Introduction
Chronic hepatitis B (CHB) is a major cause of liver
cirrhosis and hepatocellular carcinoma (1). Although persistently high hepatitis B
virus (HBV) loads have been correlated with disease progression,
the progression itself is not caused by the HBV, but by a host
immune-mediated process (2–4). An
inadequate immune response leads to CHB infection, whereas an
appropriate immune response frequently leads to viral clearance and
recovery, and an excess immune response leads to liver failure
(5–7). The mechanisms by which the immune
responses are regulated resulting in these various outcomes remain
to be elucidated.
One of the newly discovered cluster determinant
(CD)4+ T helper (Th) cells, Th17 cells, were first
isolated from CD4+ T cells. Th17 cells can secrete a
mixture of cytokines, including interleukin (IL)-17A, IL-17F and
IL-22, among which IL-17A has been characterized as a major
effector cytokine (8,9). IL-17A can stimulate chemotactic
factors, including IL-8, monocyte chemoattractant protein-1 and
growth-regulated oncogene-α, to mobilize, recruit, and activate
neutrophils and monocytes, leading to marked tissue inflammation
(10). IL-17A can also stimulate a
mixture of cytokines, including IL-6, prostaglandin E2 and other
proinflammatory factors, including IL-1β, tumor necrosis factor-α
and interferon-γ, to further activate and amplify inflammatory
reactions (11–13). Th17 cells can also activate natural
immune cells, which express IL-17R causing an increase in liver
inflammation and damage (11).
Previous studies have found that Th17 cells may be involved in the
immune responses and immunopathogenesis induced by persistent HBV
infection (14–17). Th17 has been shown to be critical
in autoimmune diseases and various infectious diseases (18).
Regulatory T (Treg) cells, a family of
immunomodulatory cell types, are critical homeostatic regulators of
immune and inflammatory responses. CD4+CD25+
Tregs suppress immune responses to maintain unresponsiveness to
self-antigens and prevent excessive immune responses against fatal
inflammatory damage (19–21). Treg cells, which have immune
incompetence and immunosuppressive characteristics, are important
in CHB and chronic hepatitis C (22–24).
Natural (n) Treg cells are involved in controlling immune
responses, and in promoting and maintaining self-tolerance
(25). They can also inhibit the
proliferative responses of conventional CD4 and CD8 T cells in
vitro (26).
The aim of the present study was to examine the
association between circulating Th17 and nTreg cell frequencies,
and the disease progression in patients infected with HBV.
Patients and methods
Patients and controls
All patients were recruited from the First
Affiliated Hospital of Chongqing Medical University (Chongqing,
China). The study was approved by the ethics committee of the First
Affiliated Hospital of Chongqing Medical University (Chongqing,
China). Written informed consent was obtained from the patients.
Blood samples (8 ml) were collected from the elbow vena mediana,
from 40 patients with chronic active hepatitis, but without
cirrhosis (CAWC), 27 patients with HBV-associated cirrhosis, 20
patients with HBV- associated liver failure (HBLF) and 20 age- and
gender-matched healthy individuals, who were enrolled as controls.
All patients were diagnosed, according to previously described
criteria (27). The inclusion
criteria were as follows: i) Healthy controls had no underlying
disease history, were normal on physical examination and had no
evidence of HBV infection within at least 6 months; ii) patients
with CHB had been diagnosed with HBV for >6 months and presented
with either persistent or recurrent elevations in serum alanine
aminotransferase (ALT), or evidence of hepatitis in liver
histology, but without cirrhosis; iii) patients with HBV-associated
cirrhosis exhibited portal hypertension (splenomegaly and
esophageal varices) on abdominal ultrasound or endoscopy, or
hypersplenism in blood tests or histology, but had no evidence of
liver failure; iv) patients with HBLF had serum levels of total
bilirubin >10 times higher than then normal limit, severe
coagulopathy (prothrombin activity ≤40%), encephalopathy, renal
insufficiency, variceal bleeding and ascites.
Patients with concurrent viral infections, human
immunodeficiency virus-1 infection, alcoholic or non-alcoholic
fatty liver diseases, hepatocellular carcinoma, genetic and
autoimmune liver diseases were excluded. None of the patients had
received antiviral or immunomodulatory therapy prior to sampling.
