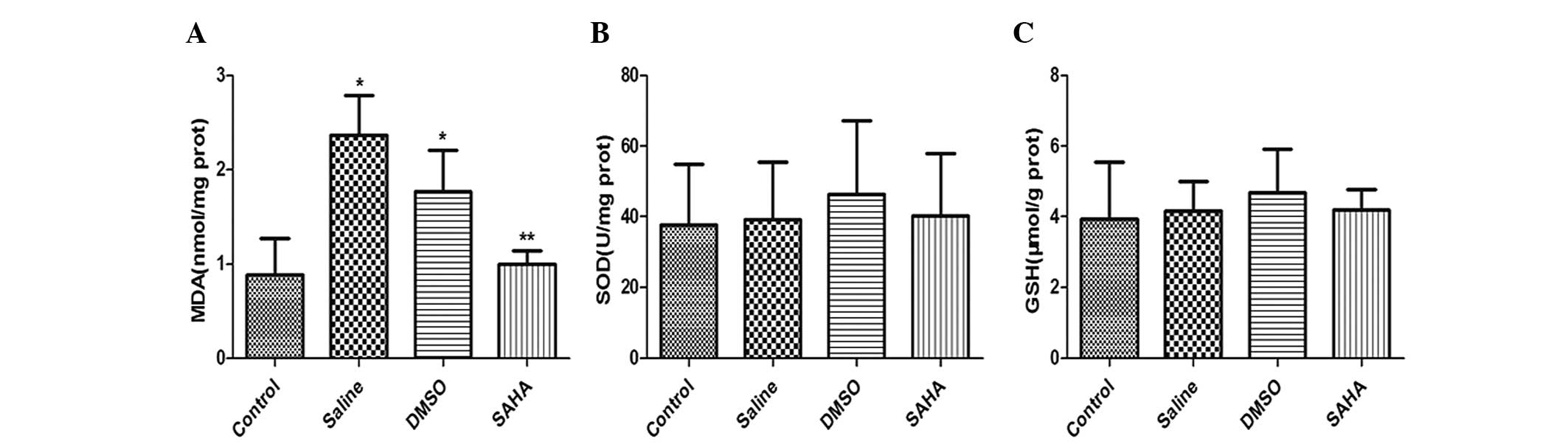
To determine whether the level of oxidative stress
was associated with SAHA treatment, the concentrations of MDA, SOD
and GSH in the kidney tissues were assessed. The results indicated
that glyoxylate caused a significant increase in the concentrations
of MDA in the saline and DMSO groups, compared with the control. By
contrast, the MDA content inthe SAHA-treated group was
significantly lower than in the saline and DMSO groups (Fig. 3A). Although SAHA did not
significantly alter the levels of SOD (Fig. 3B) or GSH (Fig. 3C) in the kidney tissues, a marginal
increase was observed.
SAHA alters the expression levels of OPN
and CD44, and reduces apoptosis induced by CaOx
