Introduction
Non-alcoholic fatty liver disease (NAFLD) is a type
of liver disorder characterized by the absence of a history of
excessive alcohol consumption, with steatosis and lipoidosis. NAFLD
is the most common cause of chronic liver disease in Western
countries, and is estimated to influence ~30% of the general
population (1,2). Various lifestyle-associated diseases,
including metabolic syndrome, obesity, type 2 diabetes, arterial
hypertension and hyperlipidemia have been shown to be associated
with NAFLD (3–5); however, the cellular mechanisms
underlying the development and progression of NAFLD have remained
elusive.
Research regarding the pathogenic mechanisms of
NAFLD has identified signal transduction and post-translational
protein modification, as well as transcriptional activation and
repression processes (6). Included
in the latter category, microRNAs (miRNAs) are short (~19–24
nucleotide) non-protein-coding RNAs that regulate hundreds of
targets, primarily through translational inhibition or messenger
RNA (mRNA) degradation (6,7). Consequently, miRNAs are able to
regulate various biological processes, including cell
proliferation, apoptosis and metabolism. It has previously been
suggested that miR-122 may regulate the majority of target genes
involved in lipid and cholesterol metabolism (8). Fatty acid synthase (FASN) is one of
the known target genes of miR-122 (9,10).
The process of fatty acid synthesis is highly coordinated and
involves numerous enzymes. Reduced expression of FASN may therefore
significantly reduce the levels of triglycerides in vivo,
facilitating the prevention and treatment of fatty liver disease
(9).
Pharmacological agents used in Western medicine to
treat NAFLD have a low efficacy, and lipid-regulating
pharmaceuticals are frequently used as adjunctive therapy (11,12).
Antioxidants, including vitamins B, C and E, lecithin,
thiazolidinediones and silymarin are often used to protect liver
tissue; however, these agents lack specificity in the treatment of
fatty liver diseases, and certain synthetic supplements have the
potential for liver toxicity (13,14).
Natural products, including Traditional Chinese Medicines, have
long been considered as alternative medicines. Nevertheless,
numerous formulas have been shown to possess beneficial therapeutic
effects against various diseases, including NAFLD (15).
Artemisia capillaris formula (ACF) is a
traditional Chinese formula, which has a long history of clinical
use for the treatment of NAFLD in China. Preliminary research by
our group in a rat model of NAFLD demonstrated that ACF was able to
significantly decrease the liver weight and alter the serum free
fatty acid levels of the rats, thus suggesting that ACF may be a
useful therapy for NAFLD (16).
However, the mechanisms underlying its anti-NAFLD activity remain
to be elucidated.
The present study used a rat model of diet-induced
NAFLD, as well as HepG2 human liver carcinoma cells cultured with a
high concentration of free fatty acid (HFFA) to investigate whether
miRNAs contribute to the pathogenesis of NAFLD. Polyene
phosphatidylcholine (PP) is a hepatoprotective therapeutic agent
that accelerates choleresis (17,18),
in the present study, PP served as a positive control.
Materials and methods
Preparation and content assay of ACF
ACF was provided by the Academy of Pharmacology of
Fujian Chinese Medical University (Fuzhou, China). For the animal
experiments, ACF powder was dissolved in physiological saline to a
working concentration of 500 mg/ml. For the cell-based experiments,
a stock solution of ACF (1 g/ml) was freshly prepared by dissolving
ACF powder in double distilled H2O to a concentration of
1 g/ml, which was subsequently stored at −20°C. Working
concentrations of ACF (1.25 mg/ml and 2.5 mg/ml) were prepared by
diluting the stock solution in culture medium of Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal
bovine serum (FBS) and 100 U/ml penicillin and 100 μg/ml
streptomycin (all obtained from Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Reagents
TRIzol® reagent, M-MLV First-Strand cDNA
Synthesis kit and Taq DNA Polymerase were obtained from Invitrogen
(Thermo Fisher Scientific, Inc.). Assay kits for alanine
aminotransferase (ALT), aspartate transaminase (AST), triglyceride
(TG), total cholesterol (TC), high-density lipoprotein cholesterol
(HDL-C), low-density lipoprotein cholesterol (LDL-C) activity and
oil red staining were obtained from the Jiancheng Institute of
Biotechnology (Nanjing, China). PP was obtained from the Tonghua
Dongbao Pharmaceutical Co., Ltd. (Tonghua, China). Trypsin-EDTA and
Fast SYBR® Green Master Mix were purchased from Thermo
Fisher Scientific, Inc.. Rabbit polyclonal FASN (cat. no. 3180;
1:1,000) and β-actin (cat. no. 4970; 1:2,000) primary antibodies,
and horseradish peroxidase (HRP)-conjugated secondary antibodies
(cat. no. 7074) were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). All other chemicals, unless otherwise stated,
were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animal model and treatment protocols
Male 8-week-old Sprague-Dawley (SD) rats (Slike Co.,
Ltd., Shanghai, China), weighing 200±10 g, were housed five per
cage in an environmentally controlled room (22±1°C) with 40–60%
relative humidity. Air exchange occurred 12–18 times/h and a 12:12
h artificial light/dark cycle of 150–300 lux was maintained. The
rats were given ad libitum access to food and water. All
experiments using animals were approved by the Animal Ethics
Committee of the Fujian Institute of Traditional Chinese Medicine
(Fuzhou, China). Experimental procedures were conducted in
accordance with the Guidelines for Animal Experimentation of Fujian
University of Traditional Chinese Medicine.
