Introduction
MicroRNAs (miRNAs) are endogenous non coding RNAs
and are key post transcriptional regulators, inhibiting the
translation or promoting the degradation of target mRNAs via
binding to the 3′ untranslated regions (UTRs) (1). These miRNAs have been identified to
be involved in complex physiological processes including
differentiation, proliferation and metabolism (2). Previous studies suggest that intronic
miRNAs, which are located in the intron regions of host genes, may
construct a fine-tuning regulatory system with their host genes and
trigger cell transition by responding to external stimuli (3,4). The
gene encoding LIM domain lipoma preferred partner (LPP) has been
reported to promote smooth muscle cell (SMC) migration in arterial
injury and atherosclerosis (5–7),
whereas its intronic miRNA, miRNA-28 (miR-28), is associated with
tumor cell migration, adhesion, proliferation and apoptosis
(8,9). A previous preliminary study (10) indicated that the transfection of
miR-28-5p mimics increased the expression of ABCA1 in HepG2 cells
and THP-1-derived macrophages. In addition, the circulating levels
of miR-28-5p were significantly increased in a relatively small
sample population of patients with unstable angina (UA). Therefore,
investigation of miR-28 may provide insight into the cholesterol
metabolism in atherosclerosis.
Extracellular signal regulated kinase 2 (ERK2) is a
member of a highly conserved family of serine threonine protein
kinases and is a key regulator in cell growth and differentiation
(11). ERK2 is activated or
overexpressed in numerous types of cancer, and may be a potential
therapeutic target (12). In
addition, ERK2 has been demonstrated to be involved in cardiac
development and protection (13).
Zhou et al (14) reported a
novel role for ERK2 activity in cholesterol trafficking,
demonstrating that inhibition of ERK1/2 markedly increases ATP
binding cassette transporter A1 (ABCA1) expression and leads to
increased cholesterol efflux in macrophages. ABCA1 is a key
mediator in the maintenance of high density lipoprotein cholesterol
(HDL-C) biosynthesis in the liver and cholesterol efflux in
macrophages (15,16). Mutations in the ABCA1 gene lead to
Tangier disease, which is characterized by impaired cholesterol
efflux and low levels of HDL, leading to an increased risk of
atherosclerotic disease (17–19).
Due to the anti atherogenic properties of ABCA1 and ERK2, they
represent potential targets through which atherosclerosis may be
attenuated via ABCA1 upregulation. miRWalk target prediction
previously indicated that miR-28-5p is the highest ranking miRNA
predicted to target ERK2 (20),
suggesting that it may regulate ABCA1 expression via ERK2
inhibition.
Girardot et al (21) validated the fact that ERK2 is an
miR-28-targeted gene using the psi-CHECK-2 luciferase reporter
system in the Mo7e human megakaryoblastic leukemia cell line. In
the current study, the inhibitory effect on ERK2 was investigated
via the transfection of miR-28-5p mimics and inhibitors into HepG2
cells and the assessment of the mechanism of miR-28-5p-mediated
ABCA1 upregulation. Additionally, the association between the
plasma levels of miR-28-5p and HDL C levels was evaluated in a
sample population of patients with UA to investigate its potential
use as a biomarker and as a therapeutic target in
atherosclerosis.
Materials and methods
Cell culture and transfection
The HepG2 human hepatoma cell line was obtained from
the Cell Culture Engineering Center of the Chinese Academy of
Medical Sciences and Peking Union Medical College (Beijing, China).
HepG2 cells were cultured in 6 well plates with 2.0 ml/well
Dulbecco's modified Eagle's medium supplemented with 10%
heat-inactivated fetal bovine serum (Invitrogen Life Technologies,
Carlsbad, CA, USA), 100 U/ml penicillin and 100 µg/ml strep
tomycin (Invitrogen Life Technologies) at 37°C in a 5%
CO2 incubator. HepG2 cells were serum starved for a
minimum of 16 h prior to treatment.
Control mimics, miR-28-5p mimics or inhibitors (100
nM; Invitrogen Life Technologies) were transiently transfected into
HepG2 cells using Lipofectamine® RNAi 2000 Reagent and
Opti MEM I Reduced Serum Medium (Invitrogen Life Technologies)
according to the manufacturer's instructions. Cells were treated
with the ERK2 inhibitor PD98059 (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) as described previously (13). Briefly, HepG2 cells were
pre-transfected with or without the mir-28-5p inhibitor and
incubated with 20 or 40 µM PD98059 overnight. Following 48 h
of transfection, cells were harvested for protein analysis or were
treated for further experiments. Briefly, the treated cells were
washed with times with phosphate buffered saline, and lysed by
radioimmunoprecipitation acid buffer (Beyotime Institute of
Biotechnology, Shanghai, China) at 4°C. All transfection
experiments were performed at least three times.
Western blotting
Treated cells were lysed in radioimmu
noprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). Protein concentrations were determined using a
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology). Protein extracts (40 µg/lane) were separated
on a 10% SDS-polyacrylamide gel (Sigma-Aldrich, St. Louis, MO, USA)
and transferred to polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA) using a Mini Trans-Blot®
system (Bio-Rad Laboratories, Inc., Shanghai, China). Membranes
were blocked with immuno-blotting blocking buffer (Beyotime
Institute of Biotechnology) for 1 h at room temperature prior to
incubation with the following primary antibodies obtained from
Santa Cruz Biotechnology, Inc.: Polyclonal rabbit anti-ABCA1
(1:500; cat no. sc-20794), polyclonal rabbit anti ERK2 (1:500; cat
no. sc-154) or polyclonal rabbit anti-β-actin (1:1,000; cat no.
sc-130656) overnight at 4°C. Following washing with Tris buffered
saline with Tween-20, the membranes were incubated for 2 h at room
temperature with horseradish peroxidase conjugated goat anti rabbit
IgG antibodies (1:5,000; cat no. sc-2004; Santa Cruz Biotechnology,
Inc.). Protein bands were visualized by chemiluminescence using the
SuperSignal™ West Dura Substrate (Thermo Fisher Scientific,
Waltham, MA, USA). Signal intensity was quantified using
AlphaEaseFC 4.0 software (Cell Biosciences, Inc., Santa Clara, CA,
USA) and normalized to β-actin levels.
