Introduction
MicroRNAs (miRNAs) are functional non-coding RNAs,
20–24 nucleotides long, which have important roles in the
regulation of the stability and translational efficiency of target
mRNAs (1). Mature miRNAs induce
post-transcriptional gene silencing by targeting the complementary
sequence motifs within the 3′-untranslated regions of target mRNAs.
Therefore, aberrant miRNA expression may affect the transcription
of target genes and profoundly influence cancer-associated
signaling pathways, which are involved in proliferation, cell cycle
control, apoptosis, differentiation, migration and metabolism
(2,3). Tumor tissue usually exhibits reduced
levels of mature miRNAs (4);
however, miRNA (miR)-21 has been reported to act as an oncomiR,
which is highly expressed and correlated with numerous types of
cancer, including pancreatic (5),
breast (6), non-small cell lung
(7), liver (8), gastric (9), ovarian (10), cervical (11), colon (12), brain (13), esophageal (14) and prostate (15) cancer. In addition, a previous study
demonstrated that miR-21 modulated paclitaxel sensitivity in
ovarian cancer (16), indicating
that miR-21 has an important role in chemoresistance.
Ionizing radiation is used extensively to treat
various types of cancer. However, despite the wide use of radiation
therapy and its association with remission, tumor recurrence is a
predominant cause of radiation treatment failure, and is frequently
associated with the acquisition of radioresistance by tumors
(17). At present, the detailed
mechanism underlying radiation resistance in human cancer remains
unclear. It has previously been reported that upregulated
phopshoinositide 3-kinase/AKT signaling may render human liver and
cervical cancer resistant to radiation (17,18).
In addition, a previous study demonstrated that high expression
levels of miR-21 may contribute to radiation resistance in breast
cancer cells (19), thus
suggesting that targeting miR-21 may be considered a therapeutic
strategy to overcome radiation resistance.
The majority of cancer cells prefer anaerobic
glycolysis for the production of their energy supply, which is
known as the 'Warburg effect'; however, anaerobic glycolysis is
less efficient at producing ATP, as compared with mitochondrial
respiration (20). As a result,
uptake of excessive glucose and generation of large quantities of
lactate by cancer cells is a well-recognized phenotype of abnormal
glycolysis. Notably, previous studies have suggested that metabolic
pathways are associated with chemoresistance (17,18,21),
suggesting that dysregulated metabolic pathways in cancer may be a
target for the development of novel therapeutic strategies. The
present study identified the role of miR-21 in radiation
resistance. The results detected a correlation between glycolysis
and radioresistance, and may provide evidence regarding the
mechanisms underlying miR-21-mediated radioresistance in non-small
cell lung cancer cells.
Materials and methods
Cell culture and ionizing radiation
treatment
The A549 lung cancer cells were obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in RPMI-1640 media (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.) and antibiotics (Gibco Antibiotic-Antimycotic
containing mphotericin B, penicillin and streptomycin; Gibco,
Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator
containing 5% CO2. The cells, at a density of
5×105, were exposed to various doses of irradiation (0,
0.5, 1, 2, 4, 5, 6, 8 and 10 Gy) using a Cs-137 irradiator (HWM
D-2000; Siemens, Waltershausen, Germany) at a dose rate of 2
Gy/min. Irradiation was administered at room temperature and the
cells were subsequently incubated at 37°C, and harvested for the
following experiments.
Antibodies
The following antibodies were used in the present
study: Anti-hexokinase 2 (HKII) (cat. no. 2867), anti-pyruvate
kinase (PK)M2 (cat. no. 4053), anti-lactate dehydrogenase (LDH)A
(cat. no. 2012), anti-β-actin (cat. no. 4967) (Cell Signaling
Technology, Inc., Danvers, MA, USA), and anti-hypoxia-inducible
factor (HIF)1α (cat. no. sc-10790; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA).
Small interfering (si)RNA and plasmid DNA
transfection
HIF-1α siRNA sequences (cat. no. 106498) and
negative control siRNA were purchased from Ambion (Thermo Fisher
Scientific, Inc.). Cells were seeded onto 6-well plates at a
density of 1×105 cells/well. A plasmid vector containing
wild type HIF1α was purchased from Addgene (Cambridge, MA, USA).
Transfection was performed using Lipofectamine® 2000 and
Opti-MEM I reduced serum medium (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Transfection temperature was 37°C, and the volume of siRNA/vector
and Lipofectamine used was 100 nM siRNA/vector and 5 µl
Lipofectamine, respectively for each transfection. A total of 48
hours post-transfection, the cells were prepared for further
analysis.
miRNA transfection
The precursor and antisense sequences of miR-21 were
chemically synthesized by GenePharma Co., Ltd. (Shanghai, China).
The cells were seeded in 6-well plates at 1×105
cells/well and cultured overnight. Subsequently, the cells were
transfected with 200 nM pre-miR-21, inhibitors or negative control,
using Lipofectamine® 2000 and Opti-MEM I reduced serum
medium (Invitrogen), according to the manufacturer's protocol. A
total of 48 hours post transfection, the cells were prepared for
further analysis.
Cell viability assay
The cells were seeded into 96-well culture plates at
a density of 5×103. Following cellular adhesion, the
cells were exposed to various doses of irradiation. Following
irradiation, 20 µl 5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide (MTT;
Sigma-Aldrich, St. Louis, MO, USA) was added to each well.
Following a 4 h incubation at 37°C, the medium was gently aspirated
and replaced with 150 µl dimethylsulfoxide. The absorbance
of each well was detected at a wavelength of 570 nm using an ELx800
Absorbance Reader (BioTek Instruments, Inc., Winooski, VT, USA).
The experiment was conducted in triplicate.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using the
Absolutely RNA RT-PCR Miniprep kit (Agilent Technologies, Inc.,
Santa Clara, CA, USA), according to the manufacturer's protocol.