The study protocol was approved by the Ethics Committee of the
First Affiliated Hospital of Chongqing Medical University, and
written informed consent was obtained from each subject. The
clinical characteristics of the subjects are listed in Table I.
 | Table IClinical data of the enrolled
subjects in the four patient groups. |
Table I
Clinical data of the enrolled
subjects in the four patient groups.
| Factor | Healthy
control | Chronic active
without cirrhosis | HBV-associated
cirrhosis | Liver failure |
|---|
| Cases (n) | 20 | 40 | 27 | 20 |
| Age (years) | 36.40±5.62 | 34.61±11.89 | 40.06±16.37 | 48.17±13.27 |
| Gender (M/F) | 6/14 | 26/14 | 15/12 | 11/9 |
| HBeAg positive | 0 | 21 | 11 | 8 |
| ALT (U/l) | – | 462.65±490.89 | 83.65±138.92 | 325.15±372.49 |
| PTA (%) | – | 90.35±19.59 | 73.80+21.97 | 39.30±11.21 |
| Log10
HBV DNA | 0 | 5.00±1.79 | 4.06±2.16 | 6.30±1.49 |
Flow cytometric analysis
All antibodies were purchased from BD Biosciences
(San Jose, CA, USA). Peripheral blood mononuclear cells (PBMCs)
were isolated from the fresh, heparinized peripheral blood samples
by Ficoll-Hypaque density-gradient centrifugation (lymphocyte
separation medium; Haoyang Bio-Technology Co., Ltd., Tianjin,
China), according to the manufacturer's protocol. The PBMCs (1 ml
of 2×106) were treated for 5 h with 50 ng/ml phorbol
myristate acetate (Shanghai DingGuo Biotech., Co., Ltd., Shanghai,
China), 1 µg/ml ionomycin (Sigma-Aldrich, St. Louis, MO,
USA) and 10 mg/ml brefeldin A (eBioscience, Inc., San Diego, CA,
USA) in complete RPMI-1640 medium (Sigma-Aldrich) supplemented with
10% fetal bovine serum (Sigma-Aldrich). The cells were
surface-stained with fluorescein isothiocyanate-conjugated mouse
anti-human CD4 antibodies (BD Biosciences; cat. no. 555346),
PerCP-Cy™ 5.5-conjugated mouse anti-human CD25 antibodies (BD
Biosciences; cat. no. 560503) and phycoerythrin-conjugated mouse
anti-human glycoprotein A repetitions predominant (GARP; cat. no.
562150) antibodies (5 µl; BD Biosciences) at 37°C for 30
min. Following antibody incubation, the cells were fixed. The
remaining cells were then permeabilized and stained with 5 ml Alexa
Fluor® 647-conjugated anti-human IL-17A (BD Biosciences;
cat. no. 560437). The cells were fixed in 1% formaldehyde
(Sigma-Aldrich), and flow cyto-metric analyses were performed using
a FACSCalibur system (BD Biosciences). The resulting data were
analyzed using BD FACSDiva software, version 6.1.2 (BD
Biosciences).
Enzyme-linked immunosorbent assay
(ELISA)
Serum concentrations of IL-22, IL-17A, TGF-β1 were
measured using a human IL-22 ELISA detection kit, human
interleukin-17 (IL-17) ELISA detection kit and human TGF-β ELISA
detection kit (R&D Systems, Inc., Minneapolis, MN, USA),
according to the protocols provided by the manufacturer. All
samples were assessed twice in order to avoid sampling error.
Virological and biochemical
assessment
The levels of HBeAg, total bilirubin, ALT,
prothrombin time (PT) and prothrombin activity were measured using
commercially available kits (Abbott Ireland Diagnostics Ltd.,
Sligo, Ireland; Roche Diagnostics, Indianapolis, IN, USA; Siemens
Healthcare Diagnostics Inc., Newark, USA) in the clinical
laboratory of the First Affiliated Hospital of Chongqing Medical
University (Chongqing, China). Serum HBV DNA levels were measured
using the Hepatitis B Viral DNA Quantitative Fluorescence
Diagnostic kit (Shengxiang Inc., Hunan, China). The HBV DNA
detection limit was 500 IU/ml. The cycling steps were as follows:
95°C for 2 sec; 94°C for 5 sec 57°C for 30 sec 45 times. The
expression levels were quantified using a standard curve.
Statistical analysis
All data were analyzed using SPSS 19 software (IBM
SPSS, Armonk, NY, USA). Numerical data are expressed as the mean ±
standard error of the mean. Comparison between two groups was
performed using two sample Student's t-test. Rates between the
groups were compared using a two-sample χ2 test.
Comparison between various individuals was performed using a
Mann-Whitney U test. Multiple comparisons of different groups were
performed using a Kruskal-Wallis H non-parametric test. Correlation
analysis was evaluated using Spearman's rank correlation test.
Two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
Increased frequencies of circulating Th17
cells correlate with the severity of liver inflammation and
fibrosis
The present study characterized
CD4+IL-17+ cells as Th17 cells, and compared
the proportions of peripheral Th17 cells among the four groups.