Study population
All patients with UA (n=39) in the current study
were enrolled from the Department of Cardiology in Tangshan Gongren
Hospital (Tangshan, China) between March 2012 and August 2013. The
diagnosis of UA was confirmed using the American College of
Cardiology and the American Heart Association 2007 guidelines
(22). Healthy subjects (n=28)
were selected if the following conditions were absent: Diabetes,
hypertension, hepatitis, kidney disease, cardiovascular disease and
medication history at the Tangshan Gongren Hospital. The current
study was approved by the Ethics Committee of Tangshan Gongren
Hospital and written informed consent was obtained from each
volunteer.
Clinical samples
The handling of blood samples was completed within 2
h of collection according to the Tangshan Gongren Hospital Biobank
Handling and Storage Protocol for the collection, processing and
archiving of human blood. Briefly, fasting blood samples were drawn
in the morning and collected into EDTA anticoagulant tubes
(Guangzhou Improve Medical Instruments Co., Ltd., Guangzhou, China)
and centrifuged at 3,000 × g then 16,000 × g for 10 min at 4°C each
to separate the plasma from blood cells, platelets and cellular
debris. Plasma was then transferred to RNase/DNase-free 1.5 ml Cryo
tubes (Sangon Biotech Co., Ltd., Shanghai, China) and stored at
−80°C until required.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total small RNAs were isolated from 500 µl
plasma using the mirVana™ RNA Isolation kit (Ambion Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. Extracted RNAs were eluted with 80 µl
diethylpyrocarbonate treated ddH2O (Sangon Biotech Co.,
Ltd.) and stored at−80°C. Quality of RNA was determined using a
NanoDrop™ 2000 Spectrophotometer (Thermo Fisher Scientific).
The expression levels of miR-28-5p and miR-423-3p
were determined by RT-qPCR. Briefly, small RNA was reverse
transcribed into DNA using the TaqMan® MicroRNA Reverse
Transcription kit (Applied Biosystems Life Technologies, Foster
City, CA, USA). In brief, the RT reaction mixture contained 0.8
µl 10X reverse transcription buffer, 4 µl small RNA
(~20 ng/µl), 0.1 µl 100 mM dNTPs, 0.1 µl RNase
inhib itor (20 U/µl), 1.5 µl RT primer, 0.5 µl
MultiScribe™ Reverse Transcriptase (50 U/µl) and 1 µl
RNase/DNase free ddH2O. The reaction mixture was
incubated at 16°C for 60 min, 42°C for 60 min and 85°C for 5 min.
RT qPCR was performed using an Applied Biosystems® 7500
Real time PCR System (Applied Biosystems Life Technologies). The
PCR reac tion mixture contained 4 µl RT product, 10
µl 2X TaqMan Universal PCR Master Mix, 1 µl TaqMan
probe (2.5 µM) and 5 µl RNase/DNase free
ddH2O. The cycling parameters were as follows:
Denaturation at 95°C for 10 min followed by 40 cycles each of
denaturation at 95°C for 15 sec and annealing and extension at 60°C
for 1 min. miRNA levels were determined using the 2-[Ct(target
miRNA) Ct(reference miRNA)] method. Hsa-miR-423 was used as a
stable internal reference in the plasma samples according to the
guidelines of ABI miRNA profiling (Applied Biosystems Life
Technologies) in serum/plasma and as previously reported (23).
miRNA target prediction
miRNA target interactions were predicted using the
miRwalk database (http://www.umm.uniheidelberg.de/apps/zmf/mirwalk).
miRNA sets for ERK2 were obtained based on the miRwalk prediction
algorithm.
Statistical analysis
Data were presented as the mean ± standard error
unless otherwise stated. Comparisons between two groups were
performed using the Mann Whitney U test or Student's t test as
appro priate. A Fisher's exact test or χ2 test was used
to compare categorical variables. Correlation analysis was
performed using Spearman's correlation analysis. Statistical
analysis was performed with GraphPad Prism software, version 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification of the target gene of
miR-28-5p in HepG2 cells
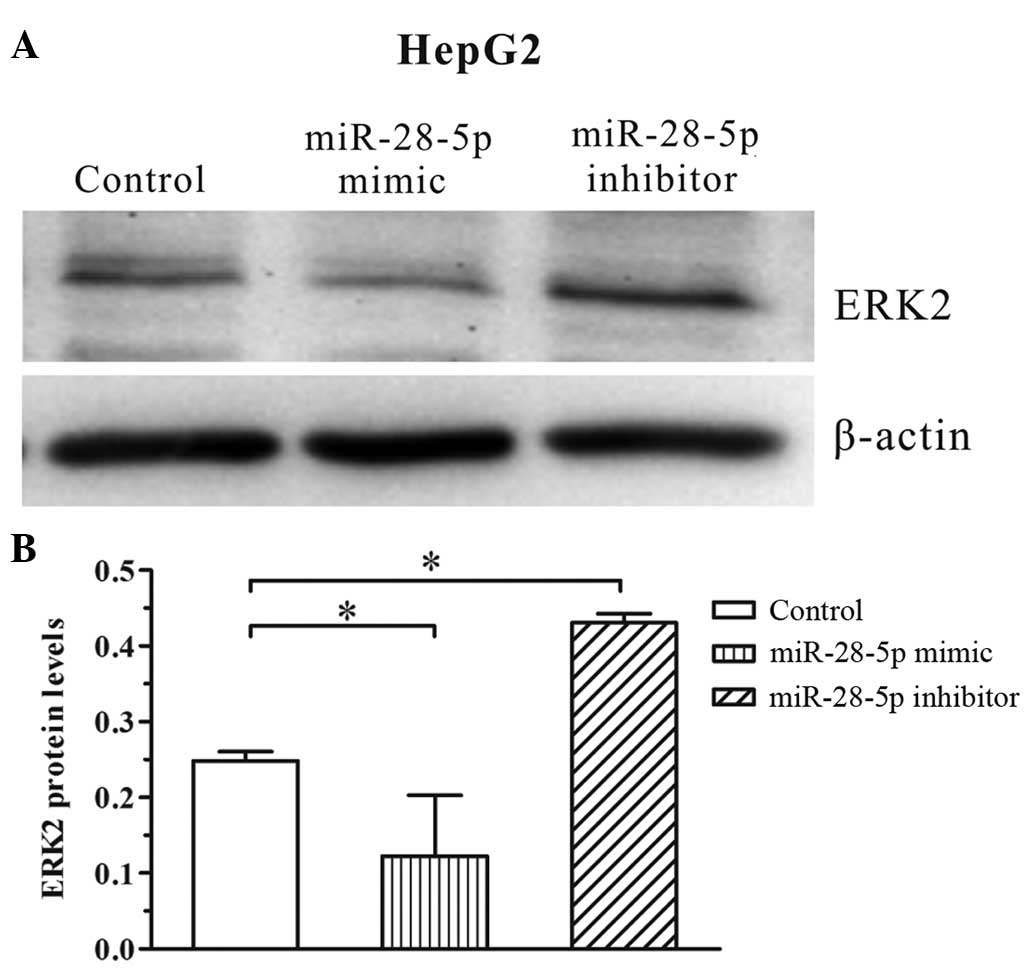
To validate the fact that miR-28-5p targets ERK2,
the protein levels of ERK2 in HepG2 cells were measured following
transfection with the miR-28-5p mimic or inhibitor. Western
blotting indicated that transfection with the miR-28-5p mimic
significantly reduced the expression levels of ERK2 in HepG2 cells,
whereas the miR-28-5p inhibitor resulted in elevated levels of ERK2
following 48 h of transfection (Fig.