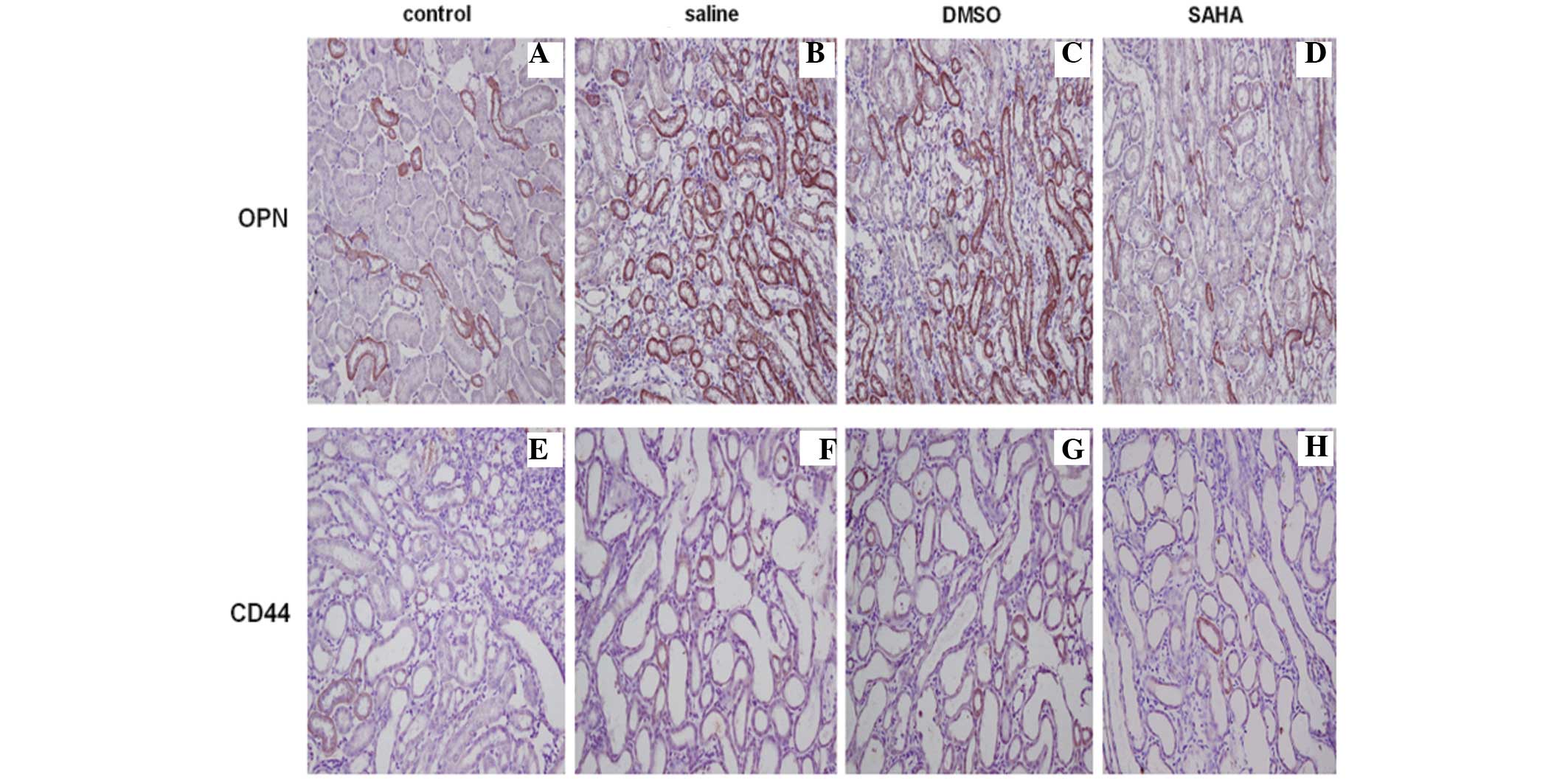
Immunohistochemical staining of the kidney tissues
revealed that the expression of OPN located in the kidney tubules
was upregulated in the saline and DMSO groups, particularly at the
corticomedullary border region. The expression of CD44 was observed
in a diffuse localization pattern in normal rat kidney and was
weaker, compared with the expression of OPN. Kidney crystal
formation resulted in high levels of expression in the proximal
tubular cells at the corticomedullary junction area, however, the
SAHA group exhibited markedly lower expression levels of OPN and
CD44, confirmed by semi-quantification (Fig. 4A–H; Table I).
