Introduction
Acute myocardial infarction (AMI) is associated with
high mortality rates worldwide (1). AMI often occurs due to rupture of an
atherosclerotic plaque in a coronary artery, which may induce
thrombosis and artery occlusion, resulting in loss of blood supply
to the affected area and necrosis. Annually, over 3,000,000
individuals suffer from acute ST-elevation myocardial infarction,
and over 4,000,000 individuals suffer from non-ST-elevation
myocardial infarction (2).
Currently, the therapeutic strategies considered most effective are
mechanical revascularization by percutaneous coronary intervention
(3), thrombolytic therapy
(4), primary angioplasty (5), coronary artery bypass grafting and
antithrombotic therapy combined with timely reperfusion (6). However, these treatments are unable
to improve cardiac function (7).
In addition, tissue ischemia followed by reperfusion initiates
systemic inflammation, which may aggravate local injury and induce
remote multi-organ dysfunction (8). Therefore, the development of a safer,
more effective strategy for reducing I/R injury and improving
postoperative survival rates is required.
Astragali Radix (AR), the root of Astragalus
membranaceus and Astragalus membranaceus var.
mongholicus (9), is a
traditional Chinese medicine (10). AR exerts various bioactivities,
including antioxidation, enhancement of cardiovascular function,
hepatoprotection, immunostimulation and myocardial protection in
diabetic nephropathy (11). AR has
also been reported to reduce myocardial ischemic injury (12), and AR extracts efficiently protect
MRC-5 cells from H2O2-induced oxidative
damage via the inhibition of superoxide dismutase (SOD) and
catalase (13). A previous
clinical report indicated that AR may be a promising agent in the
treatment of acute cerebral infarction (14). AR contains various active
components, including polysaccharides, flavonoids, astragalosides
I–VII (saponins), amino acids and trace elements (15,16).
Calycosin-7-O-β-d-glucoside (CG) is a predominant
flavonoid of AR (17–19), which is known to possess
anti-inflammatory (20) and
anti-osteoarthritic properties (21). A previous study has shown that CG
significantly reduces cerebral infarct size and histological damage
in a rat model of I/R. In addition, CG protects blood-brain barrier
integrity by inhibiting the activities of matrix
metalloproteinases, scavenging nitric oxide and promoting the
expression of caveolin-1 (22).
However, the effects of CG on myocardial I/R injury and the
underlying mechanisms remain to be fully elucidated.
In the present study, a rat model of myocardial I/R
injury was treated with CG, and the underlying molecular mechanisms
of CG on myocardial I/R injury were evaluated.
Materials and methods
Animals
Male Wistar rats (8-week-old) were purchased from
Charles River Laboratories (Beijing, China). Experiments were
performed according to the guidelines for the animal care and use
of laboratory animal protocols, and were approved by the Ethics
Committee of The Second Affiliated Hospital of Harbin Medical
University (Harbin, China; approval no. SCXK-2012-0001). The rats
were maintained in an air-conditioned room with a constant
temperature of 22°C and an alternating 12 h light/12 h dark cycle.
The rats were provided with access to water and a standard diet
ad libitum.
In vivo myocardial I/R model and
experimental groups
The rats were anesthetized with 10% chloral hydrate
(3 ml/kg body weight; Sinopharm Medicine, Shenyang, China) by
intra-peritoneal injection. The rats were intubated, and mechanical
ventilation was achieved by connecting the endotracheal tube to a
scientific ventilator (HX-300S; Chengdu Technology & Market
Co., Ltd, Chengdu, China) at a respiratory rate of 80 breaths/min
with a tidal volume of 6–8 ml/kg body weight. A left thoracotomy
was performed, in order to expose the heart and the root of the
large blood vessel. The left anterior descending (LAD) coronary
artery was subsequently transiently ligated with a nylon suture for
a 45 min ischemic period. Microsurgical scissors were used to
release the ligature, and the heart was reperfused for 3 h.
The rats were randomly divided into five groups (12
animals per group). In the sham group, the rats underwent the
described anesthetic and surgical procedures without ligation of
the LAD coronary artery; in the I/R group, the rats underwent
myocardial ischemia for 45 min and reperfusion for 3 h by ligation
of the LAD coronary artery; in the I/R+H-CG group, the rats were
pretreated with a high dose of CG (H-CG; 30 mg/kg body weight;
Dalian Meilun Biological Technology Co., Ltd., Dalian, China) via
intravenous injection 30 min prior to ligation of the LAD coronary
artery; in the I/R+L-CG group, the rats were pretreated with a low
dose of CG (L-CG; 15 mg/kg body weight) via intravenous injection
30 min prior to ligation of the LAD coronary artery; in the
sham+H-CG group, the rats were pretreated with H-CG via intravenous
injection, and then underwent the described anesthetic and surgical
procedures without ligation of the LAD coronary artery.
Detection of cardiac function
Following reperfusion, an ultrasound system (IE33;
Philips GmbH, Herrsching, Germany) was used to collect hemodynamic
parameters, including ejection fraction (EF), fractional shortening
(FS), left ventricular end-systolic pressure (LVESP) and left
ventricular end-diastolic pressure (LVEDP). Blood samples were
obtained for biochemical investigations and the hearts were removed
for Evans blue/tetrazolium chloride (TTC) staining and western
blotting.