1). These results indicate that miR-28-5p is a key mediator in
the regulation of the translation of ERK2.
Effect of miR-28-5p mimics or inhibitors
on ABCA1 expression in HepG2 cells
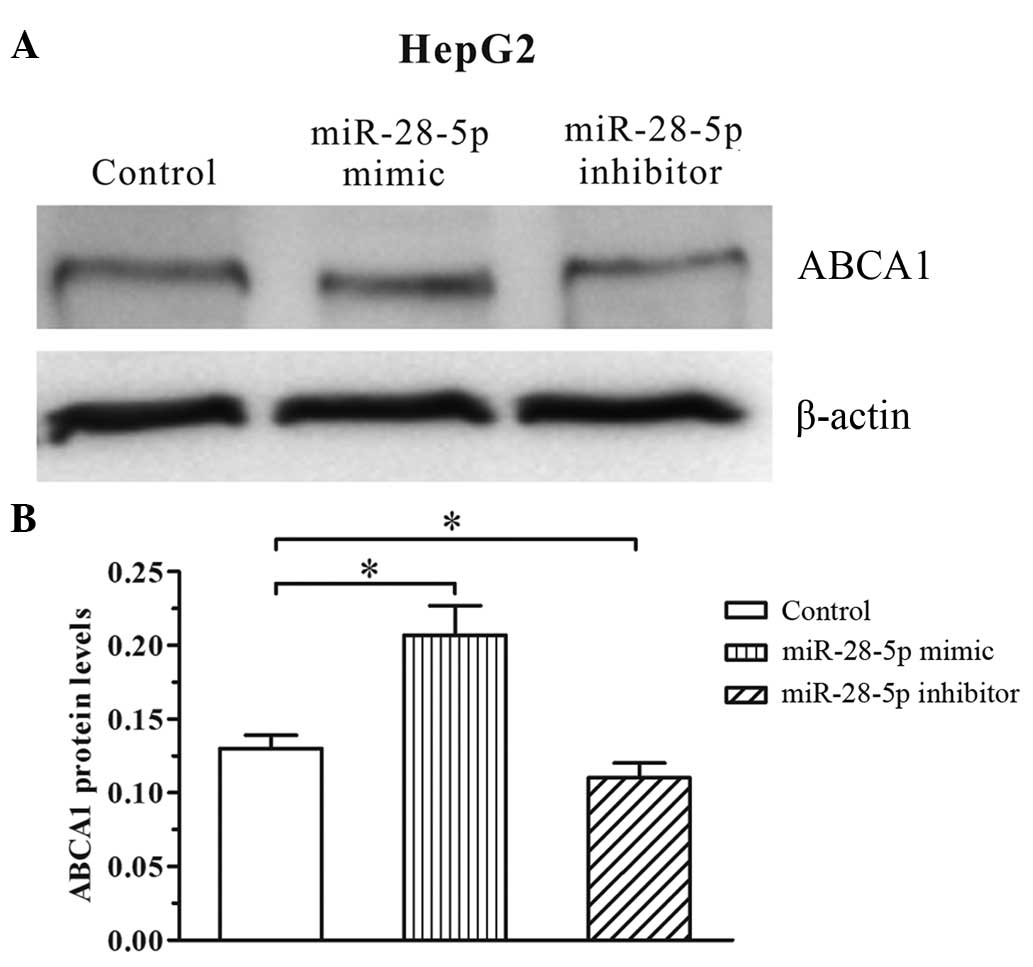
In a previous preliminary study (10), the transfection of miR-28-5p mimics
was observed to result in an increase in ABCA1 expression levels in
HepG2 cells and THP 1 derived macrophages. In the current study,
miR-28-5p inhibitors were transfected into HepG2 cells to further
investigate the miR-28-5p mediated upregulation of ABCA1. As
presented in Fig. 2, transfection
with the miR-28-5p inhibitor significantly reduced the expression
levels of ABCA1 (P<0.05) compared with control and miR-28-5p
mimics (Fig. 2).