There were significantly higher frequencies of circulating Th17
cells in patients with CHB, cirrhosis and liver failure, compared
with levels in the normal controls, with values of 6.41±2.83,
5.8±2.76 and 9.04±4.94%, respectively, compared with 3.26±2.20% in
the control (P=0.02, 0.029 and 0.000, respectively; Fig. 1A–E). The Th17 frequency was highest
in the patients in liver failure, which was significantly
different, compared with those in the patients with CAWC and
cirrhosis (P=0.026 and 0.015, respectively; Fig. 1F and Table II).
 | Table IInTreg and Th17 cell proportions in
the patient groups. |
Table II
nTreg and Th17 cell proportions in
the patient groups.
| Cell
proportion | Healthy
control | CAWC | Cirrhosis | Liver failure |
|---|
|
nTreg/CD4+ T | 3.76±1.40 | 9.40±5.81b | 6.61±3.77 | 6.26±3.64 |
|
Th17/CD4+ T | 3.26±2.20 | 6.41±2.83a | 5.80±2.76a | 9.04±4.94b |
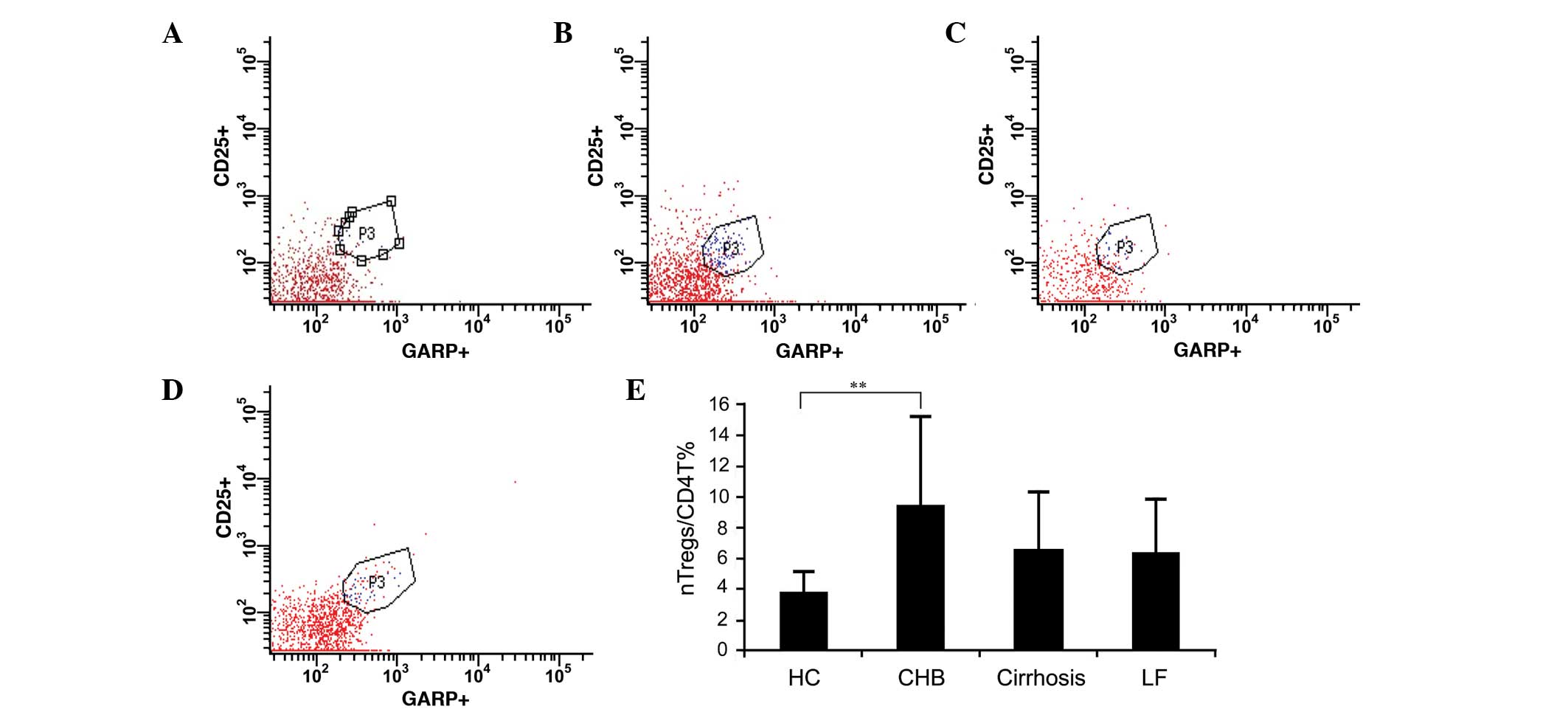
Distribution of nTreg cell subset
populations
The present study characterized
CD4+CD25+GARP+ as nTreg cells.
There were significantly higher frequencies of circulating nTreg
cells in the patients with CHB, compared with the normal controls
(9.4±5.81 vs. 3.76±1.4%; P<0.001; Fig. 2A–E). No significant differences in
nTreg cell frequencies were observed between the patients with
cirrhosis or the patients with liver failure and the normal
controls (6.61±3.77 and 6.26±3.64, respectively, vs. 3.76±1.4% in
the control; P=0.066 and P=0.144, respectively).
