Introduction
Skeletal sensory neurons form the source of a
network innervating cancellous bone. They produce various
neurotransmitters, including calcitonin gene-related peptide
(CGRP), somatostatin and substance P (1,2).
CGRP is a neuropeptide produced in specific neurons by alternative
splicing of the primary transcript from the calcitonin gene and has
important functions in numerous physiological and pathological
processes. The CGRP receptor complex, which is expressed in
osteoblasts, is a dimeric complex of the G protein-coupled
calcitonin receptor-like receptor (CRL) and G protein-coupled
activity modifying protein 1 (RAMP1), a receptor activity-modifying
protein, which are required for physiological activation by CGRP.
CRL and RAMP1 receptors are expressed in mature osteoblasts
(3–10). Numerous studies have demonstrated
that CGRP innervation is associated with bone formation in
vivo and that CGRP stimulates the differentiation of bone
marrow stromal stem cells (BMSCs) into osteoblasts in vitro
(2,11–14).
Further studies supported the bone-building action of CGRP by
demonstrating that transgenic mice show increased bone formation
and trabecular bone mass following overexpression of CGRP in their
osteoblasts, while CGRP-deficient mice displayed a decreased bone
formation rate and accelerated bone loss (4,15,16).
These studies suggested that CGRP has an important role in
maintaining bone formation in skeletal tissues; however, its
mechanism of action in osteoblastogenesis and osteoblasts has
largely remained elusive.
Canonical Wnt signaling is one of three independent
Wnt pathways activated by a receptor complex of Frizzled (Fz),
which is referred to as the Wnt/β-catenin signaling pathway. The
regulation of cytoplasmic β-catenin is a key step in numerous
cellular signal transductions (17,18).
In the Wnt/β-catenin signaling pathway, the receptors binding to
canonical Wnts include 7-transmembrane domain-spanned Fz receptor
and low-density lipoprotein 5 and -6 (LRP5/6) co-receptors
(19–21). The scaffolding protein Dishevelled
interacts with the destruction complex consisting of the scaffold
protein Axin, which binds two other key components, adenomatous
polyposis coli and glycogen synthase kinase-3, leading to the
dephosphorylation of β-catenin and subsequent translocation into
the nucleus (22–25). Accumulation of β-catenin in the
cytoplasm and nuclear localization are crucial for the activation
of the Wnt pathway. Transcription factors binding with the
β-catenin protein and activating Wnt-associated genes include
cyclin D1 and c-myc (26).
Secreted Fz-related protein (sFRP), which antagonizes the
interactions between Wnts and frizzled receptors, can inhibit the
Wnt/β-catenin signaling pathway (27). Over the past few years, the
Wnt/β-catenin-signaling pathway has been shown to be an important
regulatory factor in bone metabolism (21,28–30);
however, the involvement of the canonical Wnt/β-catenin signaling
pathway in CGRP-mediated osteogenic processes has remained to be
demonstrated, which was the purpose of the present study.
Materials and methods
Isolation of BMSCs
The study was approved by the ethics committee of
the Laboratory Animal Center of the Fourth Military Medical
University (Xi'an, China). Rats were supplied by the Laboratory
Animal Center of the Fourth Military Medical University, and
sacrificed by CO2 asphyxiation. Rat BMSCs were isolated
from the bone marrow of male rats (n=8; age, 6 weeks; weight,
80–100 g), which was obtained by flushing the femoral and tibial
medullary cavities with ice-cold low-glucose Dulbecco's modified
Eagle's medium (L-DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco). The marrow cell suspension was repeatedly aspirated through
a 22-gauge needle and filtered through a 100-µm cell
strainer prior to culture (both purchased from Corning, Inc., New
York, NY, USA). Marrow cells were plated in 25-cm2
tissue culture flasks at a density of
1×106−1×107 cells/ml. The cultures were
incubated at 37°C in a humidified atmosphere containing 5%
CO2. The media was comprised of L-DMEM supplemented with
10% FBS, 100 IU/ml penicillin, 100 µg/ml streptomycin and 1
µg/ml amphotericin, and was replaced every two days.