miR-28-5p upregulates ABCA1 expression
through the inhibition of ERK2
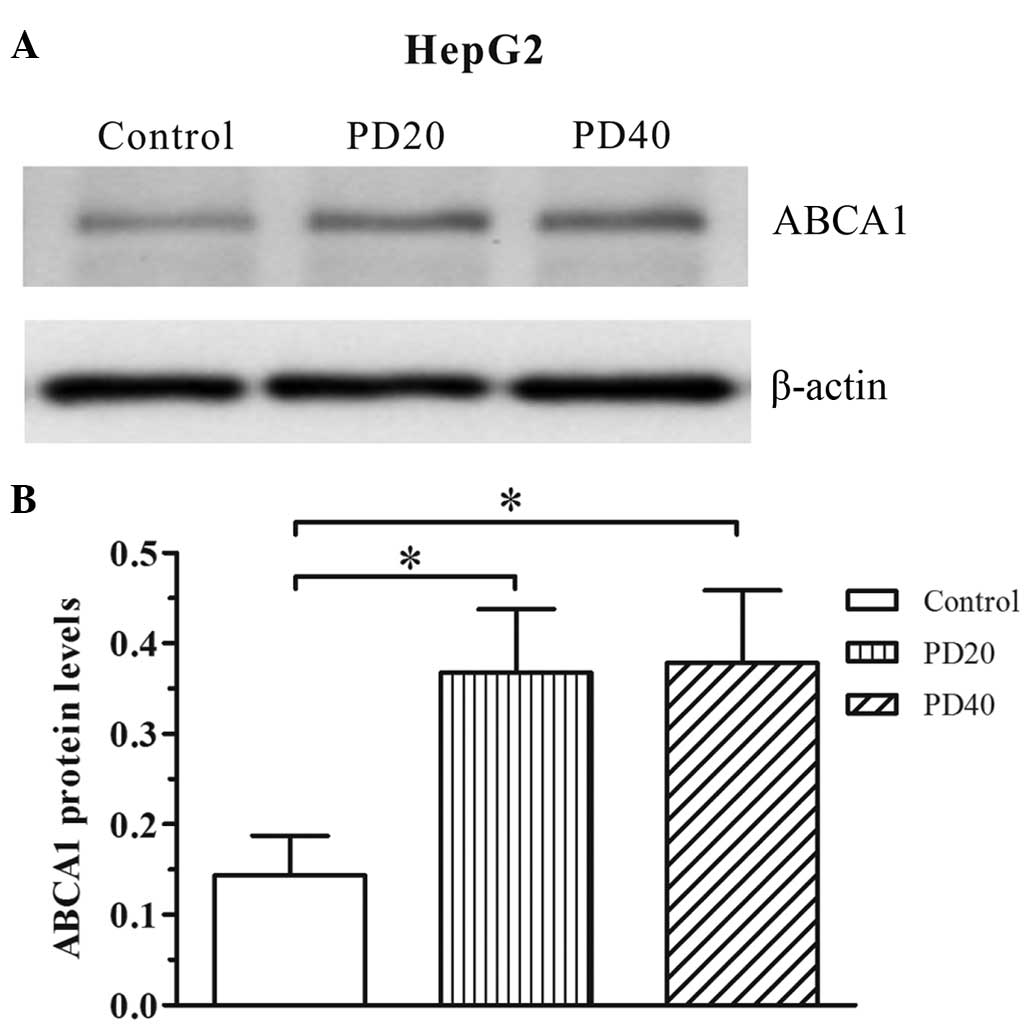
To investigate whether ERK2 alters the expression of
ABCA1, HepG2 cells were incubated with an ERK2 inhibitor, PD98059.
As previously reported, the induction of ABCA1 expression is semi
dependent on the concentration of ERK2 inhibitors, with the maximal
induction effect dose for macrophage ABCA1 expression by PD98059
being 20 µM (14). As
presented in Fig. 3, PD98059
increased the expression levels of ABCA1 in HepG2 cells.
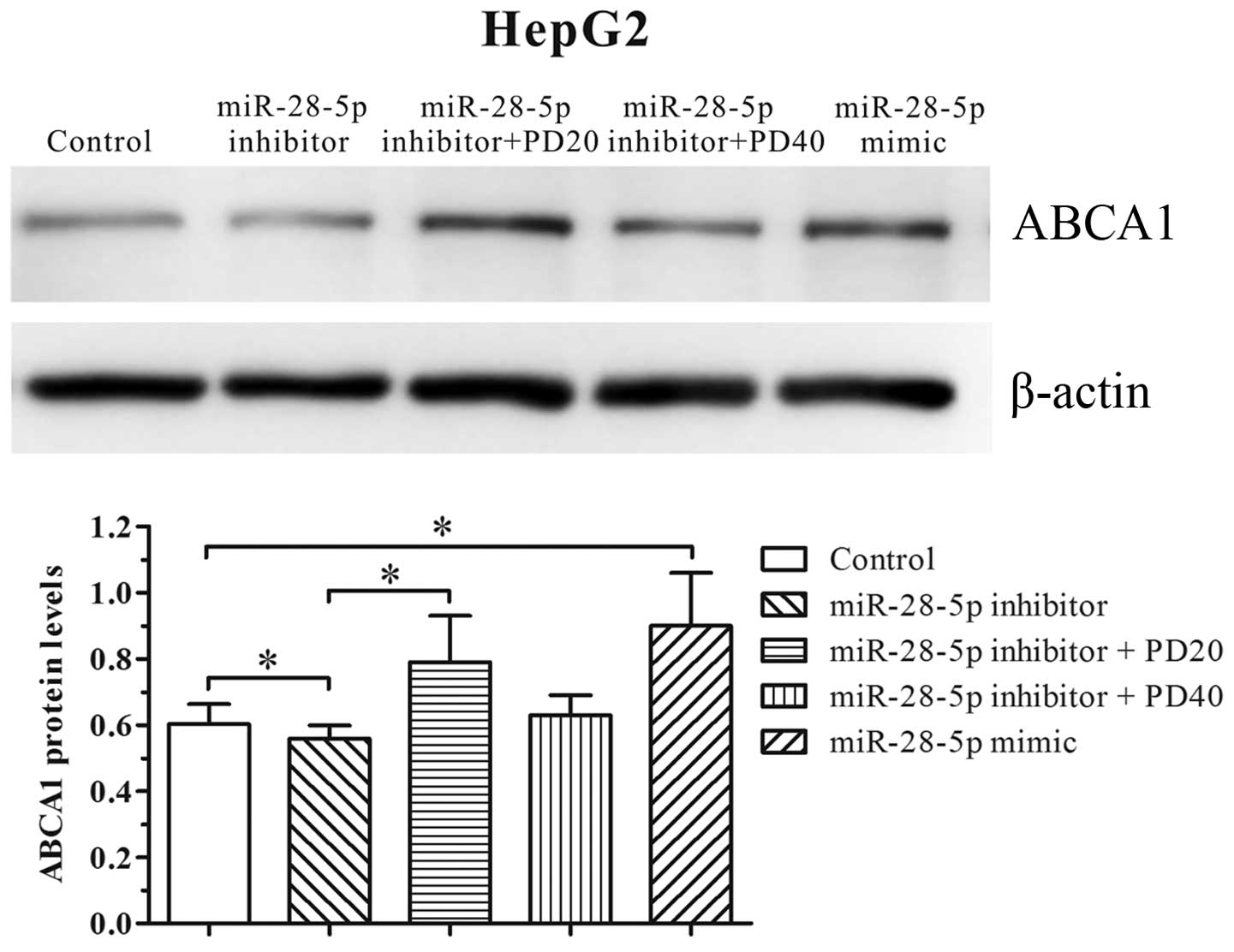
To confirm that the miR-28-5p-mediated upregulation
of ABCA1 is dependent on ERK2, HepG2 cells were transfected with
the miR-28-5p inhibitor prior to incubation with the ERK2 inhibitor
(PD98059) overnight. As presented in Fig. 4, HepG2 cells stimulated with 20
µM PD98059 antagonized the miR-28-5p inhibitor mediated
ABCA1 reduction. Taken together, these data indicate that miR-28-5p
mediates ABCA1 expression through the ERK2 signaling pathway.