Total RNA concentration was adjusted to 2 ng/µl using a
NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.).
Total RNA (1 µg) was reverse transcribed using the High
Capacity cDNA Reverse Transcription kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The cDNA was then diluted to 1:10 for use
as a template for RT-qPCR. PCR amplifications were performed in a
final reaction volume of 10 µl containing: 5.5 µl
TaqMan Universal PCR Master mix (Applied Biosystems), 0.5 µl
primers and probes mix and 4.5 µg cDNA. The cycling
conditions were as follows: One cycle at 50°C for 2 min, one cycle
at 95°C for 10 min, followed by 40 cycles of denaturation (95°C for
15 sec), and a final annealing/extension step at 60°C for 1 min.
All reactions were conducted using the Step One Plus Real-Time PCR
Systems Thermocycler (Applied Biosystems). All quantitative PCR
reactions were carried out in triplicate and repeated at least
twice. The ΔCquantification (ΔCq) for mRNA expression
was calculated relative to the Cq of 18S ribosomal RNA.
Relative mRNA expression was calculated using the formula
2(−ΔΔCq). The primers used for qPCR were as follows:
LDHA, forward ATCTTGACCTACGTGGCTTGGA and reverse
CCATACAGGCACACTGGAATCTC; HKII, forward CAAAGTGACAGTGGGTGTGG and
reverse GCCAGGTCCTTCACTGTCTC; and PKM2, forward GAGGCCTCCTTCAAGTGCT
and reverse CCAGACTTGGTGAGGACGAT. RT-qPCR quantification using the
TaqMan-miRNA assay (Applied Biosystems) was conducted to determine
the expression levels of miR-21 (cat. no. 4427975; assay ID
000397), using the cycling conditions previously described. qPCR
was performed using the Step One Plus Detection system (Applied
Biosystems), according to the manufacturer's protocol. The relative
expression values of the specific miRNA were calculated using the
2−ΔΔCq method, normalized to the control miRNA (GAPDH:
5′-AATCCCATCACCATCTTCCA-3′ forward and 5′-TGGACTCCACGACGTACTCA-3′
reverse). All reactions were performed at least twice in
duplicate.
Measurements of glucose consumption and
lactate production
The cells were seeded in 6-well plates at a density
of 5×105 cells/well in 3 ml culture medium. Then,
conditioned medium and distilled water were mixed at a 1:20 ratio
(conditioned medium:distilled water. The glucose concentration in
the diluted medium was measured using the Glucose Assay kit
(Sigma-Aldrich), according to the manufacturer's protocol. Glucose
consumption was calculated by subtracting the concentration of
glucose remaining in the medium 24 h later from the concentration
of glucose present in the fresh cell culture medium. Lactate
concentrations were determined using a Lactate Assay kit
(BioVision, Mountain View, CA, USA) according to the manufacturer's
protocol. Samples and a lactate standard were prepared with lactate
assay buffer in a 96-well plate. Subsequently, 50 µl lactate
enzyme mix was added to each well, and incubated for 30 min at room
temperature. Optical density values were measured at 570 nm using a
microplate reader (SpectraMax M2e; Molecular Devices, LLC,
Sunnyvale, CA, USA). The results were normalized to the amount of
total protein, as compared with the control cells.
Western blot analysis
The cells were harvested and washed with ice-cold
phosphate-buffered saline (PBS). Cell lysates were obtained by
re-suspending the cells in radioimmunoprecipitation assay buffer
[10 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1%
Na-deoxycholate (Kanto Chemical Co., Inc., Tokyo, Japan)] and 5 mM
EDTA supplemented with protease inhibitor cocktail (Sigma-Aldrich).
The protein concentration of the cell lysates was determined by
Bradford assay, using a Bradford kit (Beyotime Institute of
Biotechnology, Shanghai, China). Equal quantities of protein (1
µg/µl) were separated by 10% homemade sodium dodecyl
sulfate-poly-acrylamide gel electrophoresis and electrotransferred
onto a polyvinylidene difluoride membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membranes were blocked with 5%
non-fat milk and incubated overnight with primary antibodies at 4°C
at a dilution of 1:1,000. The membranes were then washed with PBS
with Tween 20 and incubated at room temperature for 1 h with
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
secondary antibodies (cat. no., 7074; dilution, 1:3,000; Nichirei
Biosciences, Inc., Tokyo, Japan). Protein bands were visualized
using Chemi-Lumi One L Western Blotting Substrate (Nacalai Tesque,
Kyoto, Japan).
Statistical analysis
Statistical analysis was performed using Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA) and the unpaired
Student's t-test was used to analyze the data. All data are
presented as the mean ± standard error. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-21 expression levels are higher in
radiation-resistant lung cancer cells
To investigate the association between miR-21
expression and radiation sensitivity, a radiation-resistant
non-small cell lung cancer cell line was generated using A549
parental cells, according to a previous study (22). The cells were treated with
increasing doses of radiation for 4 months, in order to generate
resistant cells. Resistant cell clones were developed and pooled.
To confirm that the cells were radioresistant, the A549 parental
cells and resistant cells underwent various doses of irradiation. A
total of 72 h post-irradiation cellular proliferation was
determined using an MTT assay (Fig.
1A). The A549 radiation-resistant cells were able to tolerate
higher intensities of radiation. In addition, the expression levels
of miR-21 were significantly upregulated in the radiation-resistant
cells, as compared with the sensitive cells (Fig. 1B), thus suggesting that miR-21 may
contribute to radioresistance in non-small lung cancer. To confirm
the above observation, A549 cells were transiently transfected with
pre-miR-21, in order to overexpress miR-21. The radiation
sensitivity of A549 cells with and without overexpression of miR-21
were subsequently measured following treatment with the indicated
doses. The A549 cells had an increased survival capacity in
response to irradiation following transfection with miR-21
(Fig. 1C). These data indicate a
strong correlation between miR-21 and radioresistance in lung
cancer cells, suggesting that miR-21 may be a target for overcoming
radiation resistance.