To establish the animal model, 72 rats were randomly
divided into six groups (12 rats/group), with one group of rats
used as a control and fed a normal diet that consisted of ≥18%
total protein, ≥5% total fat, ≤5% fiber and ≤8% crude ash. The
remaining rats were allowed ad libitum access to a high-fat
diet (HFD) for eight weeks. The HFD recipe conformed to NAFLD
models in SD rats (19,20), and consisted of: 87.3% basal
fodder, 10% lard, 2% cholesterol and 0.7% swine bile salt.
Following an eight-week feeding period, the HFD-fed rats were
randomly divided into five groups: The model group (model),
PP-treated group (0.076 g/kg body weight/day), ACF high-dose group
(1.848 g/kg body weight/day), ACF middle-dose group (0.924 g/kg
body weight/day) and ACF low-dose group (0.462 g/kg body
weight/day) (12 rats/group). ACF and PP were administered as
previously described (21). The
control and model groups received equal volumes of distilled water
(<5 ml). The body weight and food uptake of the rats was
recorded weekly. Following four weeks of treatment, the rats were
subjected to 4 h food depravation and subsequently sacrificed by
administration of 45 mg/kg pentobarbital. Blood samples were
collected from the aorta abdominalis for the AST, ALT, TG, TC,
HDL-C and LDL-C assays. The samples were incubated at room
temperature for 2 h and centrifuged at 3,000 × g at 4°C for 20 min
to separate the serum, which was collected and stored at -20°C.
Livers were rapidly dissected, and a section of each liver was cut
and fixed in formaldehyde saline (4%) solution for histological
analysis; the remaining tissue was snap frozen in liquid nitrogen
and stored at −80°C prior to use.
Histological examination
The liver tissue for histological evaluation was
immediately fixed in 10% buffered formalin for pathological
analysis. Formalin-fixed liver tissue was paraffin-embedded and
4–5-mm sections were prepared and subsequently stained with
hematoxylin and eosin. Histological evaluation was performed twice
by a pathologist blinded to the treatments on two separate
occasions. A semi-quantitative scoring system was used to assess
the severity of hepatic steatosis, and the inflammatory cell
infiltration in 10 different fields per section (magnification,
×100; DMI 6000; Leica Microsystems GmbH, Wetzlar, Germany)
(22). Briefly, the following
criteria were used for scoring hepatic steatosis: Grade 0 (−), no
fat; grade 1 (+), fatty hepatocytes occupying <33% of the
hepatic parenchyma; grade 2 (++), fatty hepato-cytes occupying
33–66% of the hepatic parenchyma; grade 3 (+++), fatty hepatocytes
occupying >66% of the hepatic parenchyma (19).
Biochemical assays
Serum was separated by centrifugation at 3,000 × g
for 30 min and analyzed immediately. Serum AST, ALT, TG, TC, HDL-C
and LDL-C levels were determined by spectrophotometry (BA-88A;
Mindray Bio-Medical Electronics Co., Ltd., Shenzen, China). The
serum sample (2.5 μl) was mixed with 80 μl of
solution R1 from commercially available kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). This was incubated for 5
min at 37°C and absorbance was measured (A1) at a wavelength of 546
nm. Solution R2 (60 μl) was added to the tube and incubated
for 5 min at 37°C and absorbance was measured (A2) at a wavelength
of 546 nm. The levels in the serum (mmol/l) were calculated using
an equation: [(Sample A2 − sample A1) − (blank A2 − blank A1) /
[(standard A2 − standard A1) − (blank A2 − blank A1)] × calibration
sample value. The activity of AST and ALT (U/l) were calculated
using a standard curve.
Immunohistochemical staining
Following fixation with 10% buffered formalin for 12
h, the liver samples were conventionally processed, in order to
prepare paraffin-embedded liver section slides. The slides were
incubated with antigen retrieval solution (10 mM sodium citrate; pH
6.0) at 95°C for 10 min, rinsed in cold water for 10 min and the
endogenous peroxidase activity was quenched using hydrogen
peroxide. Following blocking for non-specific proteins using normal
serum in phosphate-buffered saline (PBS; 0.1% Tween-20), the slides
were incubated with rabbit polyclonal antibodies targeting FASN
(1:200 dilution) at 4°C overnight. The slides were then washed with
PBS three times each for 5 min, and incubated with a biotinylated
secondary antibody for 30 min at room temperature. Subsequently,
the slides were incubated with horseradish peroxidase-conjugated
streptavidin at 37°C for 20 min (Dako, Glostrup, Denmark), and
washed with PBS three times each for 5 min. The slides were then
incubated with diaminobenzidine for 30–60 sec at room temperature
as the chromogen, followed by further counterstaining with diluted
Harris hematoxylin for 20–30 sec at room temperature. Following
staining, five high-power fields (magnification, ×400; DMB0004 LED;
Leica Microsystems GmbH) were randomly selected from each slide,
and the average proportion of positive cells in each field was
counted using a true color multi-functional cell image analysis
management system (Image-Pro Plus 6.0; Media Cybernetics,
Rockville, MD, USA). To rule out any nonspecific staining, PBS was
used instead of the primary antibody as a negative control.