To explore the potential miRNAs with the ability to
upreg ulate ABCA1 through ERK2 inhibition, the predicted dataset of
miRNA and ERK2 interactions were retrieved from the miRwalk
database. The top 30 predicted miRNAs are shown as Table II, and all have potential roles in
ERK2-mediated ABCA1 upregulation.
 | Table IIPredicted microRNA sites according to
mRNA selected regions. |
Table II
Predicted microRNA sites according to
mRNA selected regions.
| MicroRNA | StemLoopID | Seed length | P-value | Sites |
|---|
| hsa-miR-28-5p | hsa-mir-28 | 16 | 0.0000 | 2 |
| hsa-miR-220c | hsa-mir-220c | 11 | 0.0011 | 2 |
| hsa-miR-297 | hsa-mir-297 | 11 | 0.0011 | 2 |
|
hsa-miR-452* | hsa-mir-452 | 10 | 0.0044 | 2 |
| hsa-miR-320c | hsa-mir-320c-1 | 10 | 0.0044 | 4 |
| hsa-miR-320d | hsa-mir-320d-2 | 10 | 0.0044 | 3 |
| hsa-miR-320b | hsa-mir-320b-2 | 10 | 0.0044 | 4 |
| hsa-miR-320a | hsa-mir-320a | 10 | 0.0044 | 2 |
| hsa-miR-628-5p | hsa-mir-628 | 10 | 0.0044 | |
| hsa-miR-1183 | hsa-mir-1183 | 9 | 0.0174 | |
| hsa-miR-181a | hsa-mir-181a-2 | 9 | 0.0174 | 2 |
| hsa-miR-1229 | hsa-mir-1229 | 9 | 0.0174 | 2 |
| hsa-miR-181c | hsa-mir-181c | 9 | 0.0174 | |
|
hsa-miR-196a* | hsa-mir-196a-2 | 9 | 0.0174 | |
| hsa-miR-491-5p | hsa-mir-491 | 9 | 0.0174 | |
| hsa-miR-548l | hsa-mir-548l | 9 | 0.0174 | |
| hsa-miR-1914 | hsa-mir-1914 | 9 | 0.0174 | |
| hsa-miR-493 | hsa-mir-493 | 9 | 0.0174 | |
| hsa-miR-1250 | hsa-mir-1250 | 9 | 0.0174 | |
|
hsa-miR-195* | hsa-mir-195 | 9 | 0.0174 | |
| hsa-miR-497 | hsa-mir-497 | 9 | 0.0174 | |
| hsa-miR-300 | hsa-mir-300 | 9 | 0.0174 | |
| hsa-miR-1255a | hsa-mir-1255a | 9 | 0.0174 | |
| hsa-miR-603 | hsa-mir-603 | 9 | 0.0174 | |
| hsa-miR-1269 | hsa-mir-1269 | 9 | 0.0174 | |
| hsa-miR-1252 | hsa-mir-1252 | 9 | 0.0174 | |
|
hsa-miR-302b* | hsa-mir-302b | 9 | 0.0174 | |
| hsa-miR-628-5p | hsa-mir-628 | 9 | 0.0174 | |
|
hsa-miR-541* | hsa-mir-541 | 9 | 0.0174 | |
| hsa-miR-22 | hsa-mir-22 | 9 | 0.0174 | |
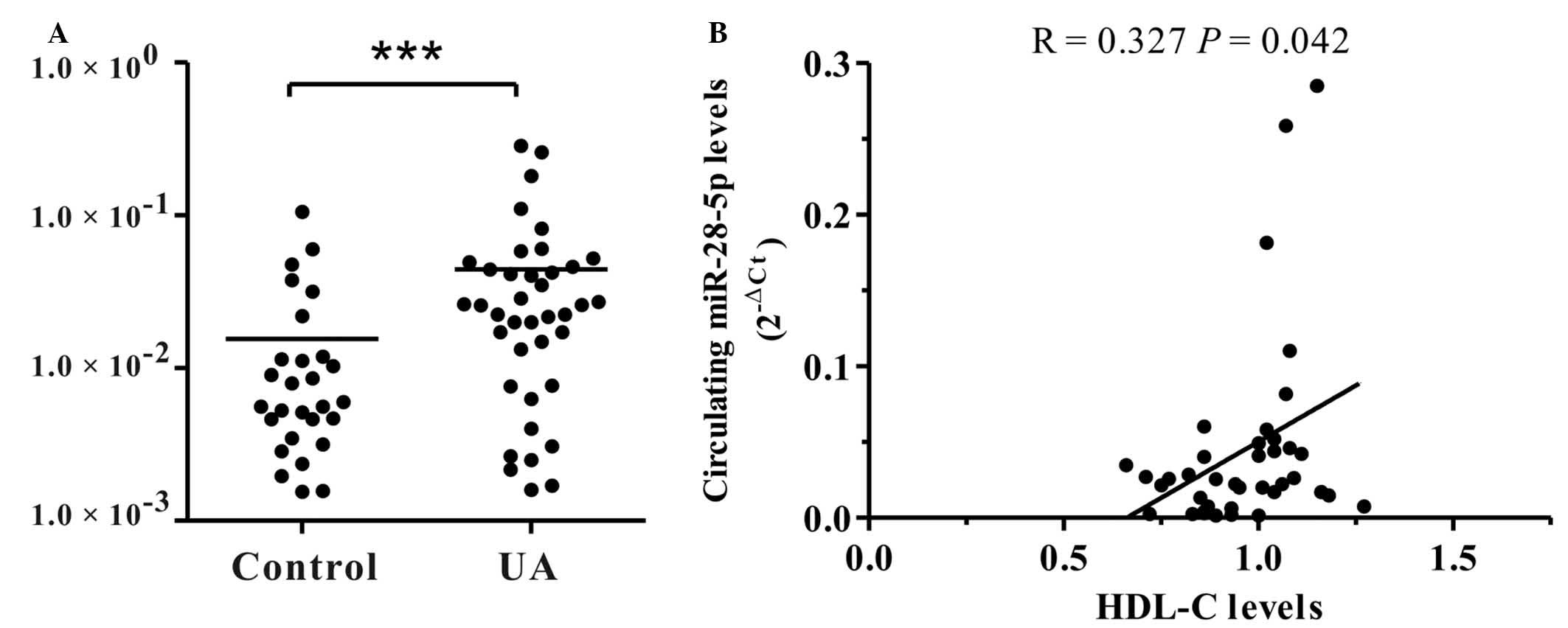
Circulating levels of miR-28-5p
positively correlate with HDL-C levels
Circulating levels of miR-28-5p in healthy subjects
(n=28) and patients with UA (n=39) were measured to investigate the
association between the circulating levels of miR-28-5p and
clinical parameters in patients with UA. Clinical parameters of
patients with UA are presented in Table I. There were no significant
differences between the groups in age, gender or risk factors
except for total cholesterol and HDL C. The circulating levels of
miR-28-5p were not significantly associated with age or gender or
with diabetic status. However, the circulating levels of miR-28-5p
in patients with UA were observed to be positively correlated with
HDL-C levels (R= −0.327; P=0.042; Fig.
5), supporting the role of miR-28-5p in the upregulation of
ABCA1 in vitro.
 | Table IClinical characteristics of subjects
between the control and UA groups. |
Table I
Clinical characteristics of subjects
between the control and UA groups.
| Characteristic | Control
(n=28) | UA
(n=39) | P value |
|---|
| Age (years) | 55±8 | 58±9 | 0.291 |
| Gender
(male/female) | 13/15 | 21/18 | 0.624 |
| Glu (mmol/l) | 5.36±0.52 | 5.33±1.39 | 0.959 |
| CHOL (mmol/l) | 5.25±095 | 4.18±0.97 | <0.001 |