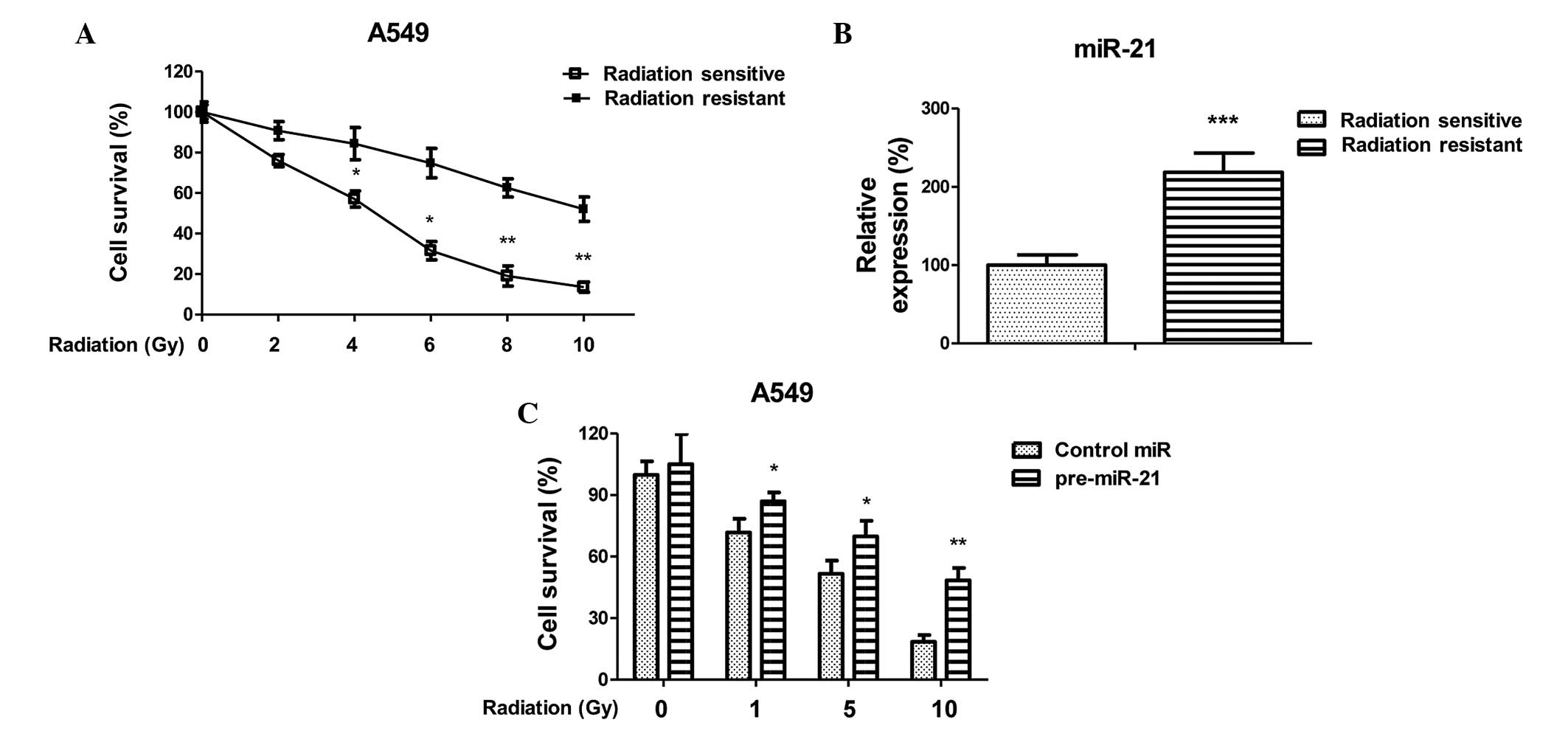 | Figure 1MicroRNA (miR)-21 is upregulated in
radioresistant cancer cells and positively correlated with
irradiation sensitivity. (A) Generation of radiation-resistant
cells from A549 lung cancer cells. Parental cells were treated with
gradually increasing doses of irradiation in regular cell culture
conditions, in order to generate resistant cells. Radioresistant
clones were pooled and analyzed following irradiation at 0, 2, 4,
6, 8 and 10 Gy, at a dose rate of 2 Gy/min. *P<0.05
and **P<0.01 versus radiation resistant. (B)
Expression levels of miR-21 in A549 parental and
radiation-resistant cells. ***P<0.001 versus
radiation sensitive. (C) A549 parental cells were transiently
transfected with pre-miR-21 for 48 h, followed by irradiation at 0,
1, 5 and 10 Gy, at a dose rate of 2 Gy/min. A cell survival assay
was subsequently performed. *P<0.05 and
**P<0.01 versus the control. Data are presented as
the mean ± standard error. |
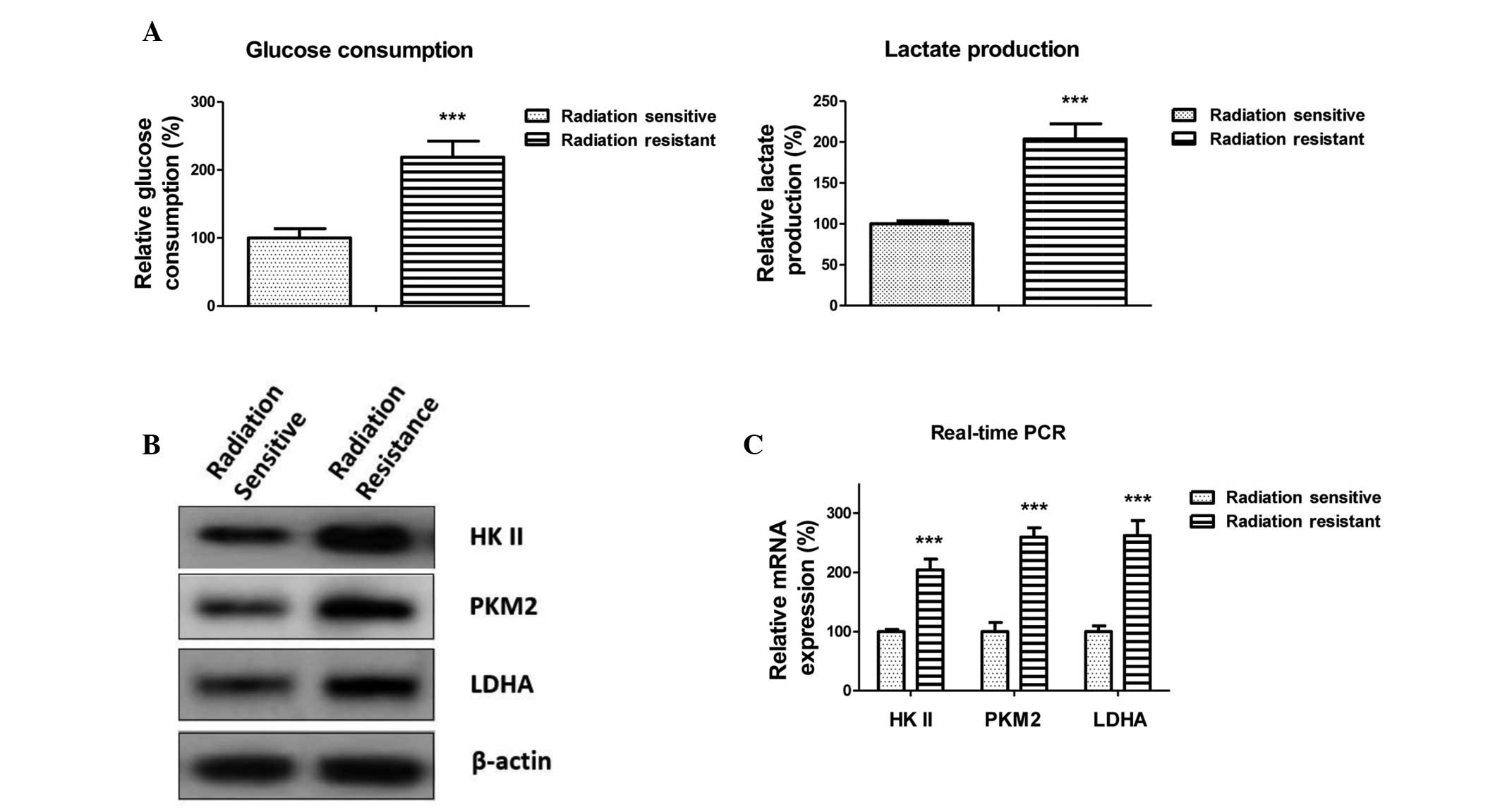
Radioresistant lung cancer cells exhibit
upregulated glycolysis
Enhanced anaerobic glycolysis often accompanies
radioresistance in cancer. To determine the mechanisms underlying
miR-21-promoted radioresistance, the glycolysis rates of
radiation-sensitive and -resistant A549 cells were detected. The
glucose consumption and lactate production were determined. As