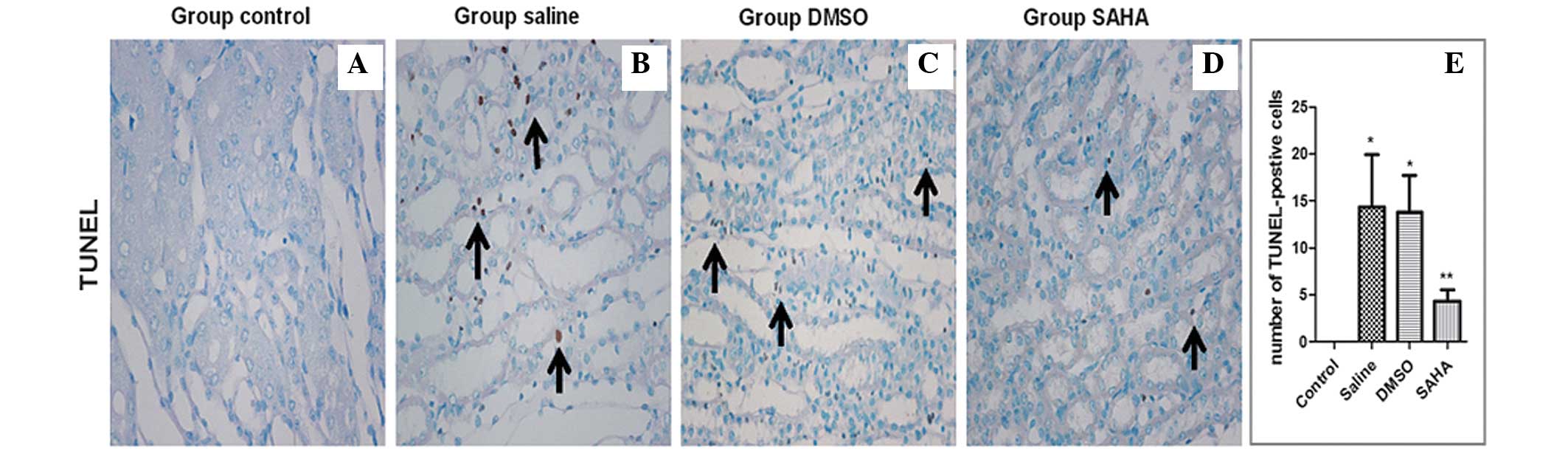
The results of the TUNEL staining revealed stained
nuclei in the area from the cortex to medulla in the saline and
DMSO groups, particularly in the corticomedullary junction. The
SAHA group exhibited weaker staining. The number of TUNEL-positive
cells in the SAHA group was significantly lower, compared with
those in the saline and DMSO groups (Fig. 5A–D), which was similar to the data
obtained in the semi-quantification analysis (Fig. 5E).
Discussion
Nephrolithiasis is one of the primary causes of
obstructive nephropathy and lower quality of life. Thus, efforts
are required to identify novel methods to prevent kidney stone
formation and improve renal function. The present study assessed
the efficacy of treatment with SAHA as a prophylactic agent for
kidney injury induced by CaOx. Serum and urine biochemical analyses
indicated a significant reduction in the level of BUN and in the
urinary concentration ratio of Ca/creatinine in the SAHA-treated
group, compared with the other treatment groups. KIM-1 is highly
specific and sensitive in identifying acute kidney injury (12). The urinary levels of KIM-1
increased in the saline and DMSO groups, however, Scr was
unchanged. These results suggested that an increase in KIM-1 may
assist in the diagnosis of kidney injury at anearlier stage,
compared with increases in Scr. Han et al (13) reported a similar finding. In
addition, data from the present study indicated that SAHA reduced
the urinary excretion of KIM-1, which may account for SAHA
protection against CaOx-induced kidney injury.
Based on the results of von Kossa staining and
calcium detection in the present study, SAHA administration reduced
renal calcium deposition, consistent with the marked
anti-nephrolithic effect of SAHA. Cho et al reported that
the two predominant stone components are calcium oxalate (71.5%)
and uric acid (15.3%). In addition, metabolic syndrome has also
been associated with a significantly increased risk of uric acid
calculi development, particularly in patients with impaired fasting
glucose and hyper-triglyceridemia (14). The possibility that SAHA reduces
calcium deposition by changing the metabolic state cannot be
excluded. In an investigation of Japanese males, inflammation was
suggested as a possible underlying mechanism of the association
between obesity and kidney stone formation (15). SAHA also exerts an
anti-inflammatory effect. Advani et al (9) demonstrated that SAHA decreases the
expression of eNOS in the kidneys of diabetic mice and in cultured
endothelial cells. Mice genetically deficient in eNOS, however, are
resistant to the attenuating effects of SAHA (9). Previous animal studies have indicated
that ROS have been recognized as a vital mediator, which can lead
to oxidative stress during crystal formation (16). Hirose et al (17) revealed that renal tubular cell
injury, particularly injury caused by mitochondrial damage and
oxidative stress, can induce the early stage of calcium oxalate
crystal formation in mice (17).
Khan et al (18) revealed
that hydroxyl-l-proline contributed to stone formation, along with
gene expression of macromolecular modulators (MMs) in hyperoxaluric
rats. Treatment with the NADPH oxidase inhibitor, apocynin,
however, resulted in a nearly complete absence of crystals and
changes of MMs. Therefore, CaOx crystallization is likely regulated
by ROS, which is produced, in part, through the activation of NADPH
oxidase (18). The present study
revealed that rats treated with SAHA exhibited decreased levels of
MDA and increased levels of SOD and GSH, suggesting that treatment
with SAHA reduced calcium oxalate crystal formation and was
associated with the decrease in ROS production, demonstrating an
anti-oxidative effect. However, no significant difference was
observed in the levels of SOD and GSH. The reason may be that SAHA
decreases ROS production more than it increases antioxidant
enzymes.