| TG (mmol/l) | 1.31±0.62 | 1.12±0.34 | 0.116 |
| HDL-C (mmol/l) | 1.19±0.24 | 1.02±0.189 | 0.002 |
| LDL-C (mmol/l) | 3.00±0.81 | 2.65±0.93 | 0.115 |
| APOA1 (g/l) | – | 1.13±0.19 | – |
| APOB100 (g/l) | – | 0.80±0.17 | – |
| LP(α) (mg/l) | – | 316.46±247.75 | – |
| CK-MB | – | 13.11±5.75 | – |
| Smokers | 13/15 | 21/18 | 0.624 |
| Hypertension | none | none | – |
| Diabetes mellitus,
n (%) | none | 31.58 | – |
Discussion
A previous study indicated that miR-28-5p inhibits
the expression of LPP (24), which
is involved in SMC phenotype transition in atherosclerosis
(6,7), suggesting that it may serve a
potential role in cardiovascular disorders. A previous preliminary
study (10) demonstrated that
miR-28-5p mimics increased ABCA1 expression in HepG2 cells.
However, the mechanism of miR-28-5p mediated ABCA1 upregulation and
its association with the clinical parameters of cardiovascular
disease remains unclear. In the current study, the translational
inhibition of ERK2 by miR-28-5p in HepG2 cells was identified, and
miR-28-5p was observed to upregulate the expression of ABCA1 via
the inhibition of ERK2. Conversely, inhibiting miR-28-5p represses
the upregulation of ABCA1 induced by the ERK2 inhibitor,
PD98059.
A number of miRNAs including miR 33a/b, miR-758 and
miR-144 have been demonstrated to serve important roles in the LXR
mediated ABCA1 pathway by post transcriptional inhibitory
regulation (25–27). These miRNAs inhibit ABCA1
expression through binding to its 3′UTR; however, certain miRNAs,
such as miR-122 and miR-370, have been reported to upregulate ABCA1
expression (25–27). Antagonism of miR-122 in mice
resulted in the sustained reduction of total plasma cholesterol
levels (28). In addition, miR-370
has been observed to directly regulate lipid metabolism via
increasing miR-122 levels (28).
The current study indicates that blockage of miR-28-5p with its
inhibitor reduced ABCA1 protein levels in HepG2 cells, whilst
miR-28-5p mimics resulted in the upregulation of ABCA1, suggesting
that miR-28-5p is a modulator miRNA for ABCA1 expression.
Numerous studies have described RNA activation,
which is the process by which miRNAs are suggested to activate
single or multiple genes via the inhibition of negative regulators
of certain genes (29–31). ERK2 is an important signaling
molecule regulating cellular proliferation and differentiation
(12). The biological functions of
ERK2 in the cardiac system focus on cardiac development,
hypertrophy and protection (32),
whereas the role of ERK2 in atherosclerosis predominantly mediates
macrophage cholesterol efflux (14). This is based on evidence that the
inhibition of ERK2 by PD098059 induces ABCA1 upregulation (14). The current study demonstrated that
transfection with miR-28-5p mimics reduced ERK2 expression levels
in HepG2 cells, whilst blockage of miR-28-5p with its inhibitor
increased ERK2 expression levels. Consequently, the effects of
miR-28-5p on ERK2 mediated ABCA1 regulation were observed. ERK2
inhibition antagonizes miR-28-5p inhibitor mediated ABCA1
reduction. Taken together, these data indicate that the miR-28-5p
mediated regulation of ABCA1 occurred via the inhibition of ERK2,
indicating a novel mechanism of the miRNA associated cholesterol
metabolism.
