Introduction
Toll-like receptors (TLRs), which exhibit homology
with the Drosophila Toll protein (1), are crucial and central in the
detection and elimination of invading pathogens (2). At present, 11 TLRs have been
identified in mammals and 10 TLRs have been found in humans
(1–3). TLR7 recognizes single-strand RNA or
synthetic agonists, including imiquimod and its derivatives
(4). Gardiquimod is a small
molecule of the imidazoquinoline compounds and a derivative of
imiquimod, which is reported as being recognized by TLR7 and is ~10
times more effective than imiquimod (4,5).
Activation of TLR7 initiates a signaling cascade, including MyD88
and nuclear factor-κB signaling molecules, which consequently leads
to activation of the downstream mitogen-activated protein kinase
(MAPK) signaling pathway, secretion of cytokines and expression of
co-stimulatory molecules (6).
Pancreatic cancer is one of the most aggressive and
life threatening types of malignancy (7). Despite its low incidence in developed
countries, pancreatic cancer is associated with poor survival rates
(8). Due to its highly invasive
and migratory potential, pancreatic cancer is ranked as the fourth
most common cause of cancer-associated mortality in the United
Kingdom (7). With the development
of molecular biology, the association between immunotherapy and
cancer, including pancreatic cancer, is improving (7,9). It
is well known that oncogenes and tumor suppressor genes control the
proliferation, differentiation, migration, invasion and apoptosis
of tumor cells (10). Interferon
type III (IFN-λ1), also termed interleukin (IL)-29 and similar to
type I and type II IFNs, is a novel broad spectrum antiviral agent,
which affects cell growth and differentiation, adjusts immune
function and exerts several types of biological activity (11). p53 is a significant tumor
suppressor, and inactivation of the p53 gene is important in tumor
formation (12). Phosphatase and
tensin homolog deleted on chromosome 10 (PTEN) is another key tumor
suppressor, which is crucial in cell migration and regulates cell
survival signaling via the phosphoinositide 3-kinase/Akt pathway
(13). Vascular endothelial growth
factor (VEGF) is the most effective promoter of vascular growth
factors, which are associated with cancer growth and metastasis
(14). Matrix metalloproteinase 9
(MMP-9) is a member of the zinc metalloproteinase family, which is
important in cell invasion (15).
Metastasis and tissue inhibitor of metalloproteinases (TIMPs) are
inhibitors of MMPs, which are involved in innate immune defense and
apoptosis (16,17), and are commonly involved in the
control of tumor development.
Several studies have demonstrated that activation of
TLRs not only provides the possibility for the resolution of
inflammation, but are also crucial in cancer (18,19).
Studies have reported that TLR7 is important in the pathogenesis of
pancreatic cancer (20); however,
the effect of TLR7 on gene expression remains to be elucidated. The
present study aimed to observe the effects of the gardiquimod TLR7
agonist on the ERK1/2 signaling pathway, and on the expression of
genes, including IFN-λ1, p53, PTEN, VEGF, MMP-9 and TIMP-1.
Materials and methods
Reagents
The BxPC-3 human pancreatic cancer cell line was
purchased from the Shanghai Cell Bank of Chinese Academy of
Sciences (Shanghai, China). Gardiquimod was obtained from
Invivogen, Inc. (San Diego, CA, USA). Antibodies targeting
phosphorylated (p)-ERK1/2 were purchased from OriGene Technologies
(Beijing, China), and those for ERK1/2 and β-actin were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Primers for
GAPDH, IFN-λ1, p53, PTEN, VEGF, MMP-9 and TIMP-1 were obtained from
Takara Biotechnology Co., Ltd. (Dalian China).
Cell culture
The BxPC-3 cells were cultured in RPMI 1640 medium
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 U/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in an atmosphere containing 5%
CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The BxPC-3 cells (3×104/ml) were seeded
into 24-well plates at 37°C in a 5% CO2 atmosphere for
48 h. Following a 48 h culture, followed by 1 day of culture in
FBS-free media, the cells were treated with gardiquimod (3
µg/ml) for a range of durations (0, 0.2, 0.5, 1, 3, 6, 12,
24 or 48 h). Total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA). Reverse transcription of the RNA to cDNA was performed
using a PrimeScript™ II 1st Strand cDNA Synthesis kit (cat. no.