Calcium oxalate crystal adherence to injured tubular
epithelial cell results in stone aggregation, and several types of
proteins are involved in the pathological process. Kanlaya et
al detected a total of 14 proteins as differentially expressed
proteins, with western blot analysis suggesting that oxalate
induced the upregulation of α-enolase and immunoblotting analysis
indicating that the downregulation of RhoA was associated with the
identified proteins (19). OPN is
a type of glycoprotein that is widely expressed in several tissues,
and is involved in several physiological and pathological
processes, including cell adhesion, migration, signaling,
inflammation and biomineralization (20). A previous report demonstrated that
OPN is expressed predominantly in the distal convoluted tubule and
collecting duct, and the results of immunohischemical staining and
in situ molecular hybridization have supported this finding
(21). A minor difference was that
Ullrich et al revealed that OPN is expressed indistal and
proximal tubules (22). Okada
et al identified OPN to be present in the tubules and
stones, and the expression of OPN is upregulated during kidney
injury (11). The present study
observed that the distribution of OPN is similar to that of crystal
retention, which is predominantly located in the tubules at the
corticomedullary junction area. Asselman et al reported the
same distribution (23). These
data indicated that OPN co-localized with CaOx crystals. Tsuji
et al reported increased renal expression of OPN in
hyperoxaluric rats and a significant reduction in expression
following transfection with OPN siRNA (24). The increased expression of OPN may
be caused by ROS production, derived from cell injury, and
inhibiting oxidative stress may reverse this. As expected, SAHA
downregulated the expression of OPN in the present study.
CD44 is a ubiquitous transmembrane glycoprotein and
serves as a cell surface receptor for hyaluronic acid and OPN, the
biological activities of which primarily depends on their
interaction with CD44. The expression of CD44 is rare in normal
kidneys, however, it is increased in kidney tissues with renal
crystals. Asselman et al revealed that CD44 is expressed at
the luminal surface of crystal-binding renal tubular cells,
however, it was not expressed in cells lacking affinity for
crystals (23). In a metabolic
syndrome mouse model, Fujii et al demonstrated that renal
crystallization contributes to upregulation of the expression
levels of OPN and CD44 (25). The
present study also demonstrated that OPN and CD44 were expressed at
sites where crystals were retained. Treatment with SAHA resulted in
a reduction in the expression levels of OPN and CD44.
In unilateral ureteral obstruction model mice, Pang
et al demonstrated that Trichostatin A inhibits caspase-3
phosphorylation and ameliorates tubular epithelial cell apoptosis
(26). In HK-2 cells, Khaskhali
et al demonstrated that exposure to high levels of Ca or
CaOx crystals causes injury to renal epithelial cells, although the
two do not work synergistically. High levels of Ca induce cell
injury and may be caused by the production of ROS, and the
development of oxidative stress (27). Niimi et al (28) observed mitochondrial collapse
within renal tubular cells and cell apoptosis was assessed using
cleaved caspase-3. In the present study, TUNEL staining indicated
that kidney crystals led to cell apoptosis and that treatment with
SAHA improved apoptosis. These data suggested that HDAC inhibitors
act in a renoprotective manner against apoptosis.
In conclusion, SAHA may be an effective prophylactic
agent against CaOx by reducing kidney injury and promoting
mechanisms involved in renoprotective functions, at least in part,
by decreasing oxidative stress and cell apoptosis, and
down-regulating the expression levels of OPN and CD44. Therefore,
HDAC inhibitors may have therapeutic potential for the treatment of
kidney stones, particularly in refractory and recurrent
nephrolithiasis. However, further investigations are required to
clarify the specific mechanism underlying this response.