The use of miRNAs in the diagnosis and treatment of
cardiovascular disease is an innovative field, with potential
strategies including the detection of circulating miRNA levels and
the delivery of miRNA inhibitors or mimics (33). The current study indicates that
elevated levels of miR-28-5p in plasma are positively correlated
with serum HDL C levels in patients with UA, supporting the role of
miR-28-5p mediated ABCA1 upregulation in the atherosclerotic
progress. In the treatment of cardiovascular diseases, there are
currently no specific drugs widely available that are able to raise
HDL levels efficiently. The use of anti-miRs against
ABCA1-associated miRs, such as miR-33 and miR-144, have been
confirmed to be potentially therapeutic in the attenuation of the
atherosclerotic progress in murine models of atheroscle rosis
(25,27). The anti atherogenic properties of
ABCA1 have been well studied, with overexpression of ABCA1 in
animal models demonstrated to reduce total cholesterol levels and
attenuate atherosclerosis (34,35),
suggesting that ABCA1 may be a candidate therapeutic target to
raise HDL C levels. The current study confirmed that miR-28-5p, as
the first positive modulator of ABCA1 via the inhibition of ERK2,
may be a potential target for the treatment of cardiovascular
disease. The top 30 predicted and 6 validated miRNAs targeted to
ERK2 are presented in Table II.
These miRs have potential roles in the ERK2-mediated ABCA1
upregulation and are suggested to act as agents which raise HDL-C
levels to relieve atherosclerotic plaques.
In conclusion, the current study demonstrated that
miR-28-5p mediated the upregulation of ABCA1 through the inhibition
of ERK2, extending the role of ERK2 in activating cholesterol
trafficking. The correlation between circulating miR-28-5p and HDL
C levels in patients with UA suggests that miR-28-5p may
participate in the development of atherosclerotic plaques. These
data provide novel insight into the cholesterol metabolism and
cardiovascular disease. Follow up studies on the correlation of
miR-28-5p with the pathophysiological alterations in patients with
UA will lead to an in depth understanding of the clinical
significance and potential use in the diagnosis, treatment and
prognosis of patients with UA. In addition, studies in animal
models will aid in the elucidation of the biological significance
of the miR-28-5p ERK2-ABCA1 signaling pathway in UA.
Acknowledgments
This study was supported by the Medical Research
Foundation of the Health Planning Commission of Hebei Province of
China (grant no. 20130315) and the Scientific Research Program for
Returned Scholars, Department of Human Resources and Social
Security of Hebei Province of China.
Abbreviations:
|
LPP
|
lipoma preferred partner
|
|
ABCA1
|
ATP binding cassette transporter
A1
|
|
ERK2
|
extracellular signal regulated kinase
2
|
|
UA
|
unstable angina
|
|
UTR
|
untranslated region
|
|
HDL C
|
high density lipoprotein
cholesterol
|
|
LDL C
|
low density lipoprotein
cholesterol
|
|
Glu
|
glucosamine
|
|
CHOL
|
cholesterol
|
|
TG
|
triglyceride
|
|
APOA1
|
apolipoprotein A1
|
|
LP(α)
|
lipoprotein α
|
|
CK MB
|
creatine kinase myocardial band
|
References
|
1
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambros V, Lee RC, Lavanway A, Williams PT
and Jewell D: MicroRNAs and other tiny endogenous RNAs in C.
elegans. Curr Biol. 13:807–818. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin SL, Miller JD and Ying SY: Intronic
microRNA (miRNA). J Biomed Biotechnol. 2006:268182006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hooper CL, Dash PR and Boateng SY: Lipoma
preferred partner is a mechanosensitive protein regulated by nitric
oxide in the heart. FEBS Open Bio. 2:135–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin L, Hastings NE, Blackman BR and Somlyo
AV: Mechanical properties of the extracellular matrix alter
expression of smooth muscle protein LPP and its partner palladin;
relationship to early atherosclerosis and vascular injury. J Muscle
Res Cell Motil. 30:41–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gorenne I, Jin L, Yoshida T, Sanders JM,
Sarembock IJ, Owens GK, Somlyo AP and Somlyo AV: LPP expression
during in vitro smooth muscle differentiation and stent induced
vascular injury. Circ Res. 98:378–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Almeida MI, Nicoloso MS, Zeng L, Ivan C,
Spizzo R, Gafà R, Xiao L, Zhang X, Vannini I, Fanini F, et al:
Strand-specific miR-28-5p and miR-28-3p have distinct effects in
colorectal cancer cells. Gastroenterology. 142:886–896.e9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang M, Yao Y, Eades G, Zhang Y and Zhou
Q: MiR-28 regulates Nrf2 expression through a Keap1 independent
mechanism. Breast Cancer Res Treat. 129:983–991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Liu Y, Sun YN, Li S, Liu XQ, Li J,
Li CM, Tian W, Zhou YT and Shang XM: miR-28-5p involved in
LXR-ABCA1 pathway is increased in the plasma of unstable angina
patients. Heat Lung Circ. 24:724–730. 2015. View Article : Google Scholar
|
|
11
|
Sebolt-Leopold JS and Herrera R: Targeting
the mitogen activated protein kinase cascade to treat cancer. Nat
Rev Cancer. 4:937–947. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
13
|
Wang Y: Mitogen-activated protein kinases
in heart development and diseases. Circulation. 116:1413–1423.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou X, Yin Z, Guo X, Hajjar DP and Han J:
Inhibition of ERK1/2 and activation of liver X receptor
synergistically induce macrophage ABCA1 expression and cholesterol
efflux. J Biol Chem. 285:6316–6326. 2010. View Article : Google Scholar :
|
|
15
|
Tall AR: Cholesterol efflux pathways and
other potential mechanisms involved in the athero protective effect
of high density lipoproteins. J Intern Med. 263:256–273. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tall AR, Yvan Charvet L, Terasaka N,
Pagler T and Wang N: HDL, ABC transporters, and cholesterol efflux:
Implications for the treatment of atherosclerosis. Cell Metab.