Cell culture
HepG2 human liver carcinoma cells were obtained from
the Cell Bank of Chinese Academy of Science (Shanghai, China). The
cells were cultured in DMEM supplemented with 10% (v/v) FBS, 100
U/ml penicillin and 100 μg/ml streptomycin at 37°C in a
humidified incubator containing 5% CO2.
To induce cellular fat overloading, the following
steps were performed in accordance with previously established
methods (23,24). Briefly, stock solutions of 0.6
mol/l oleic acid (OA) and 0.6 mol/l palm acid (PA) were prepared in
dimethyl sulfoxide. Subsequently, HepG2 cells at 75% confluence
were exposed to a mixture of OA and PA at a final ratio of 2:1, and
final concentration of 1 mmol/l in complete DMEM for 24 h at 37°C
(24,25). For ACF treatment, the cells were
treated with ACF (1.25 and 2.5 mg/ml) for 24 h at 37°C in 5%
CO2. The induction parameters were optimized in a
preliminary test, in order to achieve maximal fat overaccumulation
with minimal cytotoxic and apoptotic effects of HFFA.
Oil red O staining
HepG2 cells were seeded into 12-well plates at a
density of 6×104 cells/well in 1 ml complete DMEM and
incubated overnight at 37°C in 5% CO2. Once the
steatotic cell model had been established for 24 h, the cells were
treated with ACF (1.25 and 2.5 mg/ml) for 24 h at 37°C in 5%
CO2, and the lipid content in HepG2 cells was
subsequently determined using an oil red O staining kit, according
to the manufacturer's instructions. Briefly, at the end of
treatment, the cells were fixed with 4% polyoxymethylene for 10
min, incubated in oil red solution for 15 min in the dark at room
temperature and then incubated with Gill's or Mayer's hematoxylin
for 30 sec at room temperature. Images of stained tissue were
recorded using a phase-contrast microscope (DMI 6000; Leica
Microsystems GmbH). Images were captured at a magnification of
×400.
TC and TG analysis
HepG2 cells were seeded into six-well plates at a
density of 1.5×105 cells/well in 2 ml complete DMEM and
incubated overnight at 37°C in CO2. Once the steatotic
cell model had been established for 24h, the cells were treated
with various concentrations of ACF (1.25 and 2.5 mg/ml) for 24 h at
37°C in 5% CO2. The cells were collected via scraping in
PBS and disrupted by sonication (Sonics & Materials, Inc.,
Newtown, CT, USA), followed by centrifugation at 3,000 × g for 5
min. Subsequently, the supernatants were collected and the TG
content was measured using a commercial kit, based on the
phosphoglycerol oxidase/peroxidase enzymatic reaction according to
the manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the liver tissue and
HepG2 cells using TRIzol® reagent. Oligo(dT)-primed RNA
(1 μg) was reverse-transcribed using SuperScript II Reverse
Transcriptase, according to the manufacturer's instructions. The
obtained cDNA was used to determine the relative mRNA expression
levels of precursor miR-122, FASN and GAPDH by RT-qPCR. RT-qPCR was
performed using SYBR Green I Master mix in an ABI 7500 Fast
instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.)
under conditions as follows: Initial denaturation at 95°C for 20
sec; and 40 cycles of denaturation at 95°C for 3 sec and 60°C for
40 sec. RT-qPCR reactions were conducted according to the
manufacturer's instructions. Gene expression levels were determined
as ∆Ct=(Ct(sample)−Ct(GAPDH)) and relative quantities between
different samples were determined as ∆∆Ct=[∆Ct(sample1) −
∆Ct(sample2)] (26). RT-PCR was
performed using Dream Taq Green PCR Master mix and a GeneAmp PCR
System 9600 (Applied Biosystems; Thermo Fisher Scientific, Inc.)
under conditions as follows: Initial denaturation at 95°C for 5
min; 35 cycles of denaturation at 95°C for 30 sec, annealing at
60°C for 30 sec, elongation at 72°C for 1 min; and a final
elongation step at 72°C for 10 min. The PCR products were separated
by electrophoresis on a 1.5% agarose gel made using agarose
obtained from Thermo Fisher Scientific, Inc.. PCR reactions were
conducted according to the manufacturer's protocol. GAPDH and U6
were amplified as a control. The primers used were supplied by
Shanghai Generay Biotech Co., Ltd. (Shanghai, China) and the
sequences were as follows: Sense, 5′-AGA TCC ACA ACG GAT-3′ and
anti-sense, 5′-TCC CTC AAG ATT GTC AGCAA-3′ for rat GAPDH (308 bp);
sense, 5′-CCT TAG TAC TGC GTG GTC GTAT-3′ and antisense, 5′-CAG AGG
GTG CTT GTT AGA AAGAT-3′ for rat FASN (301 bp); sense, 5′-CTC GCT
TCG GCA GCACA-3′ and antisense, 5′-AAC GCT TCA CGA ATT TGCGT-3′ for
human U6 (130 bp); sense, 5′-CCT TAG CAG AGC TCT GGA GTG TGAC-3′
and antisense, 5′-GCC TAG CAG TAG CTG TTT AGT GTGA-3′ for human
pre-miR-122 (85 bp); and sense, 5′-CAG AGC AGC CAT GGA GGAG-3′ and
antisense, 5′-CAT CGT CCG TGA CCA TGTCC-3′ for human FASN (119
bp).