Tissue staining
The LAD coronary artery was religated following I/R
and 2–3 ml 2% Evans blue solution (Wokai, Shanghai, China) was
transcardially perfused. The rats were administered with KCl
solution via a marginal ear vein, and the heart was stopped in
diastole. The heart was subsequently removed, washed with saline,
and maintained at −20°C for 30–60 min, prior to being divided into
six 2-mm sections. The sections were stained with 1% TTC (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) at
37°C and images were captured using a digital camera (D3000; Nikon,
Tokyo, Japan). The area at risk (AAR; red staining) indicating the
ischemic area, the infarct area (IA; white staining) and
non-ischemic area (blue staining) were analyzed using an Image
Analysis system (Image Pro Plus 6.0; Media Cybernetics, Inc.,
Rockville, MD, USA). Infarct size was defined as a percentage of IA
to AAR (%).
Detection of creatine kinase (CK),
lactate dehydrogenase (LDH), SOD and malondialdehyde (MDA)
Following reperfusion, blood samples (5–8 ml/mouse)
were collected from the carotid artery and serum was obtained by
centrifugation (1,111 × g, 10 min, 4°C). Commercial assay kits
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were
used to detect the activities of CK (cat. no. A032), SOD (cat. no.
A001-3), LDH (cat. no. A020-1) and MDA (cat. no. A003-1) in the
serum, according to the manufacturer's protocol.
Western blot analysis
Total proteins were extracted from the AAR tissues
using radioimmunoprecipitation assay lysis buffer (50 mM Tris, 150
mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, pH 7.4)
(Beyotime Institute of Biotechnology, Haimen, China) and protein
concentrations were determined using a Bichinchoninic Acid Protein
Assay kit (Beyotime Institute of Biotechnology). Subsequently, 40
µg protein was separated by 10 or 13% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Bedford, MA,
USA). The membranes were blocked with 5% nonfat milk or 1% bovine
serum albumin (Amresco, Framingham, MA, USA), and then incubated
with the following primary antibodies at 4°C overnight: Rabbit
anti-rat cleaved-caspase-3 polyclonal antibody (pAb) (1:1,000
dilution; cat. no. WL0146); rabbit anti-rat cleaved-caspase-9 pAb
(1:1,000 dilution; cat. no. WL0191); rabbit anti-rat
phosphatidylinositol 3-kinase (PI3K) p85 pAb (1:1,000 dilution;
cat. no. WL0191); rabbit anti-rat phosphorylated (p)-Akt pAb
(1:1,000 dilution; cat. no. WLP001); rabbit anti-rat Akt pAb
(1:1,000 dilution; cat. no. WL0003) (all Wanleibio, Shenyang,
China) and rabbit anti-rat p-PI3K p85 pAb (1:500 dilution; cat. no.
bs-5538R; Bioss, Beijing, China). The membranes were then washed
with Tris-buffered saline containing Tween 20 (Beijing Solarbio
Science & Technology Co., Ltd.), and incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:5,000,
Beyotime Institute of Biotechnology) at 37°C for 45 min. Band
densities were analyzed using Gel-Pro Analyzer software 4.0 (Media
Cybernetics, Inc.) and normalized to β-actin.
Detection of caspase-3/9 activity
Caspase-3/9 activity was measured using a Caspase
Activity Assay kit (Beyotime Institute of Biotechnology), according
to the manufacturer's protocol. Briefly, the total cellular
proteins were quantified and reacted with the corresponding
substrates: Ac-DEVD-ρNA or Ac-LEHD-ρNA. Caspase-3/9 activity was
subsequently measured as the optical density of the cleaved
substrate ρNA at 405 nm using a microplate reader (ELX-800; Bio-Tek
Instruments, Inc., Winooski, VT, USA).
PI3K/Akt signaling pathway
The rats were randomly divided into three groups of
12: The I/R group, I/R+H-CG group and I/R+H-CG+LY294002 group. The
PI3K inhibitor, LY294002, (0.3 mg/kg body weight; Sigma-Aldrich)
was administered to the rats in the I/R+H-CG+LY294002 group via
intravenous injection 30 min prior to the administration of H-CG.
The rats were then subjected to I/R. Heart tissues from the AAR was
lysed with lysis buffer and the expression levels of PI3K p85,
p-PI3K p85, Akt and p-Akt were measured using western blot
analysis. Infarct size was assessed using Evans blue/TTC double
staining. The serum was obtained and levels of CK, SOD, LDH and MDA
were analyzed using commercial kits (Nanjing Jiancheng
Bioengineering Institute) as described above.
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, Inc.,
La Jolla, CA, USA) was used for statistical analysis and image
processing. Data are expressed as the mean ± standard deviation.
Comparisons between the experimental groups were conducted using
one-way analysis of variance, followed by a Bonferroni post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
CG ameliorates I/R-induced cardiac
dysfunction
Ultrasound analysis was performed to detect cardiac
function. As shown in Fig. 1, H-CG
had no effect on EF (Fig. 1A), FS
(Fig. 1B), LVEDP (Fig. 1C) or LVESP (Fig. 1D), in the sham+H-CG group, compared
with the sham group. However, EF, FS and LVESP levels were markedly
lower in the I/R group, compared with those in the sham group
(P<0.01), whereas LVEDP was significantly higher, compared with
the sham group (P<0.01). Following treatment with H-CG, EF, FS
and LVESP (P<0.05) were significantly increased, whereas the
LVEDP was decreased (P<0.01).
 | Figure 1Effects of CG on cardiac function.
Following reperfusion, hemodynamic parameters including (A) EF, (B)
FS, (C) LVEDP and (D) LVESP were detected, in order to determine
cardiac function. Data are expressed as the mean ± standard
deviation (n=6/group). *P<0.05 and
**P<0.01, compared with the I/R group. ns, not
significant; CG, calycosin-7-O-β-d-glucoside; I/R,
ischemia-reperfusion; H-CG, high dose CG; L-CG, low dose CG; EF,
ejection fraction; FS, fractional shortening; LVEDP, left
ventricular end-diastolic pressure; LVESP, left ventricular
end-systolic pressure. |
CG reduces myocardial infarct size
To determine whether CG affected myocardial infarct
size, the rats were pretreated with L-CG or H-CG, and then
subjected to I/R. As shown in Fig.