Serum levels of cytokines in the
peripheral blood
In the patients with CAWC, cirrhosis and liver
failure, the serum levels of IL-17 were significantly higher,
compared with those in the normal controls, with values of
104±23.8, 94±43.17 and 165±36.19 pg/ml, respectively, vs.
72.09±11.16 pg/ml in the control (P<0.05 for all; Fig. 3A). The serum levels of IL-17 were
higher, compared with the levels in the patients with CAWC and
cirrhosis, in the patients with liver failure. No significant
difference in serum levels of IL-17 were observed between the
patients with CAWC and the patients with cirrhosis.
In the patients with CAWC, cirrhosis and liver
failure, serum levels of IL-22 were significantly higher than in
the normal controls (65.32±34.12, 56.47±53.06 and 80.03±18.40
pg/ml, respectively, vs. 45.09±34.57 pg/ml in the control;
P<0.05; Fig. 3B). However, no
significant differences were observed among the three groups.
In the patients with CAWC, cirrhosis and liver
failure, serum levels of TGF-β were higher than in the normal
controls (978.76±117.21, 1,008.88±57.80 and 936.54±64.06 ng/l,
respectively, vs. 765.75±134.05 ng/l in the control). However, the
differences among the four groups were not statistically
significant (P>0.05).
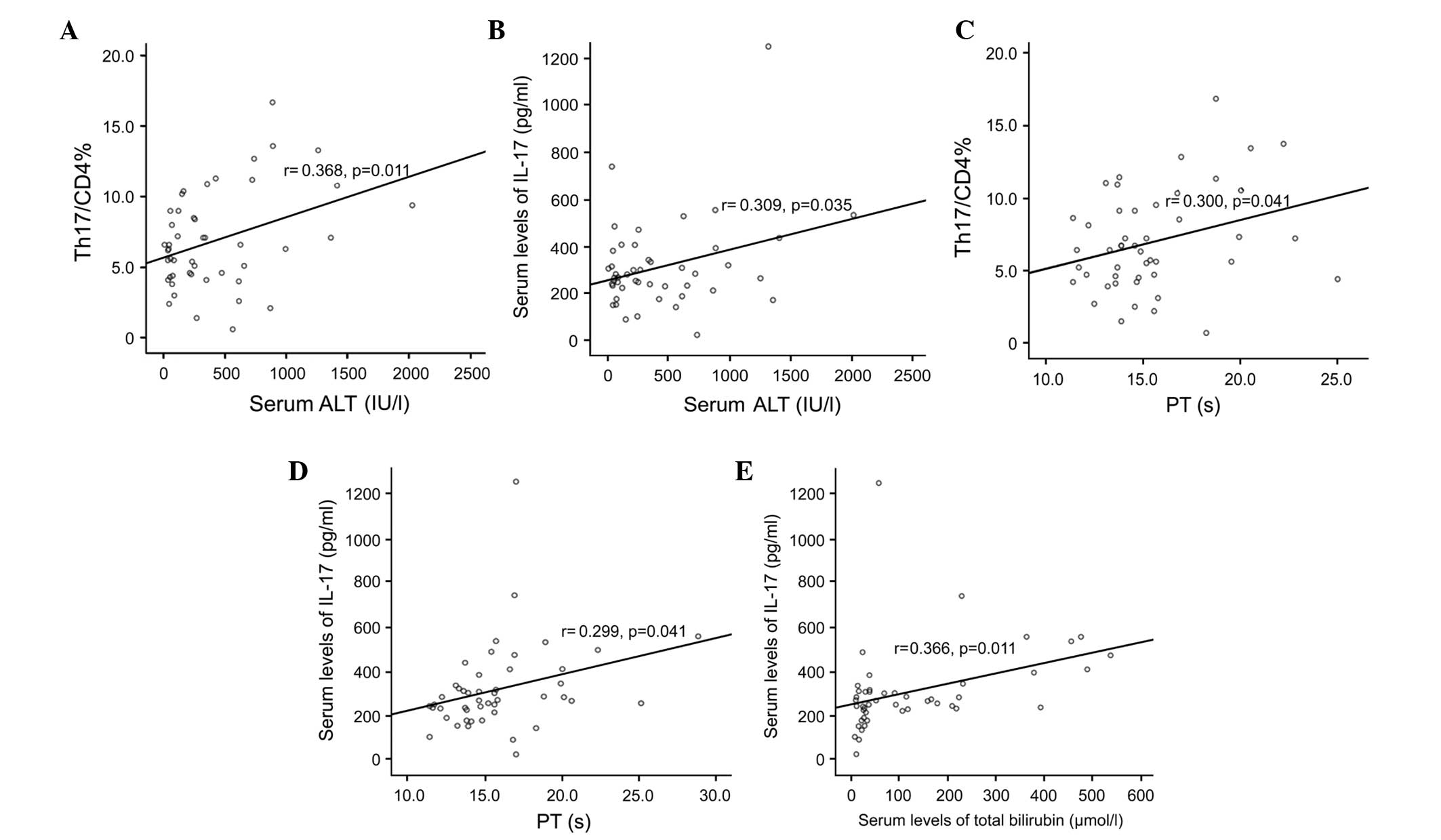
Th17 cell frequency and serum levels of
IL-17 correlate positively with ALT and PT, but not HBV DNA
The Th17 frequencies and serum levels of IL-17 were
significantly and positively correlated with the levels of ALT
(normal range, 0–40 U/l; r=0.368 and 0.309, respectively; P=0.011
and 0.035, respectively), as shown in Fig 4A and B. The serum levels of Th17 and
IL-17 were significantly and positively correlated with PT (normal
range, 11–14.5 sec; r=0.300 and 0.299, respectively; P=0.041 and
0.041, respectively), as shown in Fig.