shown in Fig. 2A, glucose
consumption and lactate production were promoted in the
radiation-resistant cells, thus indicating that upregulated
anaerobic glycolysis may be associated with the radioresistance. In
addition, the expression levels of glycolysis-associated enzymes
were detected. The protein and mRNA expression levels of HKII, PKM2
and LDHA were upregulated in the radioresistant cells (Fig. 2B and C). These results suggest that
enhancement of glycolysis by miR-21 is mediated via upregulation of
glycolysis-associated enzymes at the transcriptional level.
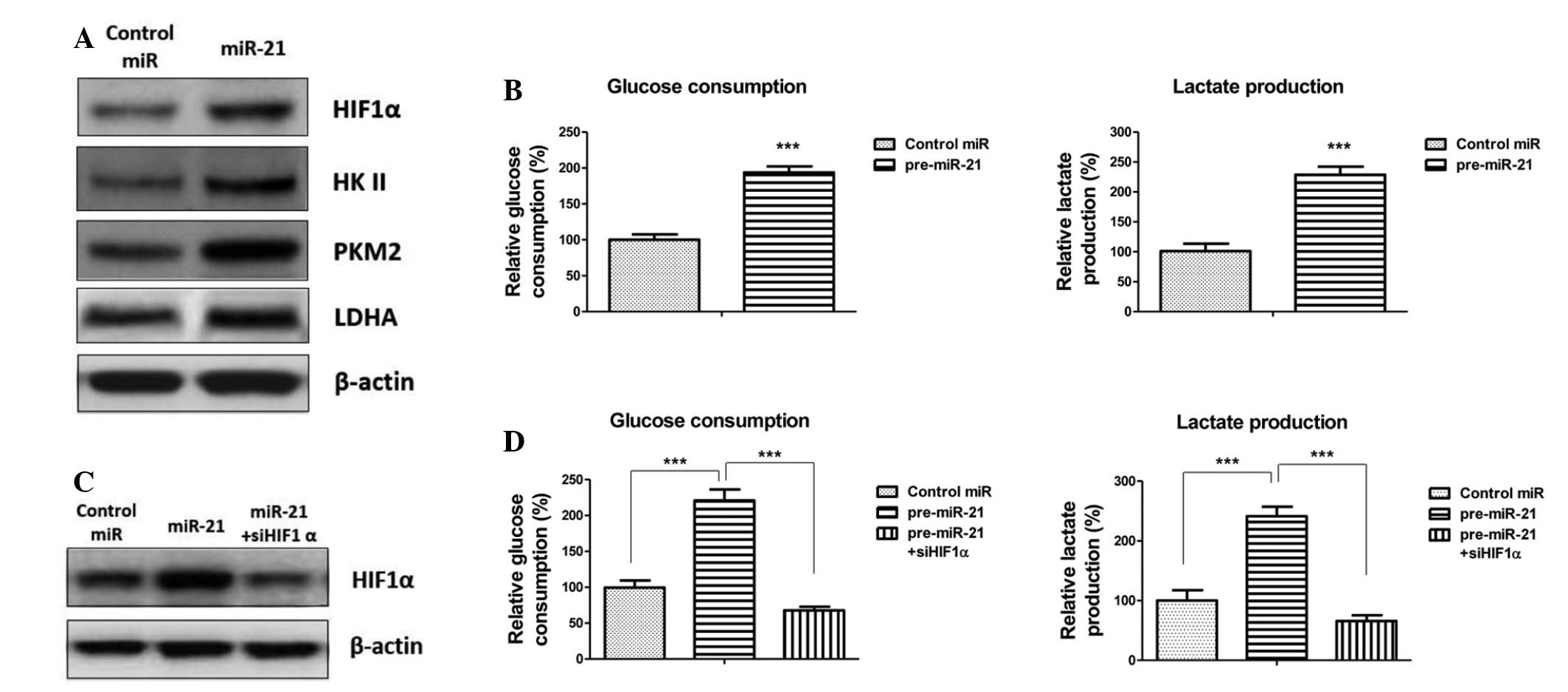
miR-21 promotes glycolysis via
upregulation of HIF1α
To further explore the mechanisms underlying the
miR-21-mediated transcriptional regulation of the key enzymes of
glycolysis in radiation-resistant cells, a literature search was
conducted, focusing on HIF1α, which is a heterodimeric
transcription factor induced by hypoxia, growth factors and
oncogenes. It has previously been reported that under hypoxic
conditions HIF1α may be activated and stimulate the transcription
of downstream target genes, including LDHA and HKII (23). In the present study, it was
hypothesized that HIF1α may be activated in radioresistant cells,
and have an important role in radioresistance by promoting the