Abbreviations:
|
ESRD
|
end-stage renal disease
|
|
CKD
|
chronic kidney disease
|
|
SAHA
|
suberoylanilide hydroxamic acid
|
|
DMSO
|
dimethyl sulfoxide
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
GSH
|
glutathione reductase
|
|
KIM-1
|
kidney injury molecular-1
|
|
HDAC
|
histone deacetylase
|
|
TSA
|
trichostatin A
|
|
CaOx
|
calcium oxalate
|
|
Scr
|
serum creatinine
|
|
BUN
|
blood urea nitrogen
|
|
OPN
|
osteopontin
|
|
HA
|
hyaluronan
|
|
ROS
|
reactive oxygen species
|
|
MMs
|
macromolecular modulators
|
|
NADPH
|
nicotinamide adenine dinucleotide
phosphate
|
Acknowledgments
This study was supported, in part, by grants from
the National Scientific Foundation of China (grant. no. 81270773),
the TCM Supported Project (grant. no. 13401900105), the Basic
Science Key Program of Science and Technology Commission of
Shanghai Municipality (grant. no. 11JC1407902), theScientific
Innovation of Shanghai Municipal Education Commission (grant. no.
14ZZ080) and the Scientific Program from Changhai Hospital (grant.
no. CH125520301).
References
|
1
|
Saigal CS, Joyce G and Timilsina AR;
Urologic Diseases in America Project: Direct and indirect costs of
nephrolithiasis in an employed population: opportunity for disease
management? Kidney Int. 68:1808–1814. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alexander RT, Hemmelgarn BR, Wiebe N,
Bello A, Morgan C, Samuel S, Klarenbach SW, Curhan GC and Tonelli
M: Alberta Kidney Disease Network: Kidney stones and kidney
function loss: A cohort study. BMJ. 345:e52872012. View Article : Google Scholar
|
|
3
|
Shoag J, Halpern J, Goldfarb DS and Eisner
BH: Goldfarb, Brian H. Eisner. Risk of chronic and end-stage kidney
disease in people with nephrolithiasis. J Urol. 192:1440–1445.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taylor EN, Stampfer MJ and Curhan GC:
Obesity, weight gain and the risk of kidney stones. JAMA.
293:455–462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang HS, Ma MC and Chen J: Low-vitamin E
diet exacerbates calcium oxalate crystal formation via enhanced
oxidative stress in rat hyperoxaluric kidney. Am J Physiol Renal
Physiol. 296:F34–F45. 2009. View Article : Google Scholar
|
|
6
|
Ma MC, Chen YS and Huang HS: Erythrocyte
oxidative stress in patients with calcium oxalate stones correlates
with stone size and renal tubular damage. Urology. 83:510e9–e17.
2014. View Article : Google Scholar
|
|
7
|
Chen S and El-Dahr SS: Histone
deacetylases in kidney development: Implications for disease and
therapy. Pediatr Nephrol. 28:689–698. 2013. View Article : Google Scholar
|
|
8
|
Marumo T, Hishikawa K, Yoshikawa M,
Hirahashi J, Kawa-chi S and Fujita T: Histone deacetylase modulates
the proinflammatory and-fibrotic changes in tubulointerstitial
injury. Am J Physiol Renal Physiol. 298:F133–F141. 2010. View Article : Google Scholar
|
|
9
|
Advani A, Huang Q, Thai K, Advani SL,
White KE, Kelly DJ, Yuen DA, Connelly KA, Marsden PA and Gilbert
RE: Long-term administration of the histone Deacetylase Inhibitor
vorinostat attenuates renal injury in experimental diabetes through
an endothelial nitric oxide synthase-dependent mechanism. Am J
Pathol. 178:2205–2214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang B, Zhu X, Kim Y, Li J, Huang S,
Saleem S, Li RC, Xu Y, Dore S and Cao W: Histone deacetylase
inhibition activates transcription factor Nrf2 and protects against
cerebral ischemic damage. Free Radic Biol Med. 52:928–936. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okada A, Nomura S, Higashibata Y, Hirose
M, Gao B, Yoshimura M, Itoh Y, Yasui T, Tozawa K and Kohri K:
Successful formation of calcium oxalate crystal deposition in mouse
kidney by intraabdominal glyoxylate injection. Urological research.
35:89–99. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonventre JV: Kidney injury molecule-1
(KIM-1): An urinary biomarker and much more. Nephrol Dial
Transplant. 24:1–4. 2009. View Article : Google Scholar
|
|
13
|
Han WK, Waikar SS, Johnson A, Betensky RA,
Dent CL, Devarajan P and Bonventre JV: Urinary biomarkers in the
early diagnosis of acute kidney injury. Kidney Int. 73:863–869.