4C and D.
The serum levels of IL-17 levels were significantly
positively correlated with the total bilirubin levels (r=0.366;
P=0.011; Fig. 4E). The Th17
frequency and serum levels of IL-17 were not correlated with HBV
DNA levels. These data suggested that peripheral Th17 cell
frequencies and serum levels of IL-17 were closely associated with
liver injury, as indicated by serum ALT levels and prothrombin
time, in the patients with HBV infection.
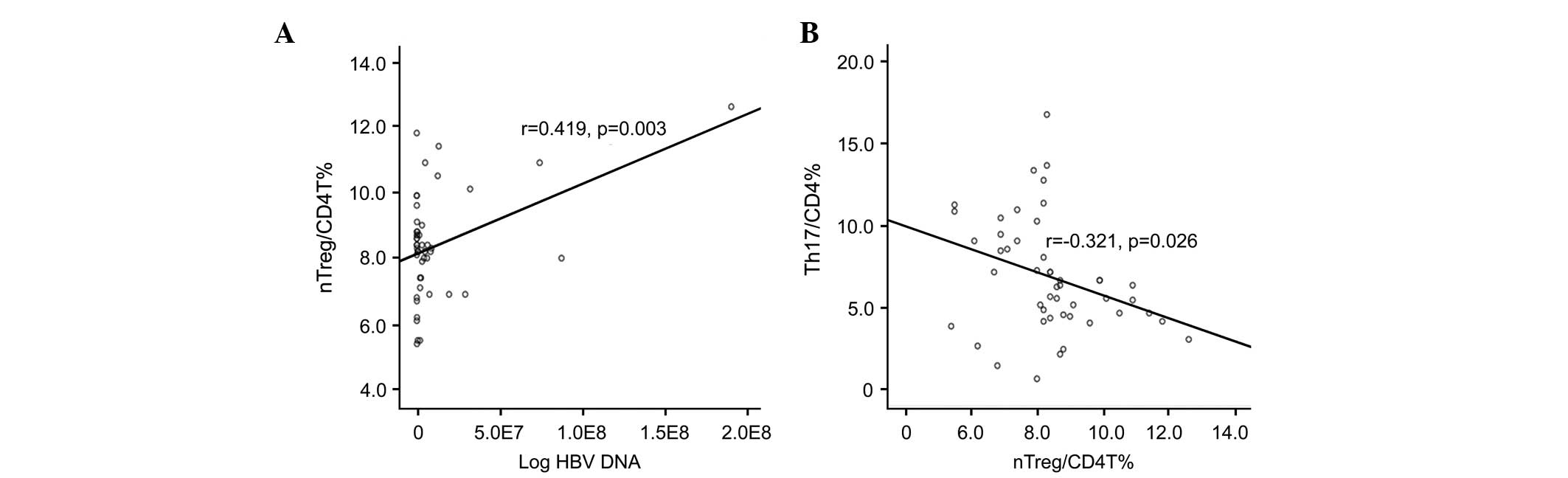
nTreg cell frequencies are positively
correlated with HBV DNA, but not with ALT or PT, and are negatively
correlated with Th17 cell frequencies
The present study analyzed the correlation between
nTreg frequency and clinical data. The nTreg frequencies were
significantly and positively correlated with plasma HBV DNA load
(r=0.419; P=0.003; Fig. 5A), but
not with the levels of ALT or the PT (P>0.05).
Further analysis of nTreg frequencies with Th17
frequencies showed that nTreg was negatively correlated with Th17
frequency (r=-0.321; P=0.026; Fig.
5B).
Discussion
There has been a focus of attention on the
significance of the Treg/Th17 balance in the progression of CHB.