Monolayer culture
When the cell confluence reached 80%, cells were
passaged by detachment with 0.02% trypsin (Gibco) and subcultured
in a fresh flask at a ratio of 1:2. The cells were cultured in
flasks and three passages of monolayer cells were divided into
24-well culture plates. At passage 3, the cells were divided into
two groups: The osteogenic induction group, which was further
cultured in differentiation medium (L-DMEM containing 10 mM
β-glycerophosphate, 100 nm dexamethasone and 50 µg/ml
ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA) to induce
osteogenic differentiation (31),
and the control group, which was cultured in normal medium. To
detect the expression of CGRP receptors in the process of
differentiation, the cells in the induction group were further
divided into four groups, which were then treated with various
concentrations of CGRP (0, 10−12, 10−10 and
10−8 M) to determine the optimal concentration to
promote cellular differentiation. In another experiment, cells from
the induction group were divided into five groups to determine the
effects of CGRP receptor antagonist and sFRP on the differentiation
of BMSCs: i) The control group was treated with phosphate-buffered
saline; ii) cells were incubated with 20 mM LiCl (Sigma-Aldrich);
iii) cells were incubated with CGRP (10−8 M); iv) cells
were incubated with a mixture of CGRP (10−8 M) and
10−6 M CGRP receptor antagonist CGRP8-37
(Sigma-Aldrich); v) cells were incubated with a mixture of CGRP
(10−8 M) and 10 µg/ml sFRP (R&D Systems, Inc.
Minneapolis, MN, USA).
Alizarin red staining
Alizarin red staining was performed to identify
osteoblasts. After three passages of monolayer culture, cells were
cultured in differentiation medium for 14 days and then fixed in 4%
paraformaldehyde (Sigma-Aldrich) for 30 min, followed by three
washes with ice-cold phosphate-buffered saline and staining for 5
min with alizarin red (Sigma-Aldrich). The cells were viewed using
a 450 fluorescent inverted phase contrast microscope (Nikon
Corporation, Tokyo, Japan).
Reverse-transcription quantitative
polymerase chain reaction (RTqPCR)
To assess the expression of various genes in BMSCs
in the experimental groups, total RNA was isolated from the cells
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and cDNA
synthesis was performed using Oligo dT (Invitrogen). The qPCR assay
was performed using SYBER Green (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with the primers (Shanghai GenePharma Co., Ltd.,
Shanghai, China) designed with Primer Express software (version
3.0; Applied Biosystems), listed in Table I. The amplification conditions were
as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 15
sec and 60°C for 30 sec. Thermal cycling and fluorescence detection
were performed using the StepOnePlus™ Real-Time PCR System (Applied
Biosystems). The mRNA levels of alkaline phosphatase, collagen type
I, osteocalcin, Runx2, c-myc, cyclin D1, Lef1, Tcf7 and β-catenin
mRNA were calculated relative to those of GAPDH using the ΔΔCt
method (32). Experiments were
performed in triplicate.
 | Table ISequences of the primers used for
polymerase chain reaction. |
Table I
Sequences of the primers used for
polymerase chain reaction.
| Gene | Sequence
(5′–3′) | Predicted length
(bp) |
|---|
| RAMP1 | F:
ACGTGAAGAGGGTGCTGTCT | 235 |
| R:
CACCCCAAAGTGCTTTGATT | |
| ALP | F:
CCTTGAAAAATGCCCTGAAA | 191 |
| R:
CTTGGAGAGAGCCACAAAGG | |