Western blot analysis
The treated cells were lysed using mammalian cell
lysis buffer containing protease and phosphatase inhibitor
cocktails. The cell lysates were separated by 8% SDS-PAGE at 80 V
for 0.5 h and 100 V for 2 h, and were then electrophoretically
transferred onto polyvinylidene fluoride membranes. The membranes
were blocked for 2 h with blocking solution at room temperature,
washed in Tris-buffered saline with 0.25% Tween-20 (TBS-T) and
exposed to primary antibodies targeting FASN (1:1,000 dilution)
overnight at 4°C. β-actin (1:1,000 dilution) was measured as an
internal control for protein loading. The following day, the
membranes were washed in TBS-T, and incubated with anti-rabbit
HRP-conjugated secondary antibody (1:2,000 dilution) for 1 h at
room temperature. Subsequently, the membranes were washed again in
TBS-T, and the blots were visualized by enhanced chemiluminescence
detection using BeyoECL Plus (Beyotime Institute of Biotechnology,
Haimen, China) and visualized with ChemiDoc XRS+ (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and Image Lab 3.0 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
All data are presented as the mean ± standard
deviation for the indicated number of independently performed
experiments. All statistical analyses were performed using the SPSS
package for Windows (version 16.0; SPSS, Inc., Chicago, IL, USA).
Statistical analyses were performed using the Student's t-test and
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
Animal toxicity analysis
To determine the potential toxicity and safety of
ACF, alterations in the physical appearance and body weight of the
rats were assessed. As shown in Fig.
1A and B, rats fed the HFD for 12 weeks did not exhibit a
significant weight change; however, the liver toxicity index was
higher, as compared with that of the rats fed a normal diet. ACF
supplementation to the HFD did not result in decreased body weight
throughout the study. In addition, the body weight of rats
administered ACF was not significantly different compared with that
of the control group; however, the liver toxicity index was
significantly decreased compared with that of the model rats. These
results indicated that ACF was safe for administration in SD
rats.
Effects of ACF on liver histology
Histological evaluation is considered to be the
'gold standard' technique for identifying the presence and severity
of NAFLD (27). Therefore, in the
present study liver sections were histologically evaluated, in
order to assess the extent of attenuation provided by ACF in the
development of hepatic steatosis. Representative photomicrographs
of liver histology of the various groups are shown in Fig. 2. Rats fed a control diet exhibited
normal liver histology; however, the rats fed a HFD exhibited
elevated levels of fat accumulation and developed steatohepatitis,
which was characterized by hepatocyte ballooning, scattered lobular
inflammatory cell infiltration and inflammatory foci. Treatment
with PP or ACF markedly abrogated hepatic steatosis in the HFD-fed
rats, and histological grading of the liver sections confirmed that
ACF treatment was able to significantly ameliorate hepatic
steatosis and necroinflammation in the HFD-fed rats.
 | Figure 2Effects of ACF on the hepatic
morphology of liver tissue from Sprague-Dawley rats fed a high-fat
diet. Control, (−) no liver fat; model, (+++) fatty hepatocytes
occupying >66% of the hepatic parenchyma, the rats demonstrated
increased fat accumulation and developed steatohepatitis, which was
characterized by hepatocyte ballooning, scattered lobular
inflammatory cell infiltration and inflammatory foci (as indicated
by the black arrow); PP, (+) fatty hepatocytes occupying <33% of
the hepatic parenchyma; ACF(L), (++) fatty hepatocytes occupying
33–66% of the hepatic parenchyma; ACF(M), (++) fatty hepatocytes
occupying 33–66% of the hepatic parenchyma, which was similar to
the effects of ACF(L); ACF(H), (+) fatty hepatocytes occupying
<33% of the hepatic parenchyma, which was similar to the effects
of PP treatment. ACF and PP treatment ameliorated hepatic steatosis
and necroinflammation in the rats fed a high fat diet. Data shown
represent the mean ± standard deviation (n=12/group).