expression of downstream glycolytic enzymes. To determine whether
miR-21 affects HIF1α expression, the A549 radiation-sensitive and
-resistant cells were transfected with an miR-21 precursor or
control miRNA, and the expression levels of HIF1α and glycolytic
key enzymes were detected, and glucose consumption and lactate
production were measured. As hypothesized, overexpression of miR-21
significantly increased the expression levels of HIFα and the key
enzymes of glycolysis (Fig. 3A).
Consistently, glucose consumption and lactate production were
increased following miR-21 transfection (Fig. 3B). To confirm that miR-21 promotes
the glycolysis of non-small cell lung cancer cells through the
upregulation of HIF1α, HIF1α expression was silenced in the
miR-21-pretransfected cells using siRNA (Fig. 3C). Glucose consumption and lactate
production were downregulated following inhibition of HIF1α
(Fig. 3D). These results indicate
that miR-21-regulated HIF1α may be associated with the underlying
mechanism of radioresistance.
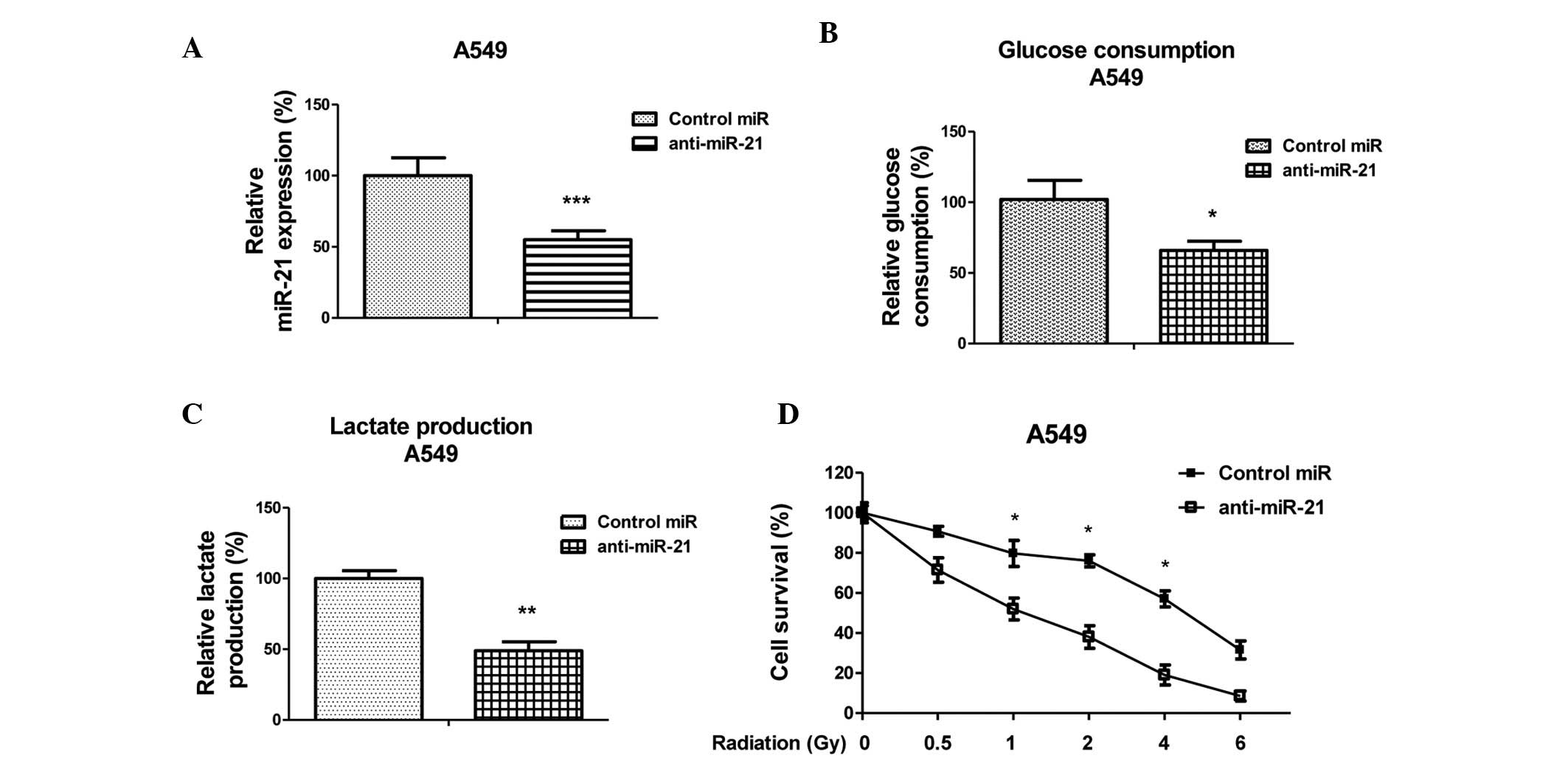
Suppression of glycolysis by inhibiting
miR-21 sensitizes lung cancer cells to radiation
To determine whether miR-21 inhibition was able to
enhance the radiosensitivity of lung cancer cells, A549 cells were
transfected with anti-sense oligonucleotides targeting miR-21 or a
scrambled miRNA control (Fig. 4A).