| OCN | F:
CATGAGGACCCTCTCTCTGC | 153 |
| R:
AGGTAGCGCCGGAGTCTATT | |
| COL1 | F:
TGGTCCTCAAGGTTTCCAAG | 123 |
| R:
TTACCAGCTTCCCCATCATC | |
| RUNX2 | F:
GAGCTACGAAATGCCTCTGC | 173 |
| R:
GGACCGTCCACTGTCACTTT | |
| CCND1 | F:
GCGTACCCTGACACCAATCT | 180 |
| R:
CTCTTCGCACTTCTGCTCCT | |
| C-MYC | F:
GCTCCTCGCGTTATTTGAAG | 152 |
| R:
TTCTCTTCCTCGTCGCAGAT | |
| β-CATENIN | F:
CTCCCCTGACAGAGTTGCTC | 187 |
| R:
ATGTCCAGTCCGAGATCAGC | |
| TCF7 | F:
GCACGGGATAACTACGGAAA | 99 |
| R:
AAAGCGAGCACGACATTTCT | |
| LEF1 | F:
TAACAAGGGCCCCTCCTACT | 198 |
| R:
CCTGGAGAAAAGTGCTCGTC | |
| GAPDH | F:
ATTGTCAGCAATGCATCCTG | 102 |
| R:
ATGGACTGTGGTCATGAGCC5 | |
Protein extraction and western blot
analysis
Following 7 and 14 days of treatment, the cells in
the experimental groups were subjected to protein extraction with
lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA)
and protein extracts were dissolved in sample buffer (Cell
Signaling Technology, Inc.) containing 2% sodium dodecyl sulfate
(SDS), 50 mM Tris-HCl, 100 mM dithiothreitol (pH 6.80) and 10%
glycerol. Protein samples (30 µg) were separated by 10%
SDS-polyacrylamide gel (Beyotime Institute of Biotechnology,
Haimen, China) electrophoresis and electrotransferred onto a
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). After
blocking with 5% non-fat milk in Tris-buffered saline solution
(Beyotime Institute of Biotechnology) for 1 h at room temperature,
blots were subsequently incubated with polyclonal goat anti-CRL
(1:5,000; sc-18007), polyclonal rabbit anti-RAMP1 (1:5,000;
sc-11379) and monoclonal mouse anti-β-catenin antibodies (1:5,000;
sc-53483) overnight at 4°C (all purchased from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Following washing for 5 min
3 times with Tris-buffered saline, horseradish peroxidase
(HRP)-conjugated polyclonal rabbit anti-mouse IgG (1:5,000;
sc-358920), HRP-conjugated polyclonal goat anti-rabbit IgG
(1:5,000; sc-2004) and HRP-conjugated polyclonal mouse anti-goat
IgG (1:5,000; sc-2345) (all purchased from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) were incubated with the
membranes for 1 h at room temperature. An Imagestation 2000 MM
(Eastman Kodak, Rochester, NY, USA) was then used for capturing
images of the blots. Blots were stripped and re-probed with
polyclonal goat anti-actin (1:500; sc-1616) and polyclonal rabbit
anti-α-tubulin (1:500; sc-5546) (both purchased from Santa Cruz
Biotechnology, Inc.) antibodies to demonstrate equal loading and
for normalization of the protein content among the groups.
Densitometric analysis of the bands was performed using Molecular
Imaging Software Version 4.0 (Eastman Kodak). Protein concentration
was determined using an Immobilon Western Chemiluminescent HRP
Substrate for enhanced chemiluminescence (EMD Millipore, Billerica,
MA, USA).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analyses were performed using SPSS software
version 13.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance was used to compare mean values between groups.
Comparisons among groups were performed using Dunnett's two-tailed
t post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Morphological changes of BMSCs
differentiating into osteoblasts
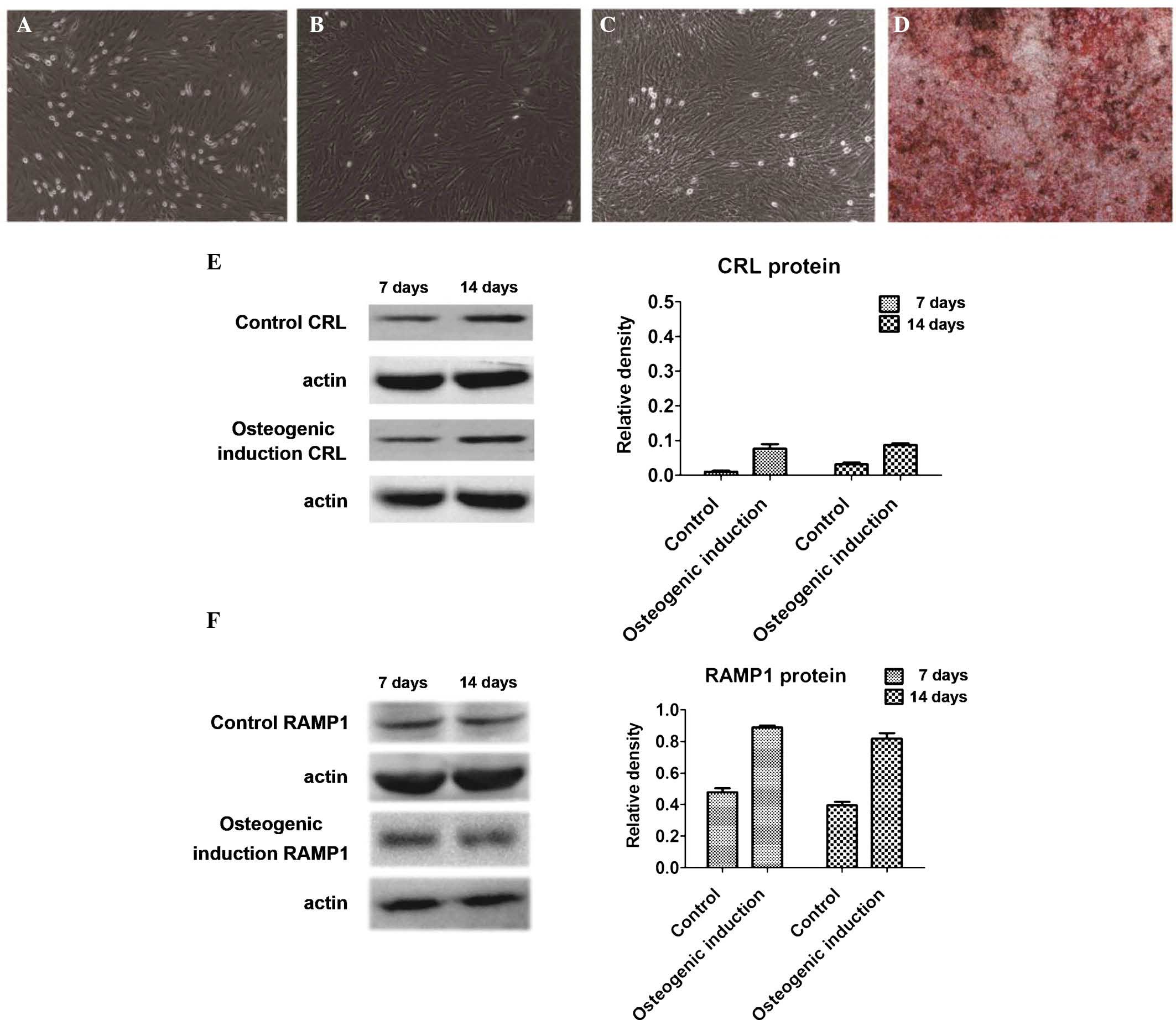
Following seven days of primary culture, BMSCs grew
in spindle-like, triangular and polygonal shapes (Fig. 1A). At passage 3 and beyond, cell
growth was accelerated (Fig. 1B).