Representative images were captured at magnification, ×400. ACF(L),
low dose Artemisia capillaris formula group; ACF(M), middle
dose ACF group; ACF(H), high dose ACF group; PP, polyene
phosphatidylcholine. |
Effects of ACF on serum AST, ALT, TG, TC,
HDL-C and LDL-C levels
As shown in Fig. 3,
rats in the model group, fed a HFD, exhibited significantly
increased serum TC, TG and LDL-C levels (P<0.05) and reduced
HDL-C levels (P<0.05) compared with those in the normal control
group. Treatment with PP or ACF significantly suppressed the
increased TC, TG and LDL-C levels induced by the HFD (P<0.05),
and upregulated the decreased HDL-C levels; however these findings
were not significant.
 | Figure 3Effects of ACF on high-fat
diet-induced increases in hepatic enzymes and serum lipids in the
various Sprague-Dawley rat groups. Data represent the mean ±
standard deviation (n=12/group). #P<0.05, as compared
with the control; *P<0.05, as compared with the model
group. PP, polyene phosphatidylcholine; ACF(L), low-dose
Artemisia capillaris formula group; ACF(M), middle-dose ACF
group; ACF(H), high-dose ACF group; ALT, alanine aminotransferase;
AST, aspartate transaminase; TG, triglyceride; TC, total
cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C,
low-density lipoprotein cholesterol. |
Effects of ACF on intracellular lipid
accumulation
As shown in Fig. 4A and
B, treatment of HepG2 cells with OA or PA resulted in the
induction of intracellular lipid accumulation, and treatment with
ACF for 24 h reduced the levels of fat deposition. Furthermore, TC
and TG levels were also significantly downregulated following
treatment with ACF.
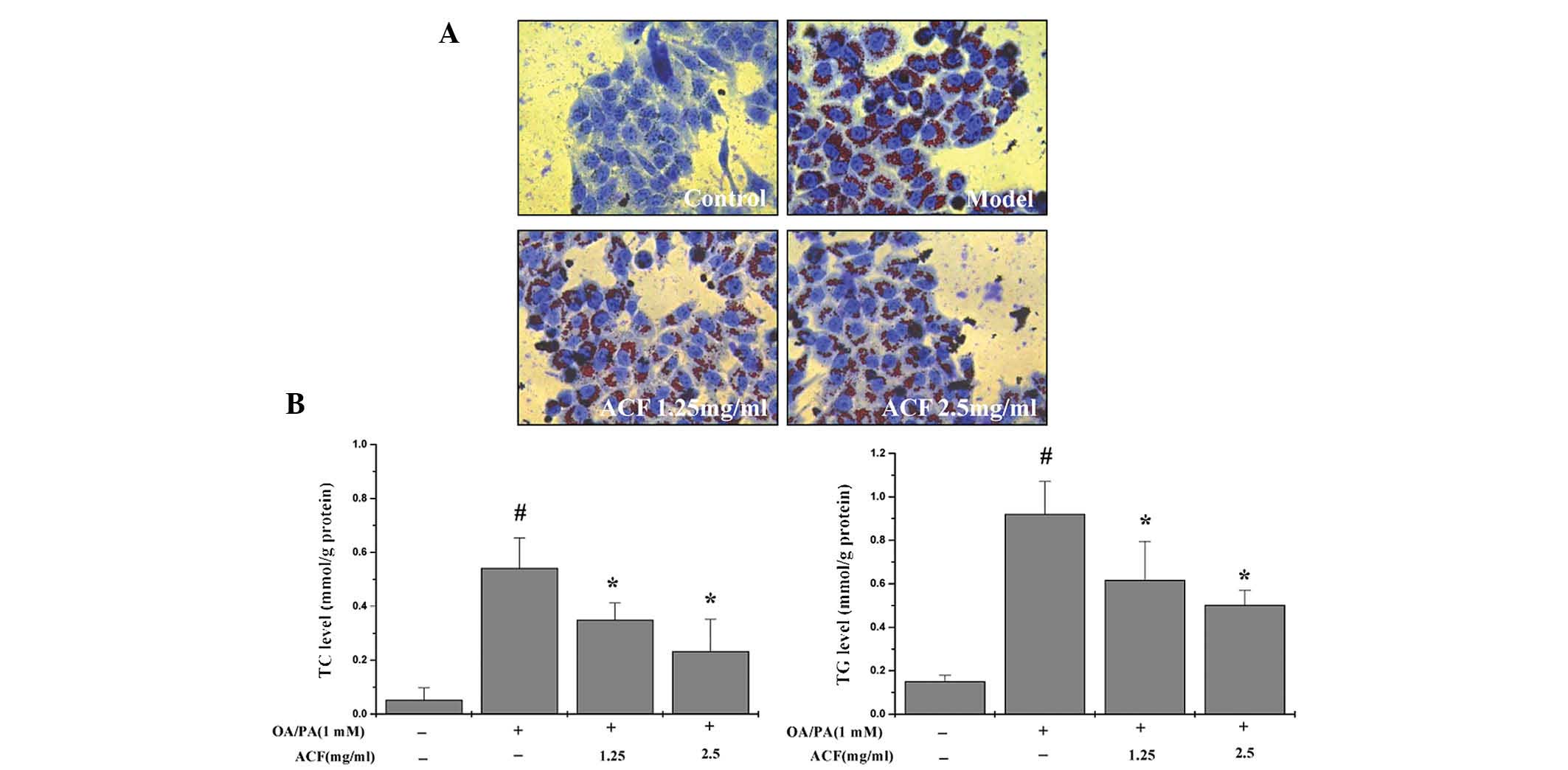 | Figure 4Effects of ACF on steatotic HepG2
human liver carcinoma cell morphology. HepG2 cells were cultured
with normal medium (control) or a high concentration of HFFA
(model) for 24 h (1.25 and 2.5 mg/ml). The steatotic HepG2 cells
were treated with the indicated concentrations of ACF for 24 h. (A)
Oil red O staining identified red droplets in the cytoplasm of
HFFA-cultured cells, and intracellular changes were observed using
phase-contrast microscopy (magnification, ×400). (B) TC and
triglyceride TG levels were measured using a phosphoglycerol
oxidase/peroxidase enzymatic assay. #P<0.05, as
compared with the control group; *P<0.05, as compared
with the model group. Values are presented as the mean ± standard
deviation and are representative of three independent experiments.