2008. View Article : Google Scholar :
|
|
14
|
Cho ST, Jung SI, Myung SC and Kim TH:
Correlation of metabolic syndrome with urinary stone composition.
Int J Urol. 20:208–213. 2013. View Article : Google Scholar
|
|
15
|
Oda E: Medical check-up center, tachikawa
medical center: Overweight and high-sensitivity C-reactive protein
are weakly associated with kidney stone formation in Japanese men.
Int J Urol. 21:1005–1011. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moriyama MT, Suga K, Miyazawa K, Tanaka T,
Higashioka M, Noda K, Oka M, Tanaka M and Suzuki K: Inhibitions of
urinary oxidative stress and renal calcium level by an extract of
Quercus salicinaBlume/Quercus stenophyllaMakino in a rat calcium
oxalate urolithiasis model. Int J Urol. 16:397–401. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirose M, Yasui T, Okada A, et al: Renal
tubular epithelial cell injury and oxidative stress induce calcium
oxalate crystal formation in mouse kidney. Int J Urol, 2010.
17:83–92. 2010.
|
|
18
|
Khan SR, Joshi S, Wang W and Peck AB:
Regulation of macromolecular modulators of urinary formation by
reactive oxygen species: Transcriptional study in an animal model
of hyperoxaluria. Am J Physiol Renal Physiol. 306:F1285–F1295.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanlaya R, Fong-Ngern K and Thongboonkerd
V: Cellular adaptive response of distal renal tubular cells to
high-oxalate environment highlights surface alpha-enolase as the
enhancer of calcium oxalate monohydrate crystal adhesion. J
Proteomics. 80:55–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie Y, Sakatsume M, Nishi S, Narita I,
Arakawa M and Gejyo F: Expression, roles, receptors and regulation
of osteopontin in the kidney. Kidney Int. 60:1645–1657. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown LF, Berse B, Van de Water L,
Papadopoulos-Sergiou A, Perruzzi CA, Manseau EJ, Dvorak HF and
Senger DR: Expression and distribution of osteopontin in human
tissues: Widespread association with luminal epithelial surfaces.
Mol Biol Cell. 3:1169–1180. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ullrich O, Mann K, Haase W and Koch-Brandt
C: Biosynthesis and secretion of an osteopontin-related 20-kDa
polypeptide in the Madin-Darby canine kidney cell line. J Biol
Chem. 266:3518–3525. 1991.PubMed/NCBI
|
|
23
|
Asselman M, Verhulst A, De Broe ME and
Verkoelen CF: Calcium oxalate crystal adherence to hyaluronan-,
osteopontin- and CD44-expressing injured/regenerating tubular
epithelial cells in rat kidneys. J Am Soc Nephrol. 14:3155–3166.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsuji H, Shimizu N, Nozawa M, Umekawa T,
Yoshimura K, De Velasco MA, Uemura H and Khan SR: Osteopontin
knockdown in the kidneys of hyperoxaluric rats leads to reduction
in renal calcium oxalate crystal deposition. Urolithiasis.
42:195–202. 2014.PubMed/NCBI
|
|
25
|
Fujii Y, Okada A, Yasu T, Niimi K,
Hamamoto S, Hirose M, Kubota Y, Tozawa K, Hayashi Y and Kohri K:
Effect of adiponectin on kidney crystal formation in metabolic
syndrome model mice via inhibition of inflammation and apoptosis.
PLoS One. 8:e613432013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pang M, Kothapally J, Mao H, Tolbert E,
Ponnusamy M, Chin YE and Zhuang S: Inhibition of histone
deacetylase activity attenuates renal fibroblast activation and
interstitial fibrosis in obstructive nephropathy. Am J Physiol
Renal Physiol. 297:F996–F1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khaskhali MH, Byer KJ and Khan SR: The
effect of calcium on calcium oxalate monohydrate crystal-induced
renal epithelial injury. Urol Res. 37:1–6. 2009. View Article : Google Scholar
|
|
28
|
Niimi K, Yasui T, Okada A, Hirose Y,
Kubota Y, Umemoto Y, Kawai N, Tozawa K and Kohri K: Novel effect of
the inhibitor of mitochondrial cyclophilin D activation,
N-methyl-4-isoleucine cyclosporin, on renalcalcium crystallization.
Int J Urol. 21:707–713. 2014. View Article : Google Scholar : PubMed/NCBI
|