After seven days of differentiation in the osteogenic medium, the
cells grew slowly and were covered with calcium deposits (Fig. 1C). After 14 days of
differentiation, alizarin red staining revealed that the cells
displayed characteristics of osteoblasts and extracellular matrix
mineralization (Fig. 1D).
CGRP receptors are expressed during
osteoblastic differentiation of BMSCs
The expression of CRL and RAMP1 protein in BMSCs on
days 7 and 14 of culture in osteogenic medium was assessed by
western blot analysis (Fig. 1E and
F). While CRL and RAMP1 protein expression was present in the
osteogenic induction group as well as in the control group on days
7 and 14, expression levels were approximately two-fold increased
in the induction group compared with those in the control
group.
CGRP enhances the expression of
osteoblastic marker genes in induced BMSCs
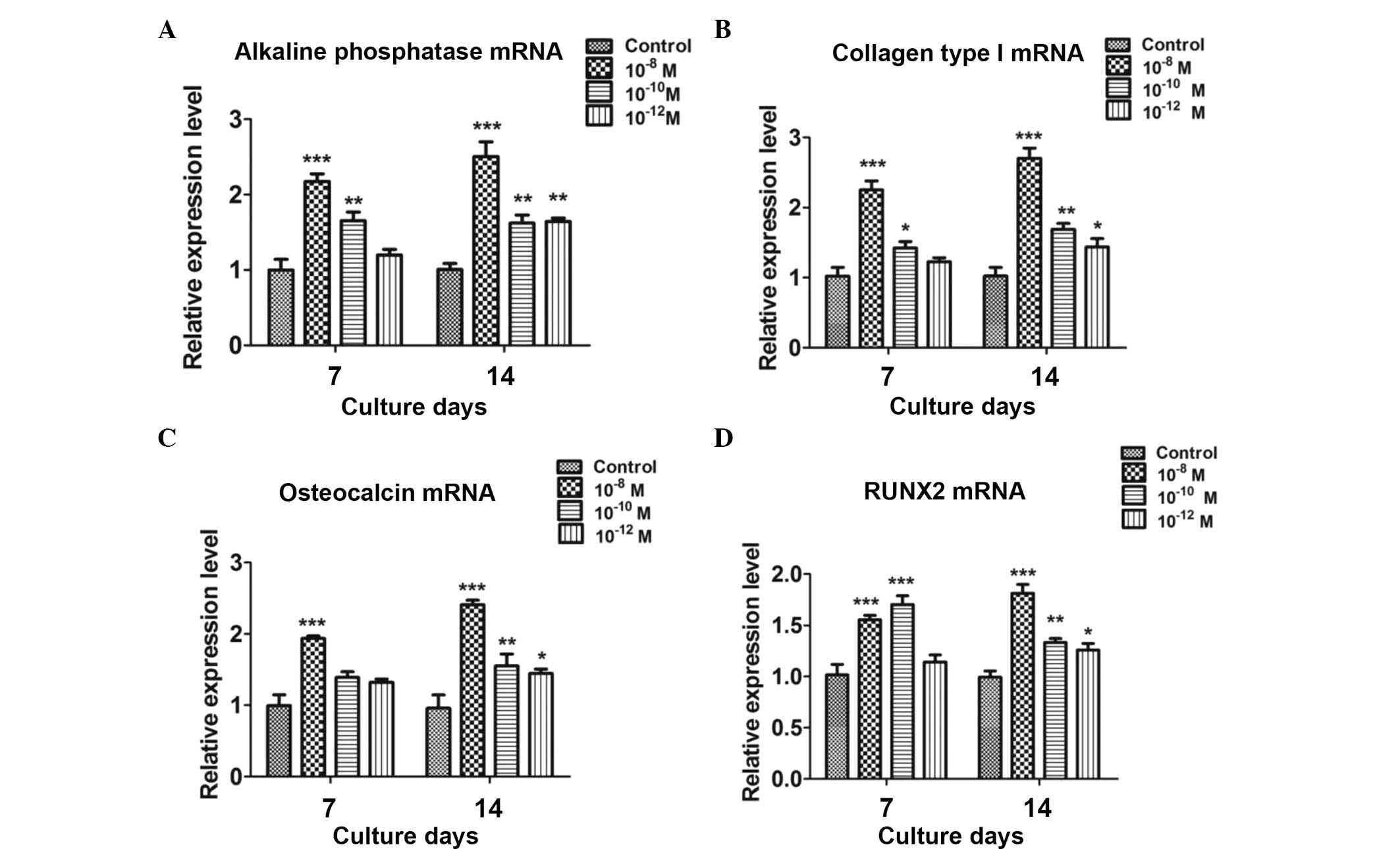
The effects of CGRP (10−8,
10−10 and 10−12 M) on the differentiation of
BMSCs were examined by assessing the mRNA expression of the early
osteoblastic markers alkaline phosphatase and collagen type I as
well as the late osteoblastic marker osteocalcin by using RT-qPCR
analysis. Runx2, a transcriptional factor necessary for osteoblast
differentiation, was also examined. Compared with the untreated
group, CGRP treatment significantly increased the levels of
osteoblastic markers at days 7 and 14, with 10−8 M CGRP
exerting a greater effect than the lower concentrations (Fig. 2).
CGRP-mediated osteoblastogenesis of BMSCs
proceeds via the Wnt/β-catenin pathway
To assess the possible involvement of the
Wnt/β-catenin pathway in BMSC differentiation into osteoclasts, the
effects of Wnt/β-catenin pathway agonist LiCl, CGRP inhibitor
CGRP8-37 and Wnt inhibitor sFRP on the expression of osteoblastic
genes in induced BMSCs were examined in the absence or presence of
CGRP. RT-qPCR revealed that CGRP (10−8 M) and LiCl alone
significantly increased the expression of osteoblastic genes. Of
note, pre-treatment with CGRP8-37 and sFRP significantly inhibited
CGRP-induced expression of the osteoblastic markers by induced