2, no ischemic and necrotic areas were detected in the sham or
the sham+H-CG groups. I/R significantly increased the infarct size
(29.98±5.28, vs. 0%; P<0.01). As expected, compared with the I/R
group (29.98±5.28%), the infarct size was significantly smaller in
the I/R+L-CG group (22.74±4.00; P<0.05) and the I/R+H-CG group
(16.22±5.15%; P<0.01)
 | Figure 2Effects of CG on infarct size.
Following 45 min ischemia and 3 h reperfusion, heart tissues were
collected and stained with Evans blue/tetrazolium chloride
staining. The AAR is characterized by red staining, indicating the
ischemic area, the IA displays white staining and the non-ischemic
area exhibits blue staining. Myocardial infarct size is expressed
as a percentage of the IA to AAR. Data are expressed as the mean ±
standard deviation (n=6/group). *P<0.05 and
**P<0.01, compared with the I/R group. CG,
calycosin-7-O-β-d-glucoside; I/R, ischemia-reperfusion; H-CG, high
dose CG; L-CG, low dose CG; IA, infarct area; AAR, area at
risk. |
CG attenuates I/R-induced myocardial
injury and oxidative stress-induced damage
The effects of CG were also evaluated on I/R-induced
myocardial injury and damaged from oxidative stress. The activities
of serum CK (Fig. 3A; P<0.01)
and LDH (Fig. 3B; P<0.01) were
markedly elevated in the I/R group, compared with those in the sham
group. Following treatment with L-CG, only CK activity was
inhibited (P<0.01); however, treatment with H-CG markedly
inhibited the activities of the two markers (P<0.01). The
activity of SOD, (Fig. 3C;
P<0.01) was significantly lower in the I/R group, compared with
the sham group. By contrast, MDA content (Fig. 3D; P<0.01) was significantly
higher, compared with the sham group. Pretreatment with H-CG
effectively increased the activity of SOD (P<0.01) and decreased
levels of MDA (P<0.01).
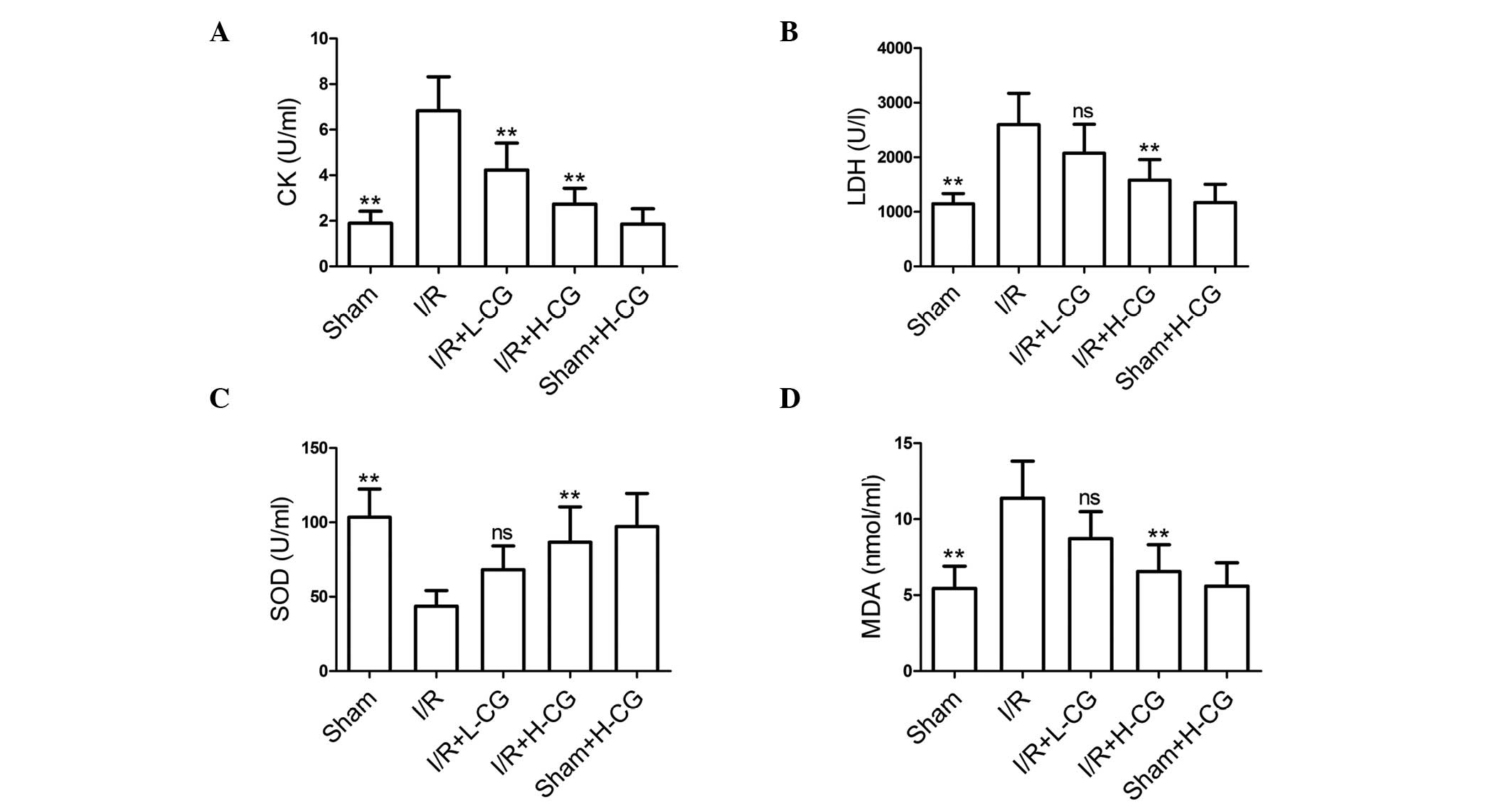 | Figure 3CG suppresses CK and LDH activities,
and ameliorates oxidative stress. Following reperfusion, blood was
collected and serum was obtained. Subsequently, the activities of
(A) CK, (B) LDH and (C) SOD, and (D) MDA content were measured.