6210A/B; Takara Biotechnology Co., Ltd.). The qPCR was subsequently
performed using SYBR Premix Ex TaqII Perfect Real Time kit (Takara
Biotechnology Co., Ltd.). The primer sequences are listed in
Table I. The cDNA samples were
amplified using the Applied Biosystems 7500 Real-Time PCR system
(Applied Biosystems Life Technologies, Foster City, CA, USA) with
the following cycling conditions: Pre-denaturation at 95°C for 5
sec, followed by 40 cycles of denaturation at 95°C for 5 sec,
annealing at 95°C for 10 sec and extension at 60°C for 60 sec.
Operation dissolution curves were then constructed using SDS
software version 2.0 (Applied Biosystems Life Technologies). The
relative gene expression was calculated using the 2−ΔΔCt
method.
 | Table IReverse transcription-quantitative
polymerase chain reaction primer sequences used in the present
study. |
Table I
Reverse transcription-quantitative
polymerase chain reaction primer sequences used in the present
study.
| Gene | Primer sequence |
|---|
| GAPDH | |
| Forward |
5′AGATCATCAGCAATGCCTCCTG |
| Reverse |
5′ATGGCATGGACTGTGGTCATG |
| IFN-λ1 | |
| Forward |
5′AAGTGGAAGAGTGTGCGGAAC |
| Reverse |
5′AATAGGGTCTTGTTTCCGGCC |
| p53 | |
| Forward |
5′CAGCCAAGTCTGTGACTTGCAC |
| Reverse |
5′AGACCATCGCTATCTGAGCAGC |
| VEGF | |
| Forward |
5′CTCTACCTCCACCATGCCAAGT |
| Reverse |
5′TCGATTGGATGGCAGTAGCTG |
| MMP-9 | |
| Forward |
5′GGTGATTGACGACGCCTTTG |
| Reverse |
5′GGACCACAACTCGTCATCGT |
| TIMP-1 | |
| Forward |
5′TCTGCAATTCCGACCTCGTC |
| Reverse |
5′CTGTTCCAGGGAGCCACAAA |
| PTEN | |
| Forward |
5′CAGCAGCTTCTGCCATCTCT |
| Reverse |
5′TGCTTTGAATCCAAAAACCTTACT |
Western blot analysis
The BxPC-3 cells (3×104/ml) were
incubated in 24-well plates at 37°C in a 5% CO2
atmosphere for 48 h. At a confluence of 70–80%, the cells were
cultured in FBS-free RPMI 1640 at 37°C in a 5% CO2
atmosphere for 1 day, prior to treatment with gardiquimod (3
µg/ml) for 0, 0.2, 0.5, 1, 3, 6, 12, 24 or 48 h. Following
three washes with cold phosphate-buffered saline, the cells were
treated with radioimmunoprecipitation assay buffer containing 1 mM
phenylmethylsulfonyl fluoride (Sigma-Aldrich, St. Louis, MO, USA)
for 30 min in an ice-water bath. The protein concentration was
determined and adjusted using a Bicinchoninic Acid kit
(Sigma-Aldrich). Equal quantities of proteins (10 µg) were
separated using 12% gel electrophoresis (Sigma-Aldrich), and
subsequently transferred to polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were then blocked
with 5% non-fat milk for 2 h at room temperature in Tris-buffered
saline with Tween (TBST; Nanjing Sunshine Biotechnology Co., Ltd.,
Nanjing, China). Following washing with TBST, the membranes were
incubated with monoclonal mouse anti-human anti-β-actin (1:500;
sc-8342), polyclonal mouse anti-human anti-p-ERK1/2 (1:500;
TA324783) and monoclonal mouse anti-human ERK1/2 (1:1,000;
sc-135900) primary antibodies overnight at 4°C. The membranes were
then incubated with secondary antibodies, and the products were
detected using enhanced chemiluminescence (SuperSignal-West Femto
Trial kit; Pierce Biotechnology, Inc., Rockford, IL, USA). Quantity
One 1-D image analysis software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to quantitatively analyze the
densitometry of the blots. This experiment was repeated at least
three times.
Statistical analysis
All experiments were repeated at least three times,
with representative results presented, data are expressed at the
mean ± standard deviation. SPSS 13.0 software (SPSS, Inc., Chicago,
IL, USA) was used for all statistical analyses. P<0.05 was
considered to indicate a statistically significant difference.
Results
Gardiquimod upregulates the expression of
IFN-λ1, p53, PTEN, VEGF, MMP-9 and TIMP-1 in BxPC-3 cells
To determine the effects of gardiquimod on gene
regulation in BxPC3 cells, RT-qPCR was performed to analyze the
mRNA expression levels of various genes. As shown in Fig. 1, these genes were upregulated at
different time-points during the 24 h treatment period. Treatment
with gardiquimod (3 µg/ml) upregulated the expression levels
of IFN-λ1, with the highest peak detected at 24 h, compared with
the control group (P<0.01; Fig.