BMSCs (Fig. 3).
To further assess the involvement of the
Wnt/β-catenin signaling pathway, the mRNA expression of signaling
molecules of this pathway, including c-myc, cyclin D1, Lef1, Tcf7
and β-catenin, was evaluated by RT-qPCR at days 7 and 14.
Incubation of BMSCs with CGRP (10−8) and LiCl in the
osteoinductive medium significantly increased the mRNA expression
of c-myc, cyclin D1, Tcf7 and Lef1 on days 7 and 14, as compared
with their expression in the control group. This upregulation was
significantly inhibited by pre-treatment with CGRP8-37 or sFRP
(Fig. 4A–D). However, the
expression of β-catenin mRNA was not significantly affected in the
experimental groups (Fig. 4E).
Therefore, the protein expression of β-catenin in all experimental
groups was examined by western blot analysis. The results revealed
that CGRP (10−8 M) and LiCl increased the protein
expression of β-catenin on days 7 and 14 compared with that in the
control group. Furthermore, pre-treatment with CGRP8-37 or sFRP
inhibited the CGRP-induced increase in β-catenin protein expression
on days 7 and 14 (Fig. 4F).
Discussion
To the best of our knowledge, the present study was
the first to demonstrate the involvement of the Wnt/β-catenin
signaling pathway in CGRP-mediated osteoblastic differentiation of
BMSCs. Sharma et al reported that Rspo 1 is involved in bone
remodeling and the activation of Wnt signaling in human as well
murine in vitro osteoblast cell models (33). The present study used an agonist
and a specific inhibitor of the Wnt/β-catenin signaling pathway as
well as an inhibitor of CGRP for mechanistic gain-and
loss-of-function studies, and their effects on the expression of
osteoblastic marker genes and the expression of Wnt signaling
molecules in induced BMSCs were assessed.
CGRP acts at the cellular level by binding to its
receptor CRL, following which it is able to regulate various
biological functions, including bone remolding, pain, biological
effects of human endothelial cells, cell differentiation and
regulation of the cardiovascular system (6,34–36).