Data are expressed as the mean ± standard deviation (n=6/group).
**P<0.01, compared with the I/R group. ns, not
significant; CG, calycosin-7-O-β-d-glucoside; I/R,
ischemia-reperfusion; H-CG, high dose CG; L-CG, low dose CG; CK,
creatine kinase; LDH, lactate dehydrogenase; SOD, superoxide
dismutase; MDA, malondialdehyde. |
CG reduces the I/R-induced increased
expression levels and activities of pro-apoptotic factors
The results of the present study demonstrated that
caspase cleavage (Fig. 4A;
P<0.01), and the activities of caspase-3 (Fig. 4B; P<0.01) and caspase-9
(Fig. 4C; P<0.01) were enhanced
in the I/R group, compared with those in the sham group. Treatment
with L-CG and H-CG markedly downregulated the levels of
cleaved-caspase-3 (P<0.01) and cleaved-caspase-9 (L-CG,
P<0.05; H-CG, P<0.01). In addition, caspase activity was
significantly inhibited following treatment with L-CG (caspase-3,
P<0.01; caspase-9, P<0.05) or H-CG (P<0.01).
CG increases the phosphorylation of PI3K
p85 and Akt
The protein expression levels of p-PI3K p85
(Fig. 5A; P<0.01) and p-Akt
(Fig. 5B, P<0.01) were
downregulated in the I/R group, compared with the sham group, and
were upregulated in the I/R+L-CG group (p-PI3K p85, P<0.05;
p-Akt, P>0.05) and I/R+H-CG group (P<0.01). No statistical
differences were observed between the groups in the expression
levels of total PI3K p85 or total Akt.
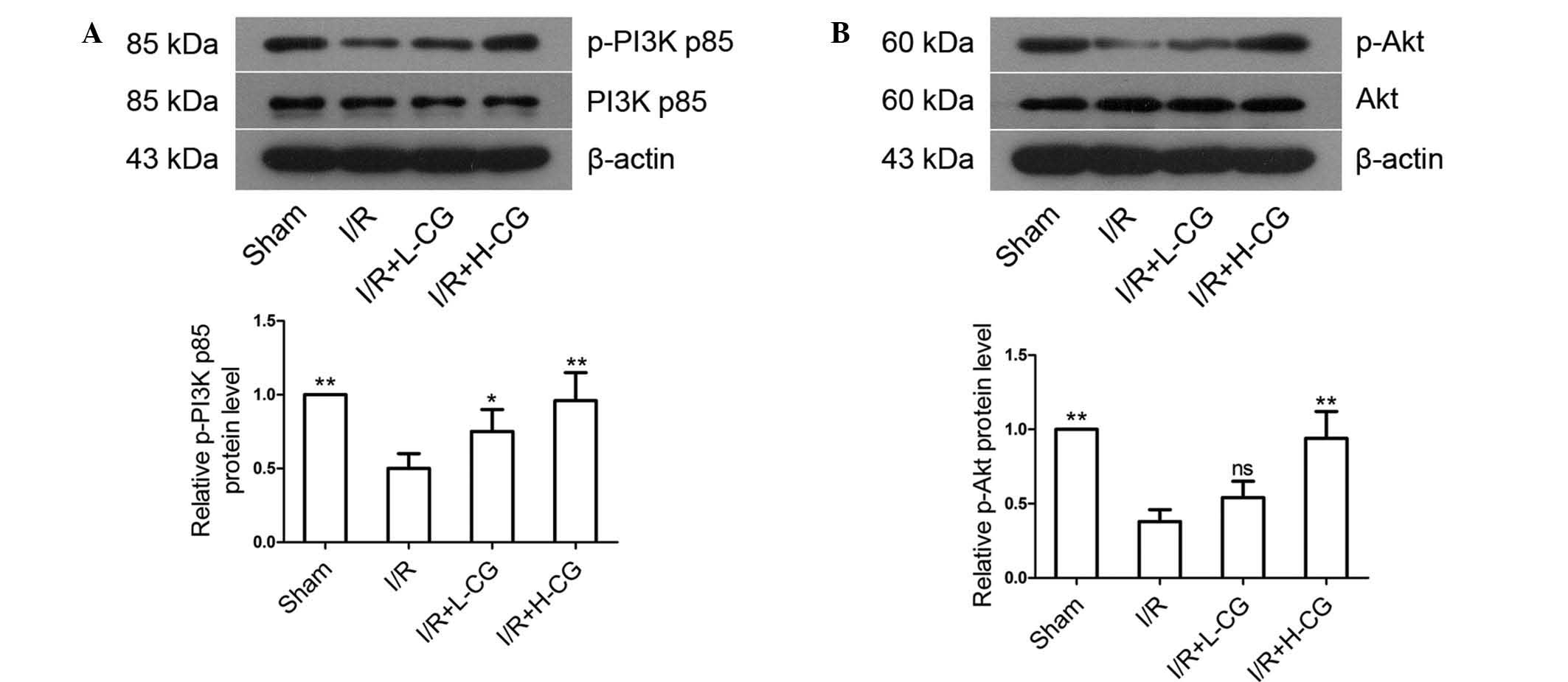 | Figure 5Effects of CG on the expression
levels of PI3K/Akt. Total proteins were extracted from the area at
risk tissues, and the expression levels of (A) PI3K p85, p-PI3K
p85, (B) Akt and p-Akt were detected using western blotting. Band
density was measured and normalized to that of β-actin. Data are
expressed as the mean ± standard deviation (n=5/group).