Th17 and Treg cells share reciprocal developmental pathways of cell
differentiation (28). The
differentiation of Treg cells from naive T cells is dependent on a
critical differentiation factor, TGF-β (29). The differentiation of pathogenic
Th17 cells from naive T cells is induced by IL-6 and TGF-β, whereas
the differentiation of Treg cells can be completely inhibited by
the induction of IL-6 during inflammation (30–32).
Not only do Th17 cells and Treg cells share the same origin, but
they are also mutually antagonistic in function. A balance between
Th17 and Treg cells, which mediates immune tolerance is crucial for
immune homeostasis (33).
The balance of Th17 and Treg cells is closely
associated with the development of several diseases, including
viral infections and autoimmune diseases (34–36).
However, little is known regarding the balance of Th17 and Treg
cells in the progression of CHB. The present study was designed to
further ascertain whether circulating Th17 cells and nTreg cells
frequency correlate with the severity of liver injury and fibrosis
in the progression of CHB.
A subset of T cells are described as Th17 due to the
fact that an IL-12 family cytokine, IL-23, induces these cell to
secrete IL-17 (33,37). It has been shown that serum levels
of IL-17 and Th17 cells are increased in patients with chronic
hepatitis C virus (38,39). However, the mechanism by which Th17
cells induce liver damage in patients with CHB remains to be
elucidated. As one of the important pro-inflammatory cytokines,
IL-17 can recruit neutrophils, which can induce liver injury
(10). In addition, IL-17 can
activate mDCs and monocytes, which produce more proinflammatory
cytokines in a dose-dependent manner. In the present study, it was
found that the Th17 frequencies in the peripheral blood of patients
with liver failure were markedly higher, compared with those of
patients with CAWC and cirrhosis. The present study provided the
first evidence, to the best of our knowledge, that Th17
cell-mediated inflammation is associated with the stages of
progression of CHB. Th17 cell frequencies may assist in predicting
the severity of liver damage and fibrosis, and Th17 cells may exert
their immune effect via IL-17 in the peripheral blood. Further
investigation is required to confirm this possibility.
Wang et al (40) were the first to report on the
association between GARP and Treg cells. GARP is an orphan
Toll-like receptor, composed of leucine-rich repeats. The study
demonstrated that GARP was selectively expressed only in activated
human nTreg and nTreg cell clones, but not in activated effector T
cells, confirming GARP as a nTreg marker. Therefore, the present
study selected GARP, rather than Foxp3, as a
CD4+CD25+ Treg-specific marker. The results
of the present study demonstrated that nTreg frequencies in the
peripheral blood of patients with CAWC were markedly higher,
compared with those in patients with cirrhosis and liver failure,
which was consistent with the results of previous studies (11,41–43)
and further supports the involvement of Treg cells in the
pathogenesis of CAWC. In the presence of cirrhosis and liver
failure, the immune tolerance status may change, enhancing HBV
clearance.
To further assess the association between the Th17
and Treg cells, the present study analyzed the Treg and Th17 cell
correlation, and found that nTreg cell frequencies were negatively
correlated with those of Th17 cells. Specifically, the decrease in
peripheral nTreg cells were accompanied by an increase in Th17
cells. These data further support the possibility that Treg/Th17
imbalance may be involved in the progression of HBV infection.
However, only peripheral blood samples were examined in the present
study. Examination of the distribution of Th17 and nTregs cells in
the liver of patients with HBV infections may provide additional
confirmatory evidence, and are planned in the future.
In conclusion, the findings of the present study
demonstrated that pro-inflammatory Th17 cell frequencies are
associated with various stages of liver injury during the
progression of HBV between simple active hepatitis and liver
failure. nTreg cell-mediated immune tolerance was associated with
levels of HBV DNA replication. This characterization of the
Treg/Th17 balance in the progression of CHB extends current
understanding of the immunopathogenesis of CHB, and supports future
investigations on pro-inflammatory and anti-inflammatory
pathways.
Abbreviations:
|
ALB
|
albumin
|
|
ALT
|
alanine aminotransferase
|
|
CAWC
|
chronic active hepatitis without
cirrhosis
|
|
CD
|
cluster determinant
|
|
CHB
|
chronic hepatitis B
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
Foxp3
|
forkhead/winged helix transcription
factor
|
|
HBV
|
hepatitis B virus
|
|
HBLF
|
HBV-associated liver failure
|
|
IFN-γ
|
interferon γ
|
|
IL
|
interleukin
|
|
NR
|
normal range
|
|
nTreg
|
natural regulatory T cell
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
PCR
|
polymerase chain reaction
|
|
PT
|
prothrombin time
|
|
TB
|
total bilirubin
|
|
Th17
|
T helper 17
|
|
TNF-α
|
tumor necrosis factor α
|
Acknowledgments
The present study was supported by grants from the
Natural Science Foundation of Chongqing City (grant no.
cstc2012jjA10052) and the National Natural Science Foundation of
China (grant no. 81271838). The authors would like to thank all the
participants involved.