However, to the best of our knowledge, changes in CRL and RAMP1
expression during the process of differentiation of BMSCs have
remained to be fully elucidated. The present study discovered that
RAMP1 and CRL protein were overexpressed in BMSCs undergoing
osteoblastic differentiation.
The osteogenic effects of LiCl, CGRP + CGRP8-37 and
CGRP + sFRP on BMSCs have not been previously described, to the
best of our knowledge. Osteoblastic differentiation in vitro
is directed by Runx2, the master transcription factor regulating
bone formation, and BMSCs can differentiate towards the
osteoblastic lineage, accompanied by the production of type I
collagen and osteocalcin and increased alkaline phosphatase
activity (37). The present study
examined the osteoblastic differentiation of rat BMSCs and
determined whether CGRP antagonist or Wnt antagonist sFRP were able
to inhibit the inductive effects of CGRP on BMSCs.
When BMSCs were treated with CGRP (10−8,
10−10 or 10−12 M) for 7 or 14 days, the
expression of osteoblastic genes, including alkaline phosphatase,
collagen type I, Runx2, and osteocalcin, was revealed to be
induced, particularly at the highest concentration of CGRP
(10−8 M). Furthermore, the stimulatory effects of CGRP
on the osteoblastic differentiation of BMSCs was inhibited by CGRP
receptor antagonist CGRP8-37 and the specific inhibitor of the
Wnt/β-catenin signaling pathway sFRP, suggesting that CGRP binds to
CGRP receptors and activates the Wnt/β-catenin signaling pathway,
leading to the differentiation of BMSCs.
To further study the roles of the Wnt/β-catenin
signaling pathway in the stimulatory effects of CGRP on the
osteoblastic differentiation of BMSC, the effects of CGRP, CGRP8-37
and sFRP on the expression of genes involved in the Wnt/β-catenin
signaling pathway in BMSCs were also examined. CGRP was found to
significantly increase the expression of the Wnt/β-catenin
signaling molecules c-myc, cyclin D1, Tcf7 and Lef1 at the mRNA
level. β-catenin, which is crucial for the activation of
Wnt/β-catenin signaling, was obviously affected at the mRNA level;
however, differences between the experimental groups were not
statistically significant. Of note, CGRP significantly enhanced the
protein expression of β-catenin at 7 and 14 days, which was
inhibited by CGRP8-37 and sFRP. The greater effects of CGRP and the
inhibitors CGRP8-37 and sFRP on β-catenin at the protein level
compared to those at the mRNA level may indicate that the
enhancement of β-catenin signaling may be complex. For instance,
activation of β-catenin via phosphorylation rather than
upregulation of its gene expression may be involved in the
activation of the Wnt/β-catenin signaling pathway.
The role of Tcf7/Lef1 in the Wnt/β-catenin signaling
pathway is controversial. While certain studies suggested that Tcf7
acts as a repressor as well as an activator, and that Lef1 is
usually an activator but occasionally a repressor, other studies
have reported that Lef1 and Tcf7 cooperatively activate the
expression of Wnt/β-catenin signaling target genes to promote cell
proliferation in the dorsal midbrain (38–40).
The present study found that treatment of BMSCs with CGRP
(10−8 M) for 7 and 14 days led to an increased
expression of Lef1 and Tcf7. Therefore, it is indicated that Lef1
and Tcf7 may be involved in the CGRP-induced activation of
Wnt/β-catenin signaling in BMSCs.
The present study also assessed the expression of
two Wnt target genes, c-myc and cyclin D1, which were markedly
increased in BMSCs following CGRP treatment, which was
significantly reduced by pre-incubation with CGRP inhibitor,
indicating that c-myc and cyclin D1 may be involved in the process
of osteoblastic differentiation of BMSCs, which was in turn
enhanced by activation of the Wnt/β-catenin signaling pathway. All
of these results suggested that BMSC differentiation into
osteoblasts by stimulation with CGRP proceeds via the Wnt/β-catenin
signaling pathway.
In conclusion, the present study demonstrated that
the CGRP ligands CRL and RAMP1 were overexpressed throughout the
osteoblastic differentiation of BMSCs, and that their interaction
with CGRP was likely to have stimulated this differentiation
process. The canonical Wnt signaling pathway was indicated to
contribute to this process.
References
|
1
|
Bjurholm A, Kreicbergs A, Brodin E and
Schultzberg M: Substance P- and CGRP-immunoreactive nerves in bone.