1A). The expression levels of p53 and PTEN exhibited an
increasing trend, reaching a peak at 24 h, compared with the
control group (P<0.05; Fig. 1B and
C). The expression level of VEGF increased marginally at 10
min; however, the expression levels reduced after 10 min, with the
lowest expression detected at 12 h (P<0.01; Fig. 1D). The expression of MMP-9 was
highest at 1 h, and the relative mRNA expression levels at 12 h
were 3.5-fold higher, compared with the control group (P<0.01;
Fig. 1E). The lowest expression
levels of MMP-9 were detected at 24 h. In addition, the expression
levels of TIMP-1 increased, compared with the blank control, with
the peak level of expression detected at 6 h (P<0.05; Fig. 1F).
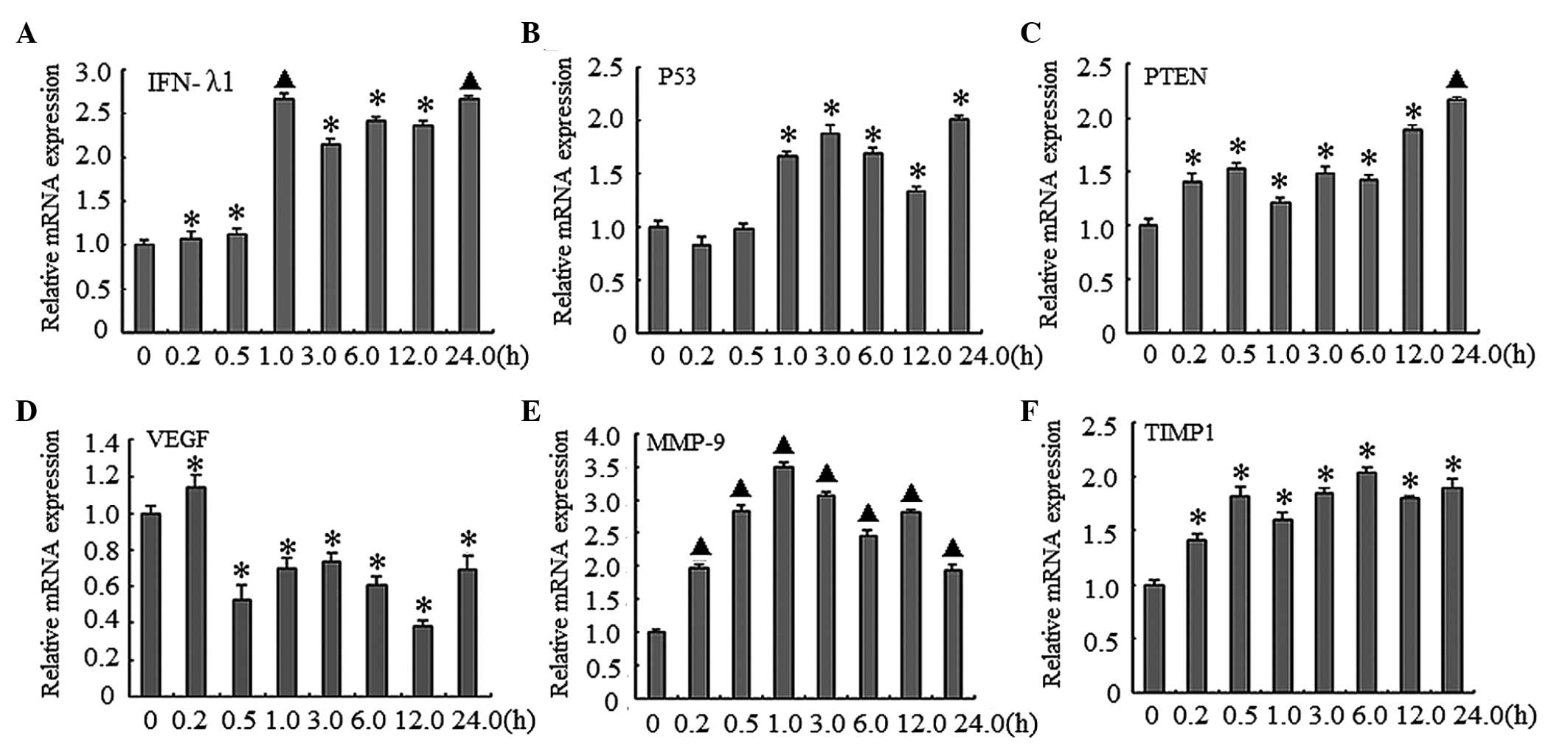 | Figure 1Gardiquimod induces the expression of
several genes, as determined using RT-qPCR. The BxPC-3 human
pancreatic cancer cells were treated with gardiquimod (3
µg/ml) for the indicated time-periods (0, 0.2, 0.5, 1.0,
3.0, 6.0, 12 and 24 h). Total cellular RNA was extracted and was
used to synthesize cDNA using reverse transcription. Subsequently,
the mRNA expression levels of genes were determined using RT-qPCR,
and GAPDH was used as a control. The mRNA expression levels (A)
IFN-λ1, (B) p53, (C) PTEN, (D) VEGF, (E) MMP-9 and (F) TIMP-1 were
determined. The results were obtained from three separate
experiments (*P<0.05 and ▲P<0.01, vs. 0
h); data are expressed as the mean ± standard deviation. RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; INF,
interferon; PTEN, phosphatase and tensin homolog deleted on
chromosome 10; VEGF, vascular endothelial growth factor; MMP,
matrix metalloproteinas; TIMP, tissue inhibitor of
metalloproteinase. |
