Introduction
Osteonecrosis of the femoral head (ONFH) is a type
of common and refractory disease (1,2).
Young adults primarily suffer from ONFH and the long-term results
of total hip arthroplasty (THA) are usually unpredictable in this
age group (3,4). A variety of surgical procedures have
been used to maintain the femoral head and avoid THA in younger
patients. These approaches include core decompression (5,6), and
various types of osteotomies (7).
Nevertheless, despite these advances, the optimal treatment for
patients with ONFH in clinical practice is not yet defined.
Previous findings have demonstrated the efficacy of
mesenchymal stem cells (MSCs) implanted into the femoral head for
the treatment of ONFH (8,9). MSCs can be isolated from bone marrow
and adipose tissues in adult stages and from umbilical cord (UC)
blood, and connective tissue (Wharton's jelly) of human UC
(10–13). MSCs can differentiate into
specialized cells to repair injured tissues, under certain
conditions based on their potential capacity of multidirectional
differentiations (14,15). Thus, stem cell transplantation has
gradually emerged as a promising approach for the treatment of ONFH
(8,16,17).
In previous studies, we grafted human UC-derived MSCs (hUC-MSCs) to
treat non-union in rats and human (11–13).
Our results demonstrated the safety and efficacy of osteoblastic
differentiation of hUC-MSCs. However, to the best of our knowledge,
few studies are available regarding the fate and distribution of
hUC-MSCs in the treatment of ONFH. Furthermore, the delivery
approaches are a wide concern and unsolved problem.
In the present study, we investigated wether
hUC-MSCs are useful in treating ONFH. As ONFH is primarily caused
by a partial obstruction of the blood supply to the femoral head,
and aggrevated by insufficient blood supply to trabecular bone and
bone cell death in the femoral head; we grafted hUC-MSCs by
intra-arterial infusion. We hypothesized that: i) the implanted
hUC-MSCs could differentiate into endothelial cells, which in turn
were able to regenerate blood vessels supplying the femoral head or
produce more collateral circulation; and ii) differentiate into
osteoblasts and reconstruct the necrotic area in femoral head. In
the present study, we retrospectively reported 9 patients with ONFH
who accepted intra-arterial infusion of hUC-MSCs treatment. The
clinical and radiographic outcomes in the subsequent 24 months were
evaluated.
Materials and methods
Eligibility criteria
This single-center randomized clinical trial was
conducted in the Siping Hospital of China Medical University
(Jilin, China) between January, 2011 and January, 2014. The aim of
the present study was to assess the efficacy of hUC-MSCs grafting
by intra-arterial infusion for the treatment of early-stage ONFH.
The protocol of the present study was approved by the Institutional
Review Board and the Ethics Committee of Siping Hospital of China
Medical University. Written informed consent was obtained from each
patient before enrollment.
Study design
Patients were evaluated clinically and
radiologically using the Association Research Circulation Osseous
(ARCO) classification (18) and
the Harris hip score (HHS) (19)
pre-operatively and at 12 and 24 months post-operation. All the
patients in this study were ≥18 years of age, and were classified
as ARCO stages II to IIIa. The patients had femoral head collapse
of <2 mm and no evidence any damage to the acetabular cartilage.
Those patients who were excluded from this study had damages caused
by a traumatic event or experienced severe hemorrhage. We also
excluded those who were not available for follow up because of
life-threatening conditions or had missing stratification
information. Those without standard care and rehabilitation were
also excluded. This exclusion criterion was chosen to ensure that
all the patients were treated according to the best medical
practice.
We included 9 patients (9 hips) for this study. The
general characteristics of the patients are shown in Table I. Blood was taken from the internal
jugular veins. The outcomes were evaluated based on the HHS and the
radiological results.
 | Table I.General characteristics of ONFH
patients. |
Table I.