*P<0.05 and **P<0.01, compared with the
I/R group. ns, not significant; CG, calycosin-7-O-β-d-glucoside;
I/R, ischemia-reperfusion; H-CG, high dose CG; L-CG, low dose CG;
PI3K, phosphatidylinositol 3-kinase; p-, phosphorylated. |
LY294002 inhibits H-CG-induced activation
of the PI3K/Akt pathway
To confirm that CG attenuated I/R injury in
vivo via activation of the PI3K/Akt pathway, the PI3K
inhibitor, LY294002, was administered to the rats prior to H-CG.
Treatment with H-CG significantly increased the phosphorylation of
PI3K and Akt (Fig. 6A; P<0.01);
however, suppressing PI3K activity with LY294002 effectively
inhibited H-CG-induced PI3K/Akt phosphorylation (P<0.01).
Treatment with H-CG significantly decreased infarct size, compared
with the I/R group (15.67±3.28, vs. 35.46±5.33%, respectively;
P<0.01), as shown in Fig. 6B,
however, infarct size was significantly higher in the
I/R+H-CG+LY294002 group, compared with that in the I/R+H-CG group
(27.81±4.10, vs. 15.67±3.28%, respectively; P<0.01). Treatment
with H-CG significantly decreased CK activity (Fig. 6C; P<0.01), LDH activity
(P<0.01) and MDA content (P<0.01), and significantly
increased SOD activity (P<0.01). However, co-treatment with
LY294002 attenuated these effects (CK, P<0.01; LDH, P<0.05;
MDA, P<0.05; SOD, P<0.05).
 | Figure 6Inhibition of PI3K by LY294002
abrogates CG-induced protection against I/R injury. LY294002 (0.3
mg/kg body weight) was administered to the rats 30 min prior to the
administration of CG. Subsequently, the rats were subjected to I/R.
Levels of (A) PI3K p85, p-PI3K p85, Akt and p-Akt were detected
using western blotting. β-actin was used as an internal control.
(B) Infarct size was examined using Evans blue/tetrazolium chloride
staining. (C) Activities of CK, LDH and SOD, and MDA content were
detected using assay kits. Data are expressed as the mean ±
standard deviation (n=6/group). **P<0.01, compared
with the I/R group; &P<0.05 and
&&P<0.01, compared with the I/R+H-CG+LY294002
group. CG, calycosin-7-O-β-d-glucoside; I/R, ischemia-reperfusion;
H-CG, high dose of CG; PI3K, phosphatidylinositol 3-kinase; p-,
phosphorylated; IA, infarct area; AAR, area at risk; CK, creatine
kinase; LDH, lactate dehydrogenase; SOD, superoxide dismutase; MDA,
malondialdehyde. |
Discussion
AMI is a leading contributor to morbidity and
mortality rates worldwide (23-25).
Reperfusion improves clinical symptoms in patients with AMI
(26); however, restoration of
blood flow following ischemia may result in I/R injury (27,28),
which is involved in the development of myocardial necrosis,
arrhythmia, myocardial stunning, endothelial dysfunction and
microvascular complications (26,29).
The present study demonstrated that CG may exert a cardioprotective
effect in a rat model of I/R-induced injury via the PI3K/Akt
signaling pathway.
Previous studies have indicated that myocardial I/R
injury alters hemodynamic parameters and affects cardiac function
(30,31). In addition, levels of EF, FS
(32,33) and LVSP are lower in I/R groups than
in sham groups, whereas, LVEDP levels are higher (34,35),
which is in agreement with the results of the present study. The
present study also demonstrated that treatment with H-CG
significantly restored the I/R-induced downregulation of EF, FS and
LVESP, and markedly lowered the levels of LVEDP in the I/R+H-CG
group, compared with the I/R group. These results suggested that CG
improved cardiac function in the rat model of I/R.
Myocardial infarct size is an indicator of
myocardial injury, and I/R has been reported to result in
infarction in MI/R groups, compared with sham groups (36,37).
Treatment with CG has been shown to significantly reduce infarct
volume in a rat model of middle cerebral artery occlusion cerebral
I/R injury (22). Consistently,
the present study observed that L-CG and H-CG efficiently decreased
infarct size.
LDH (38) and CK
(39) are often elevated in MI,
and are used to assess the degree of myocardial injury. Numerous
evidence has demonstrated that I/R often induces the generation of
reactive oxygen species (ROS) and oxidative stress (40–42).
Subsequently, ROS interacts with cell membrane lipids and produces
MDA, which impairs cardiac function and induces myocardial cell
injury (43,44). Therefore, reducing oxidative stress
is an advantageous strategy for the alleviation of I/R injury. In
the present study, CK and LDH activity, and MDA content were
increased in the I/R group, compared with in the sham group;
however, SOD activity was decreased, which was consistent with the
results of previous studies (37,45).