Gardiquimod induces the activation of
ERK1/2 in BxPC-3 cells
In order to analyze whether gardiquimod induces
activation of the ERK1/2 signaling pathway, western blot analysis
was performed to detect alterations in the expression of ERK1/2. As
shown in Fig. 2A and B gardiquimod
activated the phosphorylation of ERK1/2 to p-ERK1/2 in a
time-dependent manner in the BxPC-3 cells. The protein expression
levels of p-ERK1/2 increased after 6 h treatment with gardiquimod,
and this effect continued to 48 h. However, the effects of
gardiquimod over a longer treatment duration were not examined.
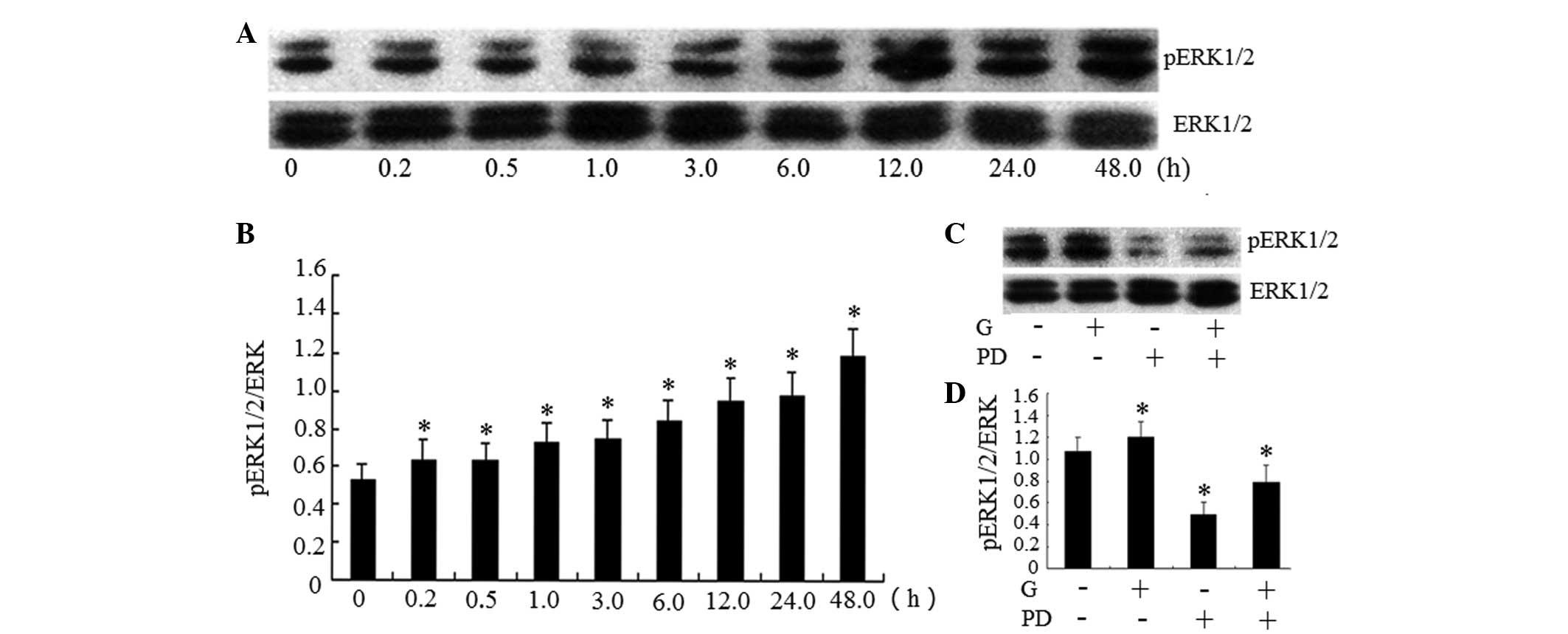 | Figure 2Activation of TLR7 by gardiquimod
induces the phosphorylation of ERK1/2, which is inhibited by
specific inhibitors. (A) BxPC-3 human pancreatic cancer cells were
treated with gardiquimod (3 µg/ml) for different durations
(0, 0.2, 0.5, 1.0, 3.0, 6.0, 12 or 24 h). Total cellular proteins
were extracted and were used to determine the protein expression
levels of pERK1/2 using western blotting, with total ERK1/2 used as
a control. (B) Band intensities of the western blot were determined
using Quantity One software. The intensity of the target bands
(pERK1/2) were normalized to the control bands (total ERK1/2). (C)
Cells were pre-treated with PD98059 (100 µmol/l) for 1 h and
treated with gardiquimod (3 µg/ml) for 6 h. The expression
levels of p-ERK1/2 and total ERK1/2 were detected using western
blotting. (D) Band intensities of the western blot were determined
using Quantity One software. The results are representative of
three separate experiments; data are expressed as the mean ±
standard deviation; *P<0.05 and ▲P<0.01
vs. 0 h. TLR, Toll-like receptor; ERK, extracellular