ACF, Artemisia capillaris formula; HFFA, high free fatty
acids; OA, oleic acid; PA, palm acid; TC, total cholesterol; TG,
triglycerides. |
ACF suppresses pre-miR-122 and FASN
expression in vivo and in vitro
As shown in Fig. 5,
mRNA and protein expression levels of FASN, as well as the
expression levels of miR-122, were determined by RT-qPCR, western
blotting and immunohistochemical analysis in vivo and in
vitro, respectively. RT-qPCR analysis demonstrated that
treatment with PP or ACF markedly increased the expression levels
of miR-122 and reduced FASN mRNA expression levels (Fig. 5A and B). Western blot analysis and
immunohistochemical assays indicated that the protein expression
levels of FASN emulated the changes of the mRNA expression levels
(Fig. 5C and D). The percentage of
cells positive for FASN was significantly greater in the model
group (95.29±9.78%) compared with that of the control group
(21.59±5.44%), as determined by immunohistochemical analysis. The
percentage of positive cells in the PP, ACF low-, middle- and
high-dose-treated rats were 39.65±7.82, 36.18±3.87, 36.09±2.96 and
28.87±3.01%, respectively.
 | Figure 5Treatment with ACF elevates miR-122
expression and reduces the expression levels of FASN in vivo
and in vitro. (A and B) mRNA expression levels of FASN and
miR-122 in HepG2 human liver carcinoma cells and rat liver tissue,
as determined by RT-qPCR. (C and D) Protein expression levels of
FASN, as determined by western blotting and immunohistochemical
analysis. GAPDH and β-actin were used as internal controls for
RT-qPCR and western blotting, respectively Magnification, ×400.
#P<0.05, as compared with the controls;
*P<0.05, as compared with the model group. Values are
presented as the mean ± standard deviation. Images are
representative of six individual mice in each group, or from three
independent cell-based experiments. ACF, Artemisia
capillaris formula; miR-122, microRNA-122; FASN, fatty acid
synthase; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction. |
Discussion
NAFLD is a multi-step process, which is widely
accepted as the hepatic manifestation of metabolic syndrome
(28). However, the pathogenesis
of NAFLD has yet to be clearly defined, although mitochondrial
dysfunction, insulin resistance and hepatic inflammation are likely
contributors (24). The present
study succeeded in establishing an NAFLD model in rats and cells.
The HFD-induced rat model used in the present study has more
physiological similarities to human NAFLD compared with those of
other models based on genetic defects or chemical-induced disease.
Steatotic HepG2 cells reproduce key features of human hepatic
steatosis, and therefore have been used in numerous studies
(9,23,24,25).
These models are ideal for testing therapies to reduce the extent
of NAFLD, as well as to elucidate the mechanisms of disease
pathogenesis. The present study investigated the ability of ACF to
prevent and treat hepatic fat accumulation in vivo and in
vitro.
ACF is a Traditional Chinese Medicine formula
composed of Artemisiae scopariae, Rhizoma Alismatis, Rhizoma
Atractylodis Macrocephalae, Radix Bupleuri, Crateagus pinnatifida,
Radix Glycyrrhizae and other components. Artemisiae scopariae is a
significant traditional Chinese herb in ACF, which has been widely
used as a remedy for liver diseases, including hepatitis, jaundice
and fatty liver diseases (16).
Previous studies have demonstrated that the aqueous extract from
Artemisia capillaris Thunb. is able to inhibit the expression of
inflammatory proteins, including inducible nitric oxide synthase,
cyclooxygenase-2 and tumor necrosis factor-α (16,29).
Together, these constituents have been shown to exhibit
hepatoprotective effects in vivo and in vitro. Understanding the
pathogenesis of liver diseases and the mechanisms underlying the
effects of traditional medicines is essential for optimizing these
forms of treatment for specific types of liver disease. miR-122 is
highly abundant in the liver, and has garnered considerable
attention for its role in the regulation of cholesterol and lipid
metabolism (1). NAFLD is known to
be associated with a reduced expression of hepatocyte miR-122,
which subsequently alters lipid metabolism (8,30,31).
miR-122 has also been shown to promote adipocyte differentiation
(8,30,31).