Glycolysis was measured 48 h post-transfection. Inhibition of
miR-21 decreased glycolysis, as detected by measuring glucose
consumption and lactate production (Fig. 4B and C). In addition, A549 cells
transfected with anti-miR-21 or control miRNA were exposed to
various doses of radiation (0, 0.5, 1, 2, 4 and 6 Gy) at a rate of
2 Gy/min, and a cell survival assay was performed. The survival
fraction of the anti-miRNA-21-transfected cells was significantly
decreased, as compared with the control group (Fig. 4D). These results indicate a
correlation between miR-21 expression and the radiosensitivity of
A549 cells to γ-radiation.
Inhibition of miR-21 resensitizes lung
cancer cells to radiation through HIF1α regulation
To further investigate whether miR-21 is capable of
modulating the sensitivity of radioresistant lung cancer cells, the
A549 radioresistant cells were transfected with anti-miR-21 or a
negative control (Fig. 5A).
Inhibition of miR-21 significantly increased the susceptibility of
the radioresistant cells to irradiation, as compared with the
negative control-transfected cells. The half maximal inhibitory
concentration (IC50) of the anti-miR-21-transfected A549
cells was 2 Gy, which was lower than the IC50 of the
negative control-transfected A549 cells, which was 15 Gy (Fig. 5B). To determine the correlation
between HIF-1α and anti-miR-21-induced sensitivity, the
anti-miR-21-transfected A549 radioresistant cells were transiently
transfected with an overexpression vector containing wild type
HIF-1α, in order to rescue its function. With the restoration of
HIF1α, the expression levels of the key enzymes of glycolysis were
rescued (Fig. 5C). In addition,
the sensitivity of HIF1α-transfected radioresistant cells to
various doses of radiation was determined. As shown in Fig. 5D HIF1α-transfected A549
radioresistant cells regained resistance to radiation. These
results suggest that inhibition of miR-21 modulates the sensitivity
of A549 radioresistant cells to irradiation via the downregulation
of HIF1α.
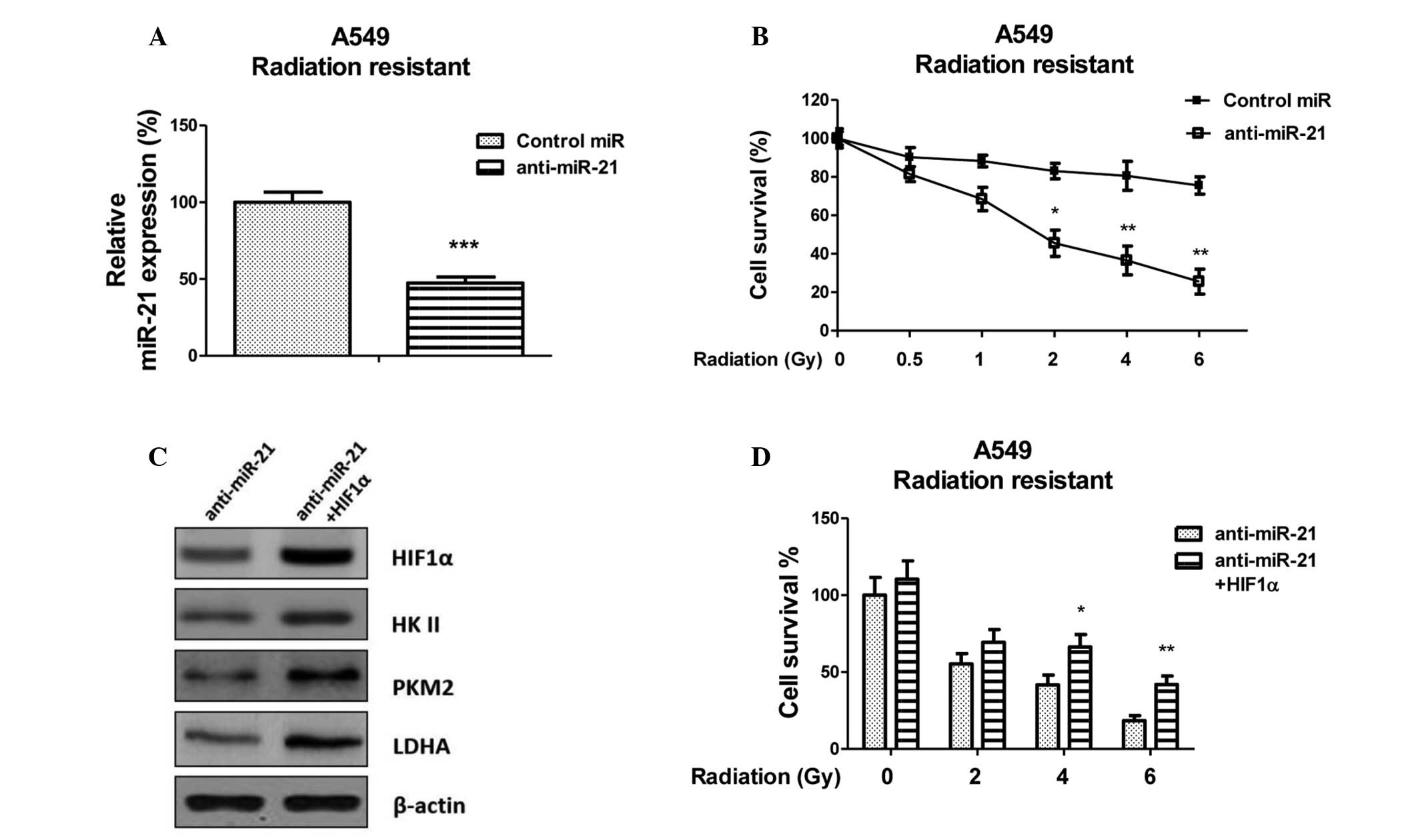 | Figure 5Resensitization of radioresistant A549
cells via the inhibition of microRNA (miR)-21-modulated
hypoxia-inducible factor (HIF)1α. (A) Expression levels of miR-21
in A549 radioresistant cells transfected with control miR or
antisense (anti)-miR-21.***P<0.001 versus the
control. (B) A549 radioresistant cells were transiently transfected
with control miRNA or anti-miR-21 for 48 h, followed by irradiation
(0, 0.5, 1, 2, 4 and 6 Gy; dose rate, 2 Gy/min), and a cell
survival assay was performed. *P<0.05 and
**P<0.01 versus the control. (C) Protein expression
of HIF1α, hexokinase (HK)II, pyruvate kinase (PK)M2 and lactate
dehydrogenase (LDH)A was rescued following the overexpression of
HIF1α in anti-miR-21-transfected cells, as detected by western
blotting. β-actin was used as a loading control. (D) A549
radioresistant cells were transiently transfected with anti-miR-21
or anti-miR-21 plus HIF1α for 48 h, followed by irradiation (0, 2,
4 and 6 Gy; dose rate, 2 Gy/min), and a cell survival assay was
performed. Data are presented as the mean ± standard error.
*P<0.05 and **P<0.01 versus
anti-miR-21. |
Discussion