These results indicated that CG exerted its cardioprotective
effects by notably decreasing CK, LDH and MDA, and increasing SOD
activity.
Apoptosis is important in development and tissue
homeostasis (46), and caspases
are considered the executioners of apoptosis (47). Once cells receive apoptotic
stimuli, the mitochondrial outer membrane becomes permeabilized and
cytochrome c is released from the mitochondria into the
cytosol (48). Cytochrome c
interacts with apoptotic protease activating factor 1 and
procaspase-9, which is cleaved into caspase-9 and initiates the
activation of caspase-3, caspase-6 and caspase-7 (49). Previous studies have reported that
I/R injury is associated with the apoptosis of cardiomyocytes
(50,51). The present study demonstrated that
the expression levels of cleaved-caspase-3 and cleaved-caspase-9,
and caspase activity were downregulated in the I/R+L-CG and
I/R+H-CG groups, compared with in the I/R group. Therefore, CG may
alleviate I/R injury by suppressing caspase activity and inhibiting
cardiomyocyte apoptosis.
PI3K consists of a catalytic subunit (p110) and a
regulatory subunit (p85) (52,53).
Akt is a serine-threonine kinase and, following phosphorylation,
performs its antiapoptotic effects via the activation of B-cell
lymphoma-2-associated death promoter and caspases (54). Previous studies have reported that
the PI3K/Akt signaling pathway is crucial in protecting the
myocardium from MI/R injury (55),
and the activation of PI3K/Akt significantly reduces cardiomyocyte
apoptosis (56). The present study
examined the expression levels of PI3K p85, p-PI3K p85, Akt and
p-Akt. Pretreatment with CG effectively activated and
phosphorylated PI3K and Akt, whereas the levels of total PI3K p85
and Akt were not changed. The PI3K inhibitor, LY294002, was used to
determine whether the PI3K/Akt pathway was involved in the
CG-mediated alleviation of I/R injury. Suppressing PI3K activity
with LY294002 reversed the beneficial effects of CG. Based on the
above results, it was hypothesized that CG alleviates I/R injury by
activating the PI3K/Akt signaling pathway.
In conclusion, the results of the present study
demonstrated that CG attenuated myocardial I/R injury in the rat
model. The protective effects may be associated with activation of
the PI3K/Akt pathway, and the inhibition of oxidative stress and
pro-apoptotic factors.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81171404
and 81201093).
References
|
1
|
Murray CJ and Lopez AD: Mortality by cause
for eight regions of the world: Global Burden of Disease Study.
Lancet. 349:1269–1276. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
White HD and Chew DP: Acute myocardial
infarction. Lancet. 372:570–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Betgem RP, de Waard GA, Nijveldt R, Beek
AM, Escaned J and van Royen N: Intramyocardial haemorrhage after
acute myocardial infarction. Nat Rev Cardiol. 12:156–167. 2015.
View Article : Google Scholar
|
|
4
|
Lincoff AM and Topol EJ: Illusion of
reperfusion. Does anyone achieve optimal reperfusion during acute
myocardial infarction? Circulation. 88:1361–1374. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartwell D, Colquitt J, Loveman E, Clegg
AJ, Brodin H, Waugh N, Royle P, Davidson P, Vale L and MacKenzie L:
Clinical effectiveness and cost-effectiveness of immediate
angioplasty for acute myocardial infarction: Systematic review and
economic evaluation. Health Technol Assess. 9:1–99. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo J, Xu H and Chen KJ: Potential
benefits of Chinese Herbal Medicine for elderly patients with
cardiovascular diseases. J Geriatr Cardiol. 10:305–309. 2013.
|
|
7
|
Clifford DM, Fisher SA, Brunskill SJ,
Doree C, Mathur A, Watt S and Martin-Rendon E: Stem cell treatment
for acute myocardial infarction. Cochrane Database Syst Rev.
2:CD0065362012.PubMed/NCBI
|
|
8
|
Abela CB and Homer-Vanniasinkham S:
Clinical implications of ischaemia-reperfusion injury.
Pathophysiology. 9:229–240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu KZ, Liu J, Guo BL, Zhao ZZ, Hong H,
Chen HB and Cai SQ: Microscopic research on a multi-source
traditional Chinese medicine, Astragali Radix. J Nat Med.
68:340–350. 2014. View Article : Google Scholar
|
|
10
|
Ismail ZM, Amin NM, Yacoub MF and Mohamed
AM: Myelo-enhancement by astragalus membranaceus in male albino
rats with chemotherapy myelo-suppression. Histological and
immunohistochemical study. Int J Stem Cells. 7:12–22. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu XB, Ma L, Zhang AH, Zhang YH, Jiang J,
Ma W, Zhang LM, Ren WC and Kong XJ: High-throughput analysis and
characterization of Astragalus membranaceus transcriptome using 454
GS FLX. PLoS One. 9:e958312014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin Y, Chen Q, Li X, Fan X and Li Z:
Astragali Radix protects myocardium from ischemia injury by
modulating energy metabolism. Int J Cardiol. 176:1312–1315. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu X, Li F, Zhang X, Li P, Zhang X, Wu Z
and Li D: In vitro synergistic antioxidant activity and
identification of antioxidant components from Astragalus
membranaceus and Paeonia lactiflora. PLoS One. 9:e967802014.
View Article : Google Scholar
|
|
14
|
Luo Y, Qin Z, Hong Z, Zhang X, Ding D, Fu
JH, Zhang WD and Chen J: Astragaloside IV protects against ischemic
brain injury in a murine model of transient focal ischemia.