signal-regulated kinase; pERK, phosphorylated ERK; G, gardiquimod;
PD, PD98059. |
Gardiquimod-induced upregulation of
downstream gene expression in BxPC-3 cells is partially dependent
on the activation of ERK1/2
As shown in Fig. 2A and
B, treatment with gardiquimod activated the ERK1/2 signaling
pathway. In order to determine the association between activation
of ERK1/2 and the expression of the activated genes, the cells were
co-treated with gardiquimod (3 µg/ml) and the
ERK1/2-specific inhibitor, PD98059 (100 µM). The effects of
PD98059 on p-ERK1/2 in the signaling pathway, and on the mRNA
expression levels of IFN-λ1, p53, PTEN, VEGF, MMP-9 and TIMP-1 were
examined. As shown in Fig. 2C and
D the ERK1/2 signal pathway was inhibited by PD98059. The mRNA
expression levels of the genes decreased markedly in the cells
treated with PD98059 either alone or in combination with
gardiquimod, compared with the cells in the gardiquimod-alone or
blank control groups (Fig. 3).
When the ERK1/2 signaling pathway was inhibited, the mRNA
expression levels of MMP-9 were markedly decreased, regardless of
whether the cells were treated with gardiquimod or not. The
expression levels of IFN-λ1, p53, PTEN and TIMP-1 also reduced,
however, these reductions in expression were lower than that of
MMP-9. These results suggested that the effect of gardiquimod on
MMP-9 may be predominantly involved with the activation of ERK1/2;
whereas the effects on IFN-λ1, p53 and PTEN and MMP-9 and TIMP-1
may be only partially associated with the ERK1/2 pathway. In
addition, as only marginal changes in the expression levels of VEGF
were observed following treatment with gardiquimod, the regulation
of VEGF by gardiquimod may not be associated with the ERK1/2
activation.