Peptides. 9:165–171. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Imai S and Matsusue Y: Neuronal regulation
of bone metabolism and anabolism: Calcitonin gene-related peptide-,
substance P- and tyrosine hydroxylase-containing nerves and the
bone. Microsc Res Tech. 58:61–69. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawase T, Okuda K and Burns DM: Immature
human osteoblastic MG63 cells predominantly express a subtype
1-like CGRP receptor that inactivates extracellular signal response
kinase by a cAMP-dependent mechanism. Eur J Pharmacol. 470:125–137.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schinke T, Liese S, Priemel M, Haberland
M, Schilling AF, Catala-Lehnen P, Blicharski D, Rueger JM, Gagel
RF, Emeson RB and Amling M: Decreased bone formation and osteopenia
in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner
Res. 19:2049–2056. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Togari A, Arai M, Mizutani S, Mizutani S,
Koshihara Y and Nagatsu T: Expression of mRNAs for neuropeptide
receptors and beta-adrenergic receptors in human osteoblasts and
human osteogenic sarcoma cells. Neurosci Lett. 233:125–128. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tuo Y, Guo X, Zhang X, Wang Z, Zhou J, Xia
L, Zhang Y, Wen J and Jin D: The biological effects and mechanisms
of calcitonin gene-related peptide on human endothelial cell. J
Recept Signal Transduct Res. 33:114–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uzan B, de Vernejoul MC and Cressent M:
RAMPs and CRLR expressions in osteoblastic cells after
dexamethasone treatment. Biochem Biophys Res Commun. 321:802–808.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Villa I, Mrak E, Rubinacci A, Ravasi F and
Guidobono F: CGRP inhibits osteoprotegerin production in human
osteoblast-like cells via cAMP/PKA-dependent pathway. Am J Physiol
Cell Physiol. 291:C529–C537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wimalawansa SJ: Calcitonin gene-related
peptide and its receptors: Molecular genetics, physiology,
pathophysiology and therapeutic potentials. Endocr Rev. 17:533–585.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wimalawansa SJ: Amylin, calcitonin
gene-related peptide, calcitonin and adrenomedullin: A peptide
superfamily. Crit Rev Neurobiol. 11:167–239. 1997. View Article : Google Scholar
|
|
11
|
Fang Z, Yang Q, Xiong W, Li GH, Liao H,
Xiao J and Li F: Effect of CGRP-adenoviral vector transduction on
the osteoblastic differentiation of rat adipose-derived stem cells.
PLoS One. 8:e727382013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YS, Wang YH, Zhao GQ and Li YB:
Osteogenic potential of human calcitonin gene-related peptide alpha
gene-modified bone marrow mesenchymal stem cells. Chin Med J
(Engl). 124:3976–3981. 2011.
|
|
13
|
Xu J, Kauther MD, Hartl J and Wedemeyer C;
Study was performed at the University of Duisburg-Essen, Germany:
Effects of alpha-calcitonin gene-related peptide on osteoprotegerin
and receptor activator of nuclear factor-κB ligand expression in
MG-63 osteoblast-like cells exposed to polyethylene particles. J
Orthop Surg Res. 5:832010. View Article : Google Scholar
|
|
14
|
Yoo YM, Kwag JH, Kim KH and Kim CH:
Effects of neuropeptides and mechanical loading on bone cell
resorption in vitro. Int J Mol Sci. 15:5874–5883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ballica R, Valentijn K, Khachatryan A,
Guerder S, Kapadia S, Gundberg C, Gilligan J, Flavell RA and
Vignery A: Targeted expression of calcitonin gene-related peptide
to osteoblasts increases bone density in mice. J Bone Miner Res.
14:1067–1074. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huebner AK, Schinke T, Priemel M,
Schilling S, Schilling AF, Emeson RB, Rueger JM and Amling M:
Calcitonin deficiency in mice progressively results in high bone
turnover. J Bone Miner Res. 21:1924–1934. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SY, Kim S, Yun-Choi HS and Jho EH:
Wnt5a potentiates U46619-induced platelet aggregation via the
PI3K/Akt pathway. Mol Cells. 32:333–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng Q, Chen P, Xu Z, Li F and Yi XP:
Expression and redistribution of β-catenin in the cardiac myocytes
of left ventricle of spontaneously hypertensive rat. J Mol Histol.
44:565–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JH, Kim BG, Ahn JM, Park HJ, Park SK,
Yoo JS, Yates JR III and Cho JY: Role of PI3K on the regulation of
BMP2-induced beta-Catenin activation in human bone marrow stem
cells. Bone. 46:1522–1532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Li J and Chen B: Oct4 was a novel
target of Wnt signaling pathway. Mol Cell Biochem. 362:233–240.
2012. View Article : Google Scholar
|
|
21
|
Liu Y, Liu Y, Zhang R, Wang X, Huang F,
Yan Z, Nie M, Huang J, Wang Y, Wang Y, et al: All-trans retinoic
acid modulates bone morphogenic protein 9-induced osteogenesis and
adipogenesis of preadipocytes through BMP/Smad and Wnt/β-catenin
signaling pathways. Int J Biochem Cell Biol. 47:47–56. 2014.