References
|
1
|
Ganem D and Prince AM: Hepatitis B virus
infection-natural history and clinical consequences. New Engl J
Med. 350:1118–1129. 2004. View Article : Google Scholar
|
|
2
|
Hernandez-Gea V and Friedman SL:
Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. 2011.
View Article : Google Scholar
|
|
3
|
Friedman SL: Evolving challenges in
hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 7:425–436. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang FS and Zhang Z: Host immunity
influences disease progression and antiviral efficacy in humans
infected with hepatitis B virus. Expert Rev Gastroenterol Hepatol.
3:499–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dancygier H and Schirmacher P: Immune
mediated liver injury. Clinical Hepatology. Dancygier H: Springer;
Berlin, Germany: pp. 191–196. 2010
|
|
6
|
Jung MC and Pape GR: Immunology of
hepatitis B infection. Lancet Infect Dis. 2:43–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang FS: Clinical immune characterization
of hepatitis B virus infection and implications for immune
intervention: Progress and challenges. Hepatol Res. 37(Suppl 3):
S339–S346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy KM and Weaver CT: Interleukin 17-producing CD4+
effector T cells develop via a lineage distinct from the T helper
type 1 and 2 lineages. Nat Immunol. 6:1123–1132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi M, Wei J, Dong J, Meng W, Ma J, Wang
T, Wang N and Wang Y: Function of interleukin-17 and -35 in the
blood of patients with hepatitis B-related liver cirrhosis. Mol Med
Rep. 11:121–126. 2015.
|
|
10
|
Jaeschke H and Hasegawa T: Role of
neutrophils in acute inflammatory liver injury. Liver Int.
26:912–919. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN,
Jin L, Fu JL, Shi F, Shi M, Wang HF and Wang FS:
Interleukin-17-producing CD4(+) T cells increase with severity of
liver damage in patients with chronic hepatitis B. Hepatology.
51:81–91. 2010. View Article : Google Scholar
|
|
12
|
Zhang Z, Zou ZS, Fu JL, Cai L, Jin L, Liu
YJ and Wang FS: Severe dendritic cell perturbation is actively
involved in the pathogenesis of acute-on-chronic hepatitis B liver
failure. J Hepatol. 49:396–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szabo G, Mandrekar P and Dolganiuc A:
Innate immune response and hepatic inflammation. Semin Liver Dis.
27:339–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ge J, Wang K, Meng QH, Qi ZX, Meng FL and
Fan YC: Implication of Th17 and Th1 cells in patients with chronic
active hepatitis B. J Clin Immunol. 30:60–67. 2010. View Article : Google Scholar
|
|
15
|
Zhao RR, Yang XF, Dong J, Zhao YY, Wei X,
Huang CX, Lian JQ and Zhang Y: Toll-like receptor 2 promotes T
helper 17 cells response in hepatitis B virus infection. Int J Clin
Exp Med. 8:7315–7323. 2015.PubMed/NCBI
|
|
16
|
Li X, Liu X, Tian L and Chen Y:
Cytokine-mediated immuno-pathogenesis of hepatitis B virus
infections. Clin Rev Allergy Immunol. 1–14, epub. 2014.
|
|
17
|
Kondo Y, Ueno Y and Shimosegawa T:
Immunopathogenesis of hepatitis B persistent infection:
Implications for immunothera-peutic strategies. Clin J
Gastroenterol. 2:71–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kondo Y, Ueno Y, Kobayashi K, Kakazu E,
Shiina M, Inoue J, Tamai K, Wakui Y, Tanaka Y, Ninomiya M, et al:
Hepatitis B virus replication could enhance regulatory T cell
activity by producing soluble heat shock protein 60 from
hepatocytes. J Infect Dis. 202:202–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan Q, Hong S, Shi B, Kers J, Li Z, Pei
X, Xu L, Wei X and Cai M: CD4(+)CD25(-)Nrp1(+) T cells synergize
with rapamycin to prevent murine cardiac allorejection in
immunocompetent recipients. PLoS One. 8:e611512013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Voelkl S, Gary R and Mackensen A:
Characterization of the immunoregulatory function of human TCR-αβ+
CD4- CD8-double-negative T cells. Eur J Immunol. 41:739–748. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang BL, Su H, Chen Y, Wang J and Xu GL: A
role for tricho-santhin in the expansion of CD4CD25 regulatory T
cells. Scand J Immunol. 71:258–266. 2015. View Article : Google Scholar
|
|
22
|
Lambotin M, Raghuraman S, Stoll-Keller F,
Baumert TF and Barth H: A look behind closed doors: Interaction of
persistent viruses with dendritic cells. Nat Rev Microbiol.