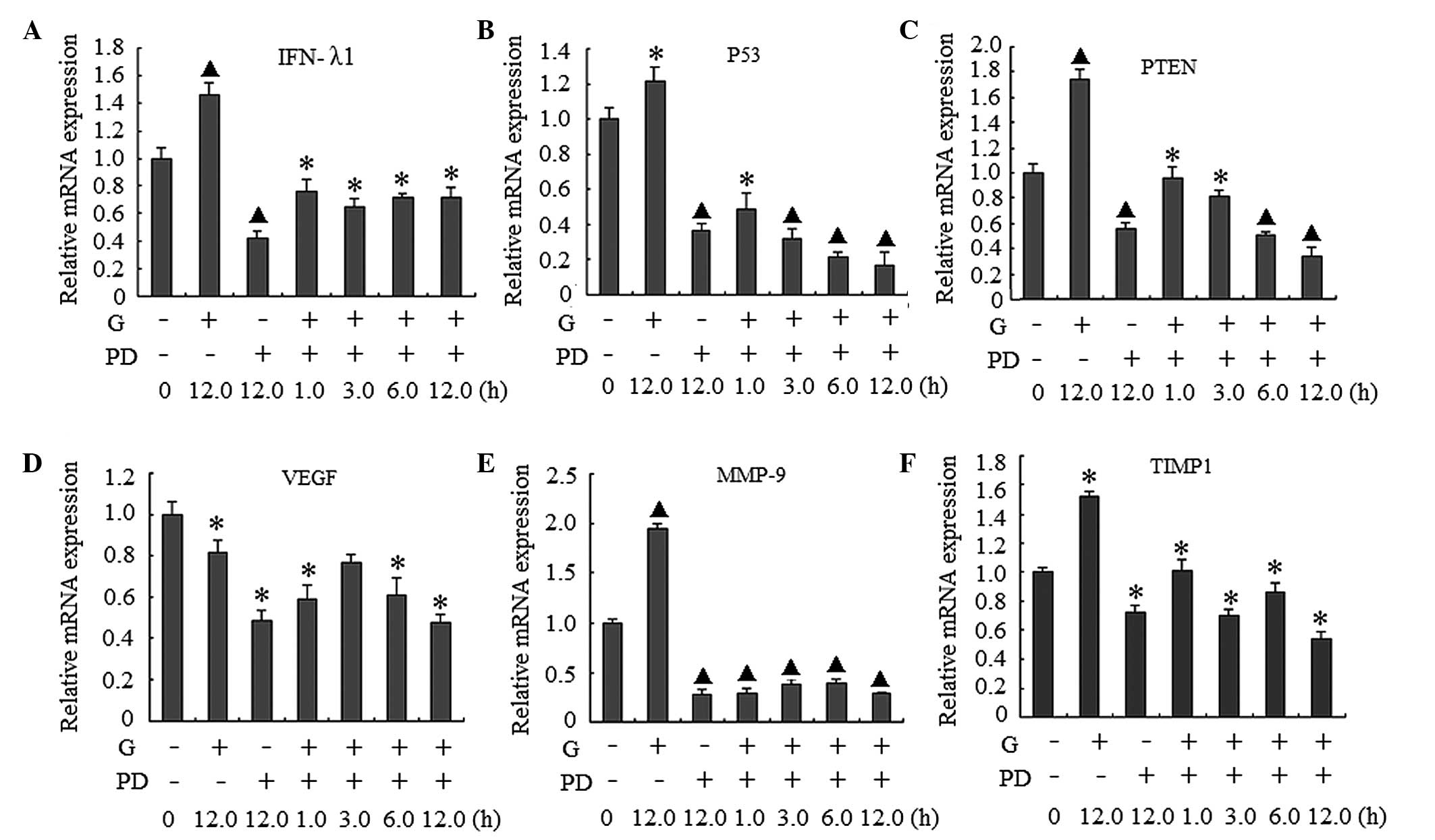 | Figure 3Effect of PD98059 (PD) on the gene
expression of BxPC-3 human pancreatic cancer cells treated with
gardiquimod (3 µg/ml). The BxPC-3 cells were pre-treated
with PD (100 µmol/l) for 1 h, followed by treatment with G
(3 µg/ml) for the indicated durations. Reverse
transcription-quantitative polymerase chain reaction was used to
detect the mRNA expression levels of (A) IFN-λ1, (B) p53, (C) PTEN,
(D) VEGF, (E) MMP-9 and (F) TIMP-1. The results were obtained from
three separate experiments; data are expressed as the mean ±
standard deviation; *P<0.05 and ▲P<0.01
vs. 0 h. INF, interferon; PTEN, phosphatase and tensin homolog
deleted on chromosome 10; VEGF, vascular endothelial growth factor;
MMP, matrix metalloproteinase; TIMP, tissue inhibitor of
metalloproteinase. |
Discussion
TLRs are an important family of pattern-recognition
receptors, which can initiate innate immunity and induce tumor cell
death directly, or activate the adaptive immune system (18,19).
TLR7 can initiate the activation and maturation of immune cells,
including dendritic cells and anti-tumor cytokines (21). In the present study, TLR7 was
activated by the TLR7 agonist gardiquimod, which resulted in a
series of changes in the expression of downstream factors.
According to previous studies, TLR7 exerts different
functions on different cells. The activation of TLR7 can inhibit
prostate cancer cells (22),
however, TLR7 can also induce the development of cervical cancer
(23). Therefore, TLR7 has
different roles in immunotherapy (24). In previous studies, TLR7 was found
to promote the expression of inflammatory cytokines, including
IL-29 and IFN-λ1, which in turn initiates the innate immune
reaction and consequently suppresses the development of cancer
(25,26). In the present study, the results
suggested that activation of TLR7 disturbed the development of
pancreatic cancer through activation of the immune response.