View Article : Google Scholar
|
|
22
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peterson-Nedry W, Erdeniz N, Kremer S, Yu
J, Baig-Lewis S and Wehrli M: Unexpectedly robust assembly of the
Axin destruction complex regulates Wnt/Wg signaling in Drosophila
as revealed by analysis in vivo. Dev Biol. 320:226–241. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salins P, Shawesh S, He Y, Dibrov A,
Kashour T, Arthur G and Amara F: Lovastatin protects human neurons
against Abeta-induced toxicity and causes activation of
beta-catenin-TCF/LEF signaling. Neurosci Lett. 412:211–216. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun YC: Examination of effects of GSK3beta
phosphorylation, beta-catenin phosphorylation and beta-catenin
degradation on kinetics of Wnt signaling pathway using
computational method. Theor Biol Med Model. 6:132009. View Article : Google Scholar
|
|
26
|
Weng X, Lin P, Liu F, Chen J, Li H, Huang
L, Zhen C, Xu H, Liu X, Ye H and Li X: Achyranthes bidentata
polysaccharides activate the Wnt/β-catenin signaling pathway to
promote chondrocyte proliferation. Int J Mol Med. 34:1045–1050.
2014.PubMed/NCBI
|
|
27
|
Wen X, Cawthorn WP, MacDougald OA, Stupp
SI, Snead ML and Zhou Y: The influence of Leucine-rich amelogenin
peptide on MSC fate by inducing Wnt10b expression. Biomaterials.
32:6478–6486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Georgiou KR, King TJ, Scherer MA, Zhou H,
Foster BK and Xian CJ: Attenuated Wnt/β-catenin signalling mediates
methotrexate chemotherapy-induced bone loss and marrow adiposity in
rats. Bone. 50:1223–1233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo J, Liu M, Yang D, Bouxsein ML, Saito
H, Galvin RJ, Kuhstoss SA, Thomas CC, Schipani E, Baron R, et al:
Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated
stromal cell response and new bone formation. Cell Metab.
11:161–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tamura M, Sato MM and Nashimoto M:
Regulation of CXCL12 expression by canonical Wnt signaling in bone
marrow stromal cells. Int J Biochem Cell Biol. 43:760–767. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu HM, Yang L, Wang Z, Liu YW, Fan JZ, Fan
J, Liu J and Luo ZJ: Overexpression of integrin a2 promotes
osteogenic differentiation of hBMSCs from senile osteoporosis
through the ERK pathway. Int J Clin Exp Pathol. 6:841–852.
2013.PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Sharma AR, Choi BS, Park JM, Lee DH, Lee
JE, Kim HS, Yoon JK, Song DK, Nam JS and Lee SS: Rspo 1 promotes
osteoblast differentiation via Wnt signaling pathway. Indian J
Biochem Biophys. 50:19–25. 2013.PubMed/NCBI
|
|
34
|
Bloom AP, Jimenez-Andrade JM, Taylor RN,
Castañeda-Corral G, Kaczmarska MJ, Freeman KT, Coughlin KA,
Ghilardi JR, Kuskowski MA and Mantyh PW: Breast cancer-induced bone
remodeling, skeletal pain and sprouting of sensory nerve fibers. J
Pain. 12:698–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eberhardt M, Dux M, Namer B, Miljkovic J,
Cordasic N, Will C, Kichko TI, de la Roche J, Fischer M, Suárez SA,
et al: H2S and NO cooperatively regulate vascular tone by
activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat
Commun. 5:43812014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sample SJ, Hao Z, Wilson AP and Muir P:
Role of calcitonin gene-related peptide in bone repair after cyclic
fatigue loading. PLoS One. 6:e203862011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shui C, Spelsberg TC, Riggs BL and Khosla
S: Changes in Runx2/Cbfa1 expression and activity during
osteoblastic differentiation of human bone marrow stromal cells. J
Bone Miner Res. 18:213–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoppler S and Kavanagh CL: Wnt signalling:
Variety at the core. J Cell Sci. 120:385–393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee JE, Wu SF, Goering LM and Dorsky RI:
Canonical Wnt signaling through Lef1 is required for hypothalamic
neurogenesis. Development. 133:4451–4461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shimizu N, Kawakami K and Ishitani T:
Visualization and exploration of Tcf/Lef function using a highly
responsive Wnt/β-catenin signaling-reporter transgenic zebrafish.
Dev Biol. 370:71–85. 2012. View Article : Google Scholar : PubMed/NCBI
|