Numerous studies have demonstrated that miR-122 inhibition in a
diet-induced mouse model of obesity results in decreased mRNA
expression levels of acetyl-CoA carboxylase 2, FASN, stearoyl CoA
desaturase-1 and HMG CoA reductase, and furthermore, significantly
reduces the plasma cholesterol levels in these mice (8,32–35).
The protein expression of FASN was increased and decreased
following overexpression and silencing of miR-122 in Huh-7 cells,
respectively (34).
The results of the present study demonstrated that
the HFD-associated upregulation of FASN was reversed by treatment
with ACF, which is a key first step in elucidating the role of
miR-122 as upregulation of miR-122 may be involved in the
underlying molecular mechanism. In addition, the observation that
upregulation of FASN in HFD-fed rats led to an increase in fatty
acid uptake and accumulation, and ultimately hepatic steatosis,
provides useful insight into the mechanisms that promote the
progression of associated liver diseases. The present study
confirmed that miR-122 was significantly downregulated in HepG2
cells that were exposed to HFFA. Furthermore, it was demonstrated
that treatment with ACF decreased the mRNA and protein expression
levels of FASN, and inhibited OA/PA-induced intracellular lipid
accumulation in HepG2 cells.
In conclusion, these results suggested that ACF
treatment may decrease ALT, AST, TC, TG and LDL-C levels in HFD-fed
rats, and TC and TG levels in steatotic HepG2 cells. Furthermore,
dietary ACF reduced hepatic steatosis by upregulating the
expression of miR-122 and downregulating the expression of FASN
in vivo and in vitro, further supporting the role of
miR-122 as a regulatory component of normal and aberrant lipid
accumulation in the liver. These results suggested that ACF
possesses significant potential for use in the prevention of NAFLD
and non-alcoholic steatohepatitis, and that further research is
required in order to clarify the correlation between miR-122 and
its regulatory functions in lipid metabolism and liver disease.
Acknowledgments
The present study was supported by the Important
Science & Technology Specific Projects of Fujian Province
(grant no. 2010YZ0001-1) and the Project Sponsored by the Education
Department of Fujian Province of China (grant no. JA11139). The
authors of the present study would like to thank Clarity Manuscript
Consultants, LLC (Indianapolis, IN, USA) for their editorial
assistance with this manuscript.
References
|
1
|
Cheung O and Sanyal AJ: Role of microRNAs
in non-alcoholic steatohepatitis. Curr Pharm Des. 16:1952–1957.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Browning JD, Szczepaniak LS, Dobbins R, et
al: Prevalence of hepatic steatosis in an urban population in the
United States: Impact of ethnicity. Hepatology. 40:1387–1395. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Charlton M: Nonalcoholic fatty liver
disease: A review of current understanding and future impact. Clin
Gastroenterol Hepatol. 2:1048–1058. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jun HJ, Kim J, Hoang MH and Lee SJ:
Hepatic lipid accumulation alters global histone h3 lysine 9 and 4
trimethylation in the peroxisome proliferator-activated receptor
alpha network. PLoS One. 7:e443452012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdelmalek MF and Diehl AM: Nonalcoholic
fatty liver disease as a complication of insulin resistance. Med
Clin North Am. 91:1125–1149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin X, Ye YF, Chen SH, Yu CH, Liu J and Li
YM: MicroRNA expression pattern in different stages of nonalcoholic
fatty liver disease. Dig Liver Dis. 41:289–297. 2009. View Article : Google Scholar
|
|
7
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esau C, Davis S, Murray SF, et al: miR-122
regulation of lipid metabolism revealed by in vivo antisense
targeting. Cell Metab. 3:87–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin J, Liu F and Jiang Y: Antisense
technologies targeting fatty acid synthetic enzymes. Recent Patents
Anticancer Drug Discov. 7:198–206. 2012. View Article : Google Scholar
|
|
10
|
Liu Y, Chen SH, Jin X and Li YM: Analysis
of differentially expressed genes and microRNAs in alcoholic liver
disease. Int J Mol Med. 31:547–554. 2013.PubMed/NCBI
|
|
11
|
Chang CY, Argo CK, Al-Osaimi AM and
Caldwell SH: Therapy of NAFLD: Antioxidants and cytoprotective
agents. J Clin Gastroenterol. 40(Suppl 1): S51–S60. 2006.PubMed/NCBI
|
|
12
|
Caldwell SH, Argo CK and Al-Osaimi AM:
Therapy of NAFLD: Insulin sensitizing agents. J Clin Gastroenterol.
40(Suppl 1): S61–S66. 2006.PubMed/NCBI
|
|
13
|
Sanyal AJ, Campbell-Sargent C, Mirshahi F,
et al: association of insulin resistance and mitochondrial
abnormalities. Gastroenterology. 120(5): 1183–1192. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pérez-Carreras M, Del Hoyo P, Martín MA,
Rubio JC, Martín A, Castellano G, Colina F, Arenas J and
Solis-Herruzo JA: Defective hepatic mitochondrial respiratory chain
in patients with nonalcoholic steatohepatitis. Hepatology.