8:350–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie Q, Shen HC, Jia NN, Wang H, Lin LY, An
BY, Gui HL, Guo SM, Cai W, Yu H, et al: Patients with chronic
hepatitis B infection display deficiency of plasmacytoid dendritic
cells with reduced expression of TLR9. Microbes Infect. 11:515–523.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carotenuto P, Artsen A, Niesters HG,
Osterhaus AD and Pontesilli O: In vitro use of autologous dendritic
cells improves detection of T cell responses to hepatitis B virus
(HBV) antigens. J Med Virol. 81:332–339. 2009. View Article : Google Scholar
|
|
25
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thimme R, Wieland S, Steiger C, Ghrayeb J,
Reimann KA, Purcell RH and Chisari FV: CD8(+) T cells mediate viral
clearance and disease pathogenesis during acute hepatitis B virus
infection. J Virol. 77:68–76. 2003. View Article : Google Scholar :
|
|
27
|
Pawlotsky JM: EASL Clinical Practice
Guidelines. J Hepatol. 50:2432009. View Article : Google Scholar
|
|
28
|
Bettelli E, Korn T, Oukka M and Kuchroo
VK: Induction and effector functions of T(H)17 cells. Nature.
453:1051–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou L, Lopes JE, Chong MM, Ivanov II, Min
R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al:
TGF-β-induced Foxp3 inhibits T(H)17 cell differentiation by
antagonizing RORgammat function. Nature. 453:236–240. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bettelli E, Carrier Y, Gao W, Korn T,
Strom TB, Oukka M, Weiner HL and Kuchroo VK: Reciprocal
developmental pathways for the generation of pathogenic effector
TH17 and regulatory T cells. Nature. 441:235–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Veldhoen M, Hocking RJ, Atkins CJ,
Locksley RM and Stockinger B: TGFbeta in the context of an
inflammatory cytokine milieu supports de novo differentiation of
IL-17-producing T cells. Immunity. 24:179–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ando DG, Clayton J, Kono D, Urban JL and
Sercarz EE: Encephalitogenic T cells in the B10. PL model of
experimental allergic encephalomyelitis (EAE) are of the Th-1
lymphokine subtype. Cell Immunol. 124:132–143. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bouchliou I, Miltiades P, Nakou E,
Spanoudakis E, Goutzouvelidis A, Vakalopoulou S, Garypidou V,
Kotoula V, Bourikas G, Tsatalas C and Kotsianidis I: Th17 and
Foxp3(+) T regulatory cell dynamics and distribution in
myelodysplastic syndromes. Clin Immunol. 139:350–359. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maek-A-Nantawat W, Buranapraditkun S,
Klaewsongkram J and Ruxrungthum K: Increased interleukin-17
production both in helper T cell subset Th17 and CD4-negative T
cells in human immunodeficiency virus infection. Viral Immunol.
20:66–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Acosta-Rodriguez EV, Rivino L, Geginat J,
Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F and Napolitani
G: Surface phenotype and antigenic specificity of human interleukin
17-producing T helper memory cells. Nat Immunol. 8:639–646. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin
17-producing CD4+ effector T cells develop via a lineage distinct
from the T helper type 1 and 2 lineages. Nat Immunol. 6:1123–1132.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lemmers A, Moreno C, Gustot T, Maréchal R,
Degré D, Demetter P, de Nadai P, Geerts A, Quertinmont E,
Vercruysse V, et al: The interleukin-17 pathway is involved in
human alcoholic liver disease. Hepatology. 49:646–657. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harada K, Shimoda S, Sato Y, Isse K, Ikeda
H and Nakanuma Y: Periductal interleukin-17 production in
association with biliary innate immunity contributes to the
pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin
Exp Immunol. 157:261–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang R, Kozhaya L, Mercer F, Khaitan A,
Fujii H and Unutmaz D: Expression of GARP selectively identifies
activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S
A. 106:13439–13444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peng G, Li S, Wu W, Sun Z, Chen Y and Chen
Z: Circulating CD4+ CD25+ regulatory T cells correlate with chronic
hepatitis B infection. Immunology. 123:57–65. 2008. View Article : Google Scholar
|
|
42
|
Niu Y, Liu H, Yin D, Yi R, Chen T, Xue H,
Zhang S, Lin S and Zhao Y: The balance between intrahepatic
IL-17(+) T cells and Foxp3(+) regulatory T cells plays an important
role in HBV-related end-stage liver disease. BMC Immunol.
12:472011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang JY, Song CH, Shi F, Zhang Z, Fu JL
and Wang FS: Decreased ratio of Treg cells to Th17 cells correlates
with HBV DNA suppression in chronic hepatitis B patients undergoing
entecavir treatment. PLoS One. 5:e138692010. View Article : Google Scholar : PubMed/NCBI
|