7:365–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bodzioch M, Orsó E, Klucken J, Langmann T,
Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C,
Porsch-Ozcürümez M, et al: The gene encoding ATP binding cassette
transporter 1 is mutated in Tangier disease. Nat Genet. 22:347–351.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brooks Wilson A, Marcil M, Clee SM, Zhang
LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO,
et al: Mutations in ABC1 in Tangier disease and familial high
density lipoprotein deficiency. Nat Genet. 22:336–345. 1999.
View Article : Google Scholar
|
|
19
|
Rust S, Rosier M, Funke H, Real J, Amoura
Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denèfle P and
Assmann G: Tangier disease is caused by mutations in the gene
encoding ATP binding cassette transporter 1. Nat Genet. 22:352–355.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk database: Prediction of possible miRNA binding sites by
“walking” the genes of three genomes. J Biomed Inform. 44:839–847.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Girardot M, Pecquet C, Boukour S, Knoops
L, Ferrant A, Vainchenker W, Giraudier S and Constantinescu SN: miR
28 is a thrombopoietin receptor targeting microRNA detected in a
fraction of myeloproliferative neoplasm patient platelets. Blood.
116:437–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anderson JL, Adams CD, Antman EM, Bridges
CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS,
Levin TN, et al American College of Cardiology; American Heart
Association Task Force on Practice Guidelines (Writing Committee to
Revise the 2002 Guidelines for the Management of Patients With
Unstable Angina/Non-ST-Elevation Myocardial Infarction); American
College of Emergency Physicians; Society for Cardiovascular
Angiography and Interventions; Society of Thoracic Surgeons;
American Association of Cardiovascular and Pulmonary
Rehabilitation; Society for Academic Emergency Medicine: ACC/AHA
2007 guidelines for the management of patients with unstable
angina/non-ST-Elevation myocardial infarction: A report of the
American College of Cardiology/American Heart Association Task
Force on Practice Guidelines (Writing Committee to Revise the 2002
Guidelines for the Management of Patients With Unstable Angina/Non
ST Elevation Myocardial Infarction) developed in collaboration with
the American College of Emergency Physicians, the Society for
Cardiovascular Angiography and Interventions, and the Society of
Thoracic Surgeons endorsed by the American Association of
Cardiovascular and Pulmonary Rehabilitation and the Society for
Academic Emergency Medicine. J Am Coll Cardiol. 50:e1–e157. 2007.
View Article : Google Scholar
|
|
23
|
Mestdagh P, Van Vlierberghe P, De Weer A,
Muth D, Westermann F, Speleman F and Vandesompele J: A novel and
universal method for microRNA RT qPCR data normalization. Genome
Biol. 10:R642009. View Article : Google Scholar
|
|
24
|
Schwindt H, Akasaka T, Zühlke Jenisch R,
Hans V, Schaller C, Klapper W, Dyer MJS, Siebert R and Deckert M:
Chromosomal translocations fusing the BCL6 gene to different
partner loci are recurrent in primary central nervous system
lymphoma and may be associated with aberrant somatic hypermutation
or defective class switch recombination. J Neuropathol Exp Neurol.
65:776–782. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rayner KJ, Sheedy FJ, Esau CC, Hussain FN,
Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y,
et al: Antagonism of miR-33 in mice promotes reverse cholesterol
transport and regression of atherosclerosis. J Clin Invest.
121:2921–2931. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramirez CM, Dávalos A, Goedeke L, Salerno
AG, Warrier N, Cirera Salinas D, Suárez Y and Fernández Hernando C:
MicroRNA-758 regulates cholesterol efflux through posttranscrip
tional repression of ATP binding cassette transporter A1.
Arterioscler Thromb Vasc Biol. 31:2707–2714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramírez CM, Rotllan N, Vlassov AV, Dávalos
A, Li M, Goedeke L, Aranda JF, Cirera Salinas D, Araldi E, Salerno
A, et al: Control of cholesterol metabolism and plasma high–density
lipoprotein levels by microRNA 144. Circ Res. 112:1592–1601. 2013.
View Article : Google Scholar
|
|
28
|
Fernández Hernando C, Ramírez CM, Goedeke
L and Suárez Y: MicroRNAs in metabolic disease. Arterioscler Thromb
Vasc Biol. 33:178–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park SY, Lee JH, Ha M, Nam JW and Kim VN:
miR 29 miRNAs activate p53 by targeting p85 α and CDC42. Nat Struct
Mol Biol. 16:23–29. 2009. View Article : Google Scholar
|
|
30
|
Liu P and Wilson MJ: miR-520c and miR-373
upregulate MMP9 expression by targeting mTOR and SIRT1, and
activate the Ras/Raf/MEK/Erk signaling pathway and NF-κB factor in
human fibrosarcoma cells. J Cell Physiol. 227:867–876. 2012.
View Article : Google Scholar
|
|
31
|
Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan
J, Wu J and Li M: MicroRNA-374a activates Wnt/β-catenin signaling
to promote breast cancer metastasis. J Clin Invest. 123:566–579.
2013.PubMed/NCBI
|
|
32
|
Xiang P, Deng HY, Li K, Huang GY, Chen Y,
Tu L, Ng PC, Pong NH, Zhao H, Zhang L and Sung RY: Dexrazoxane
protects against doxorubicin-induced cardiomyopathy: Upregulation
of Akt and Erk phosphorylation in a rat model. Cancer Chemother
Pharmacol. 63:343–349. 2009. View Article : Google Scholar
|
|
33
|
Dangwal S, Bang C and Thum T: Novel
techniques and targets in cardiovascular microRNA research.
Cardiovasc Res. 93:545–554. 2012. View Article : Google Scholar
|
|
34
|
Joyce CW, Amar MJ, Lambert G, Vaisman BL,
Paigen B, Najib Fruchart J, Hoyt RF Jr, Neufeld ED, Remaley AT,
Fredrickson DS, et al: The ATP binding cassette transporter A1
(ABCA1) modulates the development of aortic atherosclerosis in
C57BL/6 and apoE knockout mice. Proc Natl Acad Sci USA. 99:407–412.
2002. View Article : Google Scholar
|
|
35
|
Cavelier LB, Qiu Y, Bielicki JK, Afzal V,
Cheng JF and Rubin EM: Regulation and activity of the human ABCA1
gene in transgenic mice. J Biol Chem. 276:18046–18051. 2001.
View Article : Google Scholar : PubMed/NCBI
|