38:999–1007. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ji HF, Li XJ and Zhang HY: Natural
products and drug discovery. Can thousands of years of ancient
medical knowledge lead us to new and powerful drug combinations in
the fight against cancer and dementia? EMBO Rep. 10:194–200. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Zhao J, Zhong X, Zheng H, Li Y,
Liu L, Wan Y, Peng J and Hong Z: Ameliorative potential of
Artemisia capillaris formula on nonalcoholic fatty liver disease in
rats through regulation of fat metabolism. Afr J Tradit
Complementary Altern Med. 12:151–161. 2015.
|
|
17
|
Liu X, Shen T, Wang Z, Zhaung L, Zhang W,
Yu J, Wu J and Zhang S: Hepatitis E virus infection results in
acute graft failure after liver transplantation: A case report. J
Infect Dev Ctries. 2014.8:245–248. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding Y, Xing J, Qiu Z, Wang Y, Zhang Y,
Fang Y, Peng X, Long Y and Deng P: Radioactive iodine therapy
without recent antithyroid drug pretreatment for hyperthyroidism
complicated by severe hyperbilirubinemia due to hepatic
dysfunction: Experience of a Chinese medical center. Endocr Pract.
Oct. 22–2015.Epub ahead of print. PubMed/NCBI
|
|
19
|
Zhao J, Zheng H, Liu Y, Lin J, Zhong X, Xu
W, Hong Z and Peng J: Anti-inflammatory effects of total alkaloids
from Rubus alceifolius Poir [corrected]. on non-alcoholic fatty
liver disease through regulation of the NF-κB pathway. Int J Mol
Med. 31:931–937. 2013.PubMed/NCBI
|
|
20
|
Li Y, Zhao J, Zheng H, Zhong X, Zhou J and
Hong Z: Treatment of nonalcoholic fatty liver disease with total
alkaloids in Rubus aleaefolius Poir through regulation of fat
metabolism. Evid Based Complement Alternat Med.
2014:7685402014.PubMed/NCBI
|
|
21
|
Lin J, Zhao J, Li T, Zhou J, Hu J and Hong
Z: Hepatoprotection in a rat model of acute liver damage through
inhibition of CY2E1 activity by total alkaloids extracted from
Rubus alceifolius Poir. Int J Toxicol. 30:237–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonkovsky HL, Jawaid Q, Tortorelli K, et
al: Non-alcoholic steatohepatitis and iron: Increased prevalence of
mutations of the HFE gene in non-alcoholic steatohepatitis. J
Hepatol. 31:421–429. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wan Y, Liu LY, Hong ZF and Peng J: Ethanol
extract of Cirsium japonicum attenuates hepatic lipid accumulation
via AMPK activation in human HepG2 cells. Exp Ther Med. 8:79–84.
2014.PubMed/NCBI
|
|
24
|
Gómez-Lechón MJ, Donato MT,
Martínez-Romero A, Jiménez N, Castell JV and O'Connor JE: A human
hepatocellular in vitro model to investigate steatosis. Chem Biol
Interact. 165:106–116. 2007. View Article : Google Scholar
|
|
25
|
Zheng L, Lv GC, Sheng J and Yang YD:
Effect of miRNA-10b in regulating cellular steatosis level by
targeting PPAR-alpha expression, a novel mechanism for the
pathogenesis of NAFLD. J Gastroenterol Hepatol. 25:156–163. 2010.
View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Brunt EM: Nonalcoholic steatohepatitis:
Pathologic features and differential diagnosis. Semin Diagn Pathol.
22:330–338. 2005. View Article : Google Scholar
|
|
28
|
Neuschwander-Tetri BA: Nonalcoholic
steatohepatitis and the metabolic syndrome. Am J Med Sci.
330:326–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan XJ, Li Q, Chen XH, et al: Simultaneous
determination of 13 bioactive compounds in Herba Artemisiae
Scopariae (Yin Chen) from different harvest seasons by HPLC-DAD. J
Pharm Biomed Anal. 47:847–853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Esau C, Kang X, Peralta E, et al:
MicroRNA-143 regulates adipocyte differentiation. J Biol Chem.
279:52361–52365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krützfeldt J, Rajewsky N, Braich R, et al:
Silencing of microRNAs in vivo with 'antagomirs'. Nature.
438:685–689. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang J, Guo JT, Jiang D, Guo H, Taylor JM
and Block TM: Liver-specific microRNA miR-122 enhance the
replication of hepatitis C virus in nonhepatic cells. J Virol.
82:8215–8223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheung O, Puri P, Eicken C, Contos MJ,
Mirshani F, Maher JW, Keller JM, Min H, Luketic VA and Sanyal AJ:
Nonalcoholic steatohepatitis is associated with altered hepatic
microRNA expression. Hepatology. 48:1810–1820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lewis AP and Jopling CL: Regulation and
biological function of the liver-specific miR-122. Biochem Soc
Trans. 38:1553–1557. 2010. View Article : Google Scholar : PubMed/NCBI
|



