Certain anti-tumor genes were also observed to be upregulated by
gardiquimod, including TIMP-1 and PTEN. By contrast, the expression
of VEGF, which can promote proliferation, was marginally decreased
following treatment with gardiquimod. These results indicated that
TLR7 activation may have inhibited the progression of BxPC-3
pancreatic cancer cells. However, a study by Ochi et al
(20) demonstrated that TLR7
activation promotes the development of pancreatic cancer cells
carrying the G12D mutation; therefore, the effect of TLR7 on the
development of pancreatic cancer requires further
investigation.
The MAPK-ERK signaling pathway functions as a key
factor in progression of the majority of types of cancer (27). Numerous studies have demonstrated
that the activation of this pathway contributes to cancer cell
proliferation; however, this pathway can also initiate the immune
response, which defends against the transformation normal cells
into cancer cells (28–30). Therefore, the activation of the
MAPK-ERKsignaling pathway may have a pleiotropic effect on cancer
cells, however the mechanism underlying the TLR7-induced activation
of this pathway and the consequent effects on BxPC-3 cells remain
to be fully elucidated, and the role of the MAPK-ERK pathway
requires further investigation. Of note, this pathway is one of
multiple pathways activated by TLR7, and the other pathways
activated by gardiquimod also require investigation.
In conclusion, the present study demonstrated that
TLR activation induced the expression of inflammatory factors and
suppressed the expression of cancer-associated genes in BxPC-3
cells. In addition, these effects were partially dependent on
activation of the MAPK-ERK pathway. These results may provide novel
insight into the role of TLR7 in BxPC-3 pancreatic cancer
cells.
Acknowledgments
The present study was supported by grants from the
General Program of National Natural Science Foundation of China
(grant no. 81271748), and the Foundation for Doctors, Anhui Medical
University.
References
|
1
|
McGettrick AF and O'Neill LA: Toll-like
receptors: Key activators of leucocytes and regulator of
haematopoiesis. Br J Haematol. 139:185–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sada T, Ota M, Katsuyama Y, Meguro A,
Nomura E, Uemoto R, Nishide T, Okada E, Ohno S, Inoko H and Mizuki
N: Association analysis of Toll-like receptor 7 gene polymorphisms
and Behçet's disease in Japanese patients. Hum Immunol. 72:269–272.
2011. View Article : Google Scholar
|
|
3
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aspord C, Tramcourt L, Leloup C, Molens
JP, Leccia MT, Charles J and Plumas J: Imiquimod inhibits melanoma
development by promoting pDC cytotoxic functions and impeding tumor
vascularization. J Invest Dermatol. 134:2551–2561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diebold SS, Kaisho T, Hemmi H, Akira S and
Reis e Sousa C: Innate antiviral response by means of TLR7-mediated
recognition of single-stranded RNA. Science. 303:1529–1531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma F, Zhang J, Zhang J and Zhang C: The
TLR7 agonists imiquimod and gardiquimod improve DC-based
immunotherapy for melanoma in mice. Cell Mol Immunol. 7:381–388.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steele CW, Jamieson NB, Evans TR, McKay
CJ, Sansom OJ, Morton JP and Carter CR: Exploiting inflammation for
therapeutic gain in pancreatic cancer. Br J Cancer. 108:997–1003.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raimondi S, Maisonneuve P and Lowenfels
AB: Epidemiology of pancreatic cancer: An overview. Nat Rev
Gastroenterol Hepatol. 6:699–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Zhao J, Huang J, Tang H, Yu S and
Chen Y: The regulatory roles of miRNA and methylation on oncogene
and tumor suppressor gene expression in pancreatic cancer cells.
Biochem Biophys Res Commun. 425:51–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meager A, Heath A, Dilger P, Zoon K and
Wadhwa M; Participants of the Collaborative Study: Standardization
of human IL-29 (IFN-λ1): establishment of a World Health
Organization international reference reagent for IL-29 (IFN-λ1). J
Interferon Cytokine Res. 34:876–884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koido S, Homma S, Takahara A, Namiki Y,
Tsukinaga S, Mitobe J, Odahara S, Yukawa T, Matsudaira H, Nagatsuma
K, et al: Current immunotherapeutic approaches in pancreatic
cancer. Clin Dev Immunol. 2011:2675392011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A,
Takahashi H, Wakasugi T, Funahashi H, Sato M and Takeyama H: IGF-1
mediates PTEN suppression and enhances cell invasion and
proliferation via activation of the IGF-1/PI3K/Akt signaling
pathway in pancreatic cancer cells. J Surg Res. 60:90–101. 2010.