Neurosci Lett. 363:218–223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ko JK and Chik CW: The protective action
of radix Astragalus membranaceus against hapten-induced colitis
through modulation of cytokines. Cytokine. 47:85–90. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma XQ, Shi Q, Duan JA, Dong TT and Tsim
KW: Chemical analysis of Radix Astragali (Huangqi) in China: A
comparison with its adulterants and seasonal variations. J Agric
Food Chem. 50:4861–4866. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu H, Zhang WL, Ding X, Zheng KY, Ho CM,
Tsim KW and Lee YK: Optimizing combinations of flavonoids deriving
from astragali radix in activating the regulatory element of
erythropoietin by a feedback system control scheme. Evid Based
Complement Alternat Med. 2013:5414362013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng KY, Choi RC, Cheung AW, Guo AJ, Bi
CW, Zhu KY, Fu Q, Du Y, Zhang WL, Zhan JY, et al: Flavonoids from
Radix Astragali induce the expression of erythropoietin in cultured
cells: A signaling mediated via the accumulation of
hypoxia-inducible factor-1α. J Agric Food Chem. 59:1697–1704. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin LZ, He XG, Lindenmaier M, Nolan G,
Yang J, Cleary M, Qiu SX and Cordell GA: Liquid
chromatography-electrospray ionization mass spectrometry study of
the flavonoids of the roots of Astragalus mongholicus and A.
membranaceus. J Chromatogr A. 876:87–95. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Sun YN, Yan XT, Yang SY, Kim S, Lee
YM, Koh YS and Kim YH: Flavonoids from Astragalus membranaceus and
their inhibitory effects on LPS-stimulated pro-inflammatory
cytokine production in bone marrow-derived dendritic cells. Arch
Pharm Res. 37:186–192. 2014. View Article : Google Scholar
|
|
21
|
Pan H, Wang Y, Zhang Y, Zhou T, Fang C,
Nan P, Wang X, Li X, Wei Y and Chen J: Phenylalanine ammonia lyase
functions as a switch directly controlling the accumulation of
calycosin and calycosin-7-O-beta-D-glucoside in Astragalus
membranaceus var. mongholicus plants. J Exp Bot. 59:3027–3037.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu S, Gu Y, Jiang JQ, Chen X, Xu M, Chen X
and Shen J: Calycosin-7-O-β-D-glucoside regulates nitric
oxide/caveolin-1/matrix metalloproteinases pathway and protects
blood-brain barrier integrity in experimental cerebral
ischemia-reperfusion injury. J Ethnopharmacol. 155:692–701. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ozaki K, Inoue K, Sato H, Iida A, Ohnishi
Y, Sekine A, Sato H, Odashiro K, Nobuyoshi M, Hori M, et al:
Functional variation in LGALS2 confers risk of myocardial
infarction and regulates lymphotoxin-alpha secretion in vitro.
Nature. 429:72–75. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Timmers L, Sluijter JP, van Keulen JK,
Hoefer IE, Nederhoff MG, Goumans MJ, Doevendans PA, van Echteld CJ,
Joles JA, Quax PH, et al: Toll-like receptor 4 mediates maladaptive
left ventricular remodeling and impairs cardiac function after
myocardial infarction. Circ Res. 102:257–264. 2008. View Article : Google Scholar
|
|
25
|
Zou Y, Takano H, Mizukami M, Akazawa H,
Qin Y, Toko H, Sakamoto M, Minamino T, Nagai T and Komuro I:
Leukemia inhibitory factor enhances survival of cardiomyocytes and
induces regeneration of myocardium after myocardial infarction.
Circulation. 108:748–753. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moens AL, Claeys MJ, Timmermans JP and
Vrints CJ: Myocardial ischemia/reperfusion-injury, a clinical view
on a complex pathophysiological process. Int J Cardiol.
100:179–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prasad A, Stone GW, Holmes DR and Gersh B:
Reperfusion injury, microvascular dysfunction and cardioprotection:
The 'dark side' of reperfusion. Circulation. 120:2105–2112. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahmed LA, Salem HA, Attia AS and El-Sayed
ME: Enhancement of amlodipine cardioprotection by quercetin in
ischaemia/reperfusion injury in rats. J Pharm Pharmacol.
61:1233–1241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buja LM and Weerasinghe P: Unresolved
issues in myocardial reperfusion injury. Cardiovasc Pathol.
19:29–35. 2010. View Article : Google Scholar
|
|
30
|
Ali N, Rizwi F, Iqbal A and Rashid A:
Induced remote ischemic pre-conditioning on ischemia-reperfusion
injury in patients undergoing coronary artery bypass. J Coll
Physicians Surg Pak. 20:427–431. 2010.PubMed/NCBI
|
|
31
|
Toldo S, Seropian IM, Mezzaroma E, Van
Tassell BW, Salloum FN, Lewis EC, Voelkel N, Dinarello CA and
Abbate A: Alpha-1 antitrypsin inhibits caspase-1 and protects from
acute myocardial ischemia-reperfusion injury. J Mol Cell Cardiol.
51:244–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan Q, Chen M, Zuo L, Shang X, Huang MZ,
Ciccarelli M, Raake P, Brinks H, Chuprun KJ, Dorn GW II, et al:
Myocardial ablation of G protein-coupled receptor kinase 2 (GRK2)
decreases ischemia/reperfusion injury through an anti-intrinsic
apoptotic pathway. PLoS One. 8:e662342013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou YC, Liu B, Li YJ, Jing LL, Wen G,
Tang J, Xu X, Lv ZP and Sun XG: Effects of buyang huanwu decoction
on ventricular remodeling and differential protein profile in a rat
model of myocardial infarction. Evid Based Complement Alternat Med.