The present study demonstrated the importance of
miR-21-mediated enhanced aerobic glycolysis on acquired
radioresistance in non-small cell lung cancer cells. Cancer cells
use anaerobic glycolysis to assist in their growth, and a shift
toward anaerobic glycolysis occurs with active suppression of
oxidative phosphorylation, which results from aberrant regulation
of glycolytic enzymes. The present study demonstrated that
glycolysis was upregulated in radioresistant cancer cells,
suggesting that specific inhibition of glycolysis may contribute to
overcoming radioresistance. It has previously been reported that
ionizing radiation and various chemotherapeutic drugs induce
oxidative stress in targeted cells, leading to genomic instability
and lipid peroxidation (24). In
addition, excessive lactate production in cancer cells via
glycolysis has been reported to act as an antioxidant (25). Therefore, an accumulation of
lactate in the tumor microenvironment may jeopardize the
sensitivity of cancer cells to radiation and cause
chemoresistance.
Upregulation of miR-21 has been detected in numerous
types of human cancer. It has previously been reported that miR-21
expression is higher in lung cancer serum samples compared with
normal tissues, and high serum miR-21 levels are significantly
correlated with lung tumors (19).
Furthermore, miR-21 is involved in chemoresistance and
radioresistance. A previous study demonstrated that miR-21 was
upregulated in human liver cancer following exposure to radiation
(26). In human ovarian cancer
cells, miR-21 has been show to modulate paclitaxel sensitivity
(16). The present study detected
miR-21 expression in radiation-resistant and radiation-sensitive
non-small cell lung cancer cells following exposure to
γ-irradiation. The results demonstrated that miR-21 was upregulated
in radioresistant non-small cell lung cancer cells, and inhibition
of miR-21 led to resensitization of the radioresistant cells to
irradiation, which was consistent with a previous study (19). These data support the hypothesis
that miR-21 is not only associated with oncogenesis, but can act as
a radioresistant miRNA, which may be considered a therapeutic
target for the development of anticancer drugs.
The putative target of miR-21 was screened and HIF1α
was demonstrated to be a potential target. HIF1α is a
multifunctional transcription factor that has been shown to
regulate glucose metabolism in a growth factor-dependent manner
(23). Previous studies have
demonstrated that HIF1α may contribute to the development of
radioresistance (27). As HIF1α is
an upstream signaling molecule of glycolysis, upregulation of HIF1α
promotes glycolysis (23). The
results of the present study revealed that HIF1α was upregulated by
miR-21, resulting in the promotion of the key enzymes of
glycolysis. Inhibition of HIF1α by siRNA suppressed glycolysis and
resensitized the cancer cells to radiation, thus suggesting that
miR-21-induced radioresistance was mediated through the
upregulation of HIF1α. However, the detailed mechanisms underlying
the miR-21-mediated regulation of HIF1α remain unclear. In our next
project, we aim to investigate this phenotype, and to determine by
which pathway miR-21 regulates HIF1α.