View Article : Google Scholar
|
|
14
|
Shi Y, Tong M, Wu Y, Yang Z, Hoffman RM,
Zhang Y, Tian Y, Qi M, Lin Y, Liu Y, et al: VEGF-C ShRNA inhibits
pancreatic cancer growth and lymphangiogenesis in an orthotopic
fluorescent nude mouse model. Anticancer Res. 33:409–417.
2013.PubMed/NCBI
|
|
15
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.
|
|
16
|
Lorente L, Martín MM, Solé-Violán J,
Blanquer J, Labarta L, Díaz C, Borreguero-León JM, Orbe J,
Rodríguez JA, Jiménez A and Páramo JA: Association of
sepsis-related mortality with early increase of TIMP-1/MMP-9 ratio.
PLoS One. 9:e943182014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zampini R, Argañaraz ME, Miceli DC and
Apichela SA: Detection of the matrix metalloproteinases MMP-2 and
MMP-9 and tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2
in llama (Lama glama) oviduct. Reprod Domest Anim. 49:492–498.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fehres CM, Bruijns SC, van Beelen AJ,
Kalay H, Ambrosini M, Hooijberg E, Unger WW, de Gruijl TD and van
Kooyk Y: Topical rather than intradermal application of the TLR7
ligand imiquimod leads to human dermal dendritic cell maturation
and CD8+ T-cell cross-priming. Eur J Immunol. 44:2415–2424. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu X, Wang Y, Zhao W, Zhou H, Yang W and
Guan X: Toll-like receptor 7 promotes the apoptosis of
THP-1-derived macrophages through the CHOP-dependent pathway. Int J
Mol Med. 34:886–893. 2014.PubMed/NCBI
|
|
20
|
Ochi A, Graffeo CS, Zambirinis CP, Rehman
A, Hackman M, Fallon N, Barilla RM, Henning JR, Jamal M, Rao R, et
al: Toll-like receptor 7 regulates pancreatic carcinogenesis in
mice and humans. J Clin Invest. 122:4118–4129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu J, Lai K, Brownile R, Babiuk LA and
Mutwiri GK: Porcine TLR8 and TLR7 are both activated by a selective
TLR7 ligand, imiquimod. Mol Immunol. 45:3238–3243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han JH, Park SY, Kim JB, Cho SD, Kim B,
Kim BY, Kang MJ, Kim DJ and Park JH and Park JH: TLR7 expression is
decreased during tumour progression in transgenic adenocarcinoma of
mouse prostate mice and its activation inhibits growth of prostate
cancer cells. Am J Reprod Immunol. 70:317–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Cheng FW, Wang F, Jia B, Luo X and
Zhang SQ: The activation of TLR7 regulates the expression of VEGF,
TIMP1, MMP2, IL-6 and IL-15 in Hela cells. Mol Cell Biochem.
389:43–49. 2014. View Article : Google Scholar
|
|
24
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the Drosophila Toll protein
signals activation of adaptive immunity. Nature. 388:394–397. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harvey RD and Morgan ET: Cancer,
inflammation, and therapy: Effects on cytochrome p450-mediated drug
metabolism and implications for novel immunotherapeutic agents.
Clin Pharmacol Ther. 96:449–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Cigliano A, Delogu S, Armbruster
J, Dombrowski F, Evert M, Chen X and Calvisi DF: Functional
crosstalk between AKT/mTOR and Ras/MAPK pathways in
hepatocarcinogenesis: Implications for the treatment of human liver
cancer. Cell Cycle. 12:1999–2010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pivarcsi A, Müller A, Hippe A, Rieker J,
van Lierop A, Steinhoff M, Seeliger S, Kubitza R, Pippirs U, Meller
S, et al: Tumor immune escape by the loss of homeostatic chemokine
expression. Proc Natl Acad Sci USA. 104:19055–19060. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang HT, Cohen P and Rousseau S:
IL-1beta-stimulated activation of ERK1/2 and p38alpha MAPK mediates
the transcriptional up-regulation of IL-6, IL-8 and GRO-alpha in
HeLa cells. Cell Signal. 20:375–380. 2008. View Article : Google Scholar
|
|
30
|
Haydn JM, Hufnagel A, Grimm J, Maurus K,
Schartl M and Meierjohann S: The MAPK pathway as an apoptosis
enhancer in melanoma. Oncotarget. 5:5040–5053. 2014. View Article : Google Scholar : PubMed/NCBI
|

