2012:3852472012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song CL, Liu B, Diao HY, Shi YF, Li YX,
Zhang JC, Lu Y, Wang G, Liu J, Yu YP, et al: The protective effect
of microRNA-320 on left ventricular remodeling after myocardial
ischemia-reperfusion injury in the rat model. Int J Mol Sci.
15:17442–17456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao G, Wang S, Wang Z, Sun A, Yang X, Qiu
Z, Wu C, Zhang W, Li H, Zhang Y, et al: CXCR6 deficiency
ameliorated myocardial ischemia/reperfusion injury by inhibiting
infiltration of monocytes and IFN-γ-dependent autophagy. Int J
Cardiol. 168:853–862. 2013. View Article : Google Scholar
|
|
36
|
Wang Y, Li X, Wang X, Lau W, Wang Y, Xing
Y, Zhang X, Ma X and Gao F: Ginsenoside Rd attenuates myocardial
ischemia/reperfusion injury via Akt/GSK-3β signaling and inhibition
of the mitochondria-dependent apoptotic pathway. PLoS One.
8:e709562013. View Article : Google Scholar
|
|
37
|
Han J, Wang D, Yu B, Wang Y, Ren H, Zhang
B, Wang Y and Zheng Q: Cardioprotection against
ischemia/reperfusion by licochalcone B in isolated rat hearts. Oxid
Med Cell Longev. 2014:1348622014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kemp M, Donovan J, Higham H and Hooper J:
Biochemical markers of myocardial injury. Br J Anaesth. 93:63–73.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh G, Rohilla A, Singh M and Balakumar
P: Possible role of JAK-2 in attenuated cardioprotective effect of
ischemic preconditioning in hyperhomocysteinemic rat hearts.
Yakugaku Zasshi. 129:523–535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Petrosillo G, Ruggiero FM, Di Venosa N and
Paradies G: Decreased complex III activity in mitochondria isolated
from rat heart subjected to ischemia and reperfusion: Role of
reactive oxygen species and cardiolipin. FASEB J. 17:714–716.
2003.PubMed/NCBI
|
|
41
|
Shimouchi A, Yokota H, Ono S, et al:
Neuroprotective effect of water-dispersible hesperetin in retinal
ischemia reperfusion injury. Jpn J Ophthalmol. Sept 25–2015.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang AL, Niu Q, Shi N, et al: Glutamine
ameliorates intestinal ischemia-reperfusion Injury in rats by
activating the Nrf2/Are signaling pathway. Int J Clin Exp Pathol.
8:7896–7904. 2015.PubMed/NCBI
|
|
43
|
Kalaycioglu S, Sinci V, Imren Y and Oz E:
Metoprolol prevents ischemia-reperfusion injury by reducing lipid
peroxidation. Jpn Circ J. 63:718–721. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dhalla NS, Elmoselhi AB, Hata T and Makino
N: Status of myocardial antioxidants in ischemia-reperfusion
injury. Cardiovasc Res. 47:446–456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang S, Li H and Yang SJ: Tribulosin
protects rat hearts from ischemia/reperfusion injury. Acta
Pharmacol Sin. 31:671–678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Waterhouse NJ, Goldstein JC, von Ahsen O,
Schuler M, Newmeyer DD and Green DR: Cytochrome c maintains
mitochondrial transmembrane potential and ATP generation after
outer mitochondrial membrane permeabilization during the apoptotic
process. J Cell Biol. 153:319–328. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Denault JB, Eckelman BP, Shin H, Pop C and
Salvesen GS: Caspase 3 attenuates XIAP (X-linked inhibitor of
apoptosis protein)-mediated inhibition of caspase 9. Biochem J.
405:11–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hamacher-Brady A, Brady NR, Logue SE,
Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA and Gustafsson AB:
Response to myocardial ischemia/reperfusion injury involves Bnip3
and autophagy. Cell Death Differ. 14:146–157. 2007. View Article : Google Scholar
|
|
51
|
Zhang W, Xing B, Yang L, Shi J and Zhou X:
Icaritin attenuates myocardial ischemia and reperfusion injury via
anti-inflammatory and anti-oxidative stress effects in rats. Am J
Chin Med. 43:1083–1097. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shin YK, Liu Q, Tikoo SK, Babiuk LA and
Zhou Y: Influenza A virus NS1 protein activates the
phosphatidylinositol 3-kinase (PI3K)/Akt pathway by direct
interaction with the p85 subunit of PI3K. J Gen Virol. 88(Pt 1):
13–18. 2007. View Article : Google Scholar
|
|
53
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Massion PP, Taflan PM, Shyr Y, Rahman SM,
Yildiz P, Shakthour B, Edgerton ME, Ninan M, Andersen JJ and
Gonzalez AL: Early involvement of the phosphatidylinositol
3-kinase/Akt pathway in lung cancer progression. Am J Respir Crit
Care Med. 170:1088–1094. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Y, Wei L, Sun D, Cao F, Gao H, Zhao
L, Du J, Li Y and Wang H: Tanshinone IIA pretreatment protects
myocardium against ischaemia/reperfusion injury through the
phosphatidylinositol 3-kinase/Akt-dependent pathway in diabetic
rats. Diabetes Obes Metab. 12:316–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fujio Y, Nguyen T, Wencker D, Kitsis RN
and Walsh K: Akt promotes survival of cardiomyocytes in vitro and
protects against ischemia-reperfusion injury in mouse heart.
Circulation. 101:660–667. 2000. View Article : Google Scholar : PubMed/NCBI
|




