In conclusion, the present study detected a strong
correlation between the upregulation of miR-21 and radio-resistance
in non-small cell lung cancer cells. In addition, overexpression of
miR-21 activated HIF1α, which stimulated the expression of
glycolytic enzymes. Notably, inhibition of miR-21 resensitized
radioresistant cancer cells, via the suppression of HIF1α-mediated
glycolysis. The present study may provide evidence regarding the
targeting of miRNAs for the resensitization of radiation-resistant
cancer cells.
Acknowledgments
The authors of the present study would like to thank
the staff and faculty working in the Department of Radiation
Oncology, Shandong Cancer Hospital and Institute (Jinan, China),
and Dr. Hongjiang Yan for editorial assistance. The present study
was supported by a grant from the Science and Technology Department
of Shandong Province of China (grant no. 2012YD18087).
References
|
1
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brodersen P and Voinnet O: Revisiting the
principles of microRNA target recognition and mode of action. Nat
Rev Mol Cell Biol. 10:141–148. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sicard F, Gayral M, Lulka H, Buscail L and
Cordelier P: Targeting miR-21 for the therapy of pancreatic cancer.
Mol Ther. 21:986–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma Y, Xia H, Liu Y and Li M: Silencing
miR-21 sensitizes non-small cell lung cancer A549 cells to ionizing
radiation through inhibition of PI3K/Akt. Biomed Res Int.
2014:6178682014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pineau P, Volinia S, McJunkin K, Marchio
A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM and
Dejean A: miR-221 overexpression contributes to liver
tumorigenesis. Proc Natl Acad Sci USA. 107:264–269. 2010.
View Article : Google Scholar :
|
|
9
|
Chan SH, Wu CW, Li AF, Chi CW and Lin WC:
miR-21 microRNA expression in human gastric carcinomas and its
clinical association. Anticancer Res. 28:907–911. 2008.PubMed/NCBI
|
|
10
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao T and Lin Z: MiR-21 is involved in
cervical squamous cell tumorigenesis and regulates CCL20. Biochim
Biophys Acta. 1822:248–260. 2012. View Article : Google Scholar
|
|
12
|
Kanaan Z, Rai SN, Eichenberger MR, Roberts
H, Keskey B, Pan J and Galandiuk S: Plasma miR-21: A potential
diagnostic marker of colorectal cancer. Ann Surg. 256:544–551.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu C, Dong W, Wang Z, Li H, Qin Q and Li
B: The expression of miR-21 and miR-375 predict prognosis of
esophageal cancer. Biochem Biophys Res Commun. 446:1197–1203. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Folini M, Gandellini P, Longoni N, Profumo
V, Callari M, Pennati M, Colecchia M, Supino R, Veneroni S,
Salvioni R, et al: miR-21: An oncomir on strike in prostate cancer.
Mol Cancer. 9:122010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Z, Cao L and Zhang J: miR-21 modulates
paclitaxel sensitivity and hypoxia-inducible factor-1α expression
in human ovarian cancer cells. Oncol Lett. 6:795–800.
2013.PubMed/NCBI
|
|
17
|
Fang J, Zhou SH, Fan J and Yan SX: Roles
of glucose transporter-1 and the phosphatidylinositol
3-kinase/protein kinase B pathway in cancer radioresistance
(review). Mol Med Rep. 11:1573–1581. 2015.
|
|
18
|
Shimura T, Noma N, Sano Y, Ochiai Y,
Oikawa T, Fukumoto M and Kunugita N: AKT-mediated enhanced aerobic
glycolysis causes acquired radioresistance by human tumor cells.
Radiother Oncol. 112:302–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang XC, Wang W, Zhang ZB, Zhao J, Tan XG
and Luo JC: Overexpression of miRNA-21 promotes
radiation-resistance of non-small cell lung cancer. Radiat Oncol.
8:1462013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ganapathy-Kanniappan S and Geschwind JF:
Tumor glycolysis as a target for cancer therapy: Progress and
prospects. Mol Cancer. 12:1522013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Young A, Berry R, Holloway AF, Blackburn
NB, Dickinson JL, Skala M, Phillips JL and Brettingham-Moore KH:
RNA-seq profiling of a radiation-resistant and radiation sensitive
prostate cancer cell line highlights opposing regulation of DNA
repair and targets for radiosensitization. BMC Cancer. 14:8082014.
View Article : Google Scholar
|
|
23
|
Lu H, Forbes RA and Verma A:
Hypoxia-inducible factor 1 activation by aerobic glycolysis
implicates the Warburg effect in carcinogenesis. J Biol Chem.
277:23111–23115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Azzam EI, Jay-Gerin JP and Pain D:
Ionizing radiation-induced metabolic oxidative stress and prolonged
cell injury. Cancer Lett. 327:48–60. 2012. View Article : Google Scholar
|
|
25
|
Le A, Cooper CR, Gouw AM, Dinavahi R,
Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL and Dang
CV: Inhibition of lactate dehydrogenase A induces oxidative stress
and inhibits tumor progression. Proc Natl Acad Sci USA.
107:2037–2042. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang G, Li G, Wang Y, Lei W and Xiao Z:
Aberrant miRNA expression response to UV irradiation in human liver
cancer cells. Mol Med Rep. 9:904–910. 2014.PubMed/NCBI
|
|
27
|
Harada H, Inoue M, Itasaka S, Hirota K,
Morinibu A, Shinomiya K, Zeng L, Ou G, Zhu Y, Yoshimura M, et al:
Cancer cells that survive radiation therapy acquire HIF-1 activity
and translocate towards tumour blood vessels. Nat Commun.
3:7832012. View Article : Google Scholar : PubMed/NCBI
|