General characteristics of ONFH
patients.
| Characteristics | Data |
|---|
| Gender (no. of
pts.) |
|
| Male | 4 |
|
Female | 5 |
| Invasive hip (no. of
pts.) |
|
|
Unilateral | 9 |
|
Bilateral | 0 |
| Etiology (no. of
pts.) |
|
|
Idiopathic | 1 |
|
Corticosteroids | 6 |
|
Alcohol | 2 |
|
Trauma | 0 |
| ARCO staging (no. of
hips) |
|
| II | 5 |
| IIIa | 4 |
Harvesting of UC
Six human equally sized UC were collected after
informed consent was obtained from mothers in accordance with the
Ethics Committee of the Institute of Siping Hospital of China
Medical University. Informed consent was obtained from all the
cases. Experiments and laboratory procedures were carried out in
the Siping Hospital of China Medical University. From each sample,
sections of 8–10 cm of the UCs were internally washed with
phosphate-buffered saline (PBS) containing 300 U/ml penicillin and
0.3 mg/ml streptomycin and immediately immersed in Dulbecco's
modified Eagle's medium-low glucose (DMEM-LG) supplemented with 10%
AB-human serum (all from Gibco, Grand Island, NY, USA), 300 U/ml
penicillin, and 0.3 mg/ml streptomycin. The samples were processed
within 12–15 h after collection.
Isolation and culture of adherent
cells from UC (11–13)
UCs were filled with 0.1% collagenase
(Sigma-Aldrich, St. Louis, MO, USA) in PBS and incubated at 37°C
for 20 min. Each UC was washed with proliferation medium (a-MEM +
10% AB-human serum), and the detached cells were harvested after
gentle massage of the UC. The cells were centrifuged at 300 × g for
10 min, resuspended in proliferation medium, and seeded in
75-cm2 flasks at a density of 5×107 cells/ml.
After 24 h of incubation, non-adherent cells were removed, and
culture medium was replaced every 3 days. Adherent cells were
cultured until they reached 80–90% confluence. The cells were
observed using a phase contrast microscope (Olympus, Tokyo,
Japan).
Flow cytometry
To analyze the cell-surface expression of typical
protein markers, adherent cells were passaged with 0.25% trypsin
(Gibco). The cells were washed in PBS and fixed in a 4%
paraformaldehyde solution (both from Sigma-Aldrich, Milwaukee, WI,
USA) for 10 min; and incubated with the following anti-human
primary antibodies: mouse monoclonal CD45-phycoerythrin (PE)
(Abcam, Cambridge, MA, USA; catalog no.: ab25603; dilution: 0.2
µg/106 cells), mouse monoclonal CD31-fluorescein
isothiocyanate (FITC) (Abcam; catalog no.: ab33858; dilution:10
µl/106 cells), mouse monoclonal CD90-FITC (Abcam;
catalog no.: ab11155; dilution: 10 µl/106 cells), mouse
monoclonal HLA-DR-PE (Abcam; catalog no.: ab95830; dilution: 5
µl/106 cells). A total of 10,000 labeled cells were
analyzed using a Guava easyCyte flow cytometer running Guava
Express Plus software (Guava Technologies, Inc., Chicago, IL,
USA).
hUC-MSC osteogenic
differentiation
For osteocyte differentiation, the cells were plated
at 104 cells/cm2 on uncoated plastic
(Permanox) chamber slides (Lab-Tek; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and allowed to adhere for 24 h in tissue culture
media (DMEM-LG and 10% human AB serum), after which the media were
replaced with osteogenic induction media, composed of DMEM-LG
supplemented with 10% human AB serum, 1% penicillin/streptomycin,
50 µg/ml L-ascorbic acid (Wako Chemicals GmbH, Neuss, Germany), 10
mM glycerol phosphate disodium salt (β-glycerophosphate), 10 nM
dexamethasone, and 10 nM calcitriol (1α,25-dihydroxyvitamin D3)
(Sigma, Irvine, UK).
hUC-MSC osteogenic mineralization
Alizarin Red S (ARS) staining was used to examine
osteogenic differentiation and mineralization. Specimens were
washed with PBS, fixed with 10% formaldehyde, and stained with ARS
(Millipore Corp., Billerica, MA, USA), which stained calcium
minerals into a red color.
Red blood cell related parameters
measurements
Red blood cell-related parameters were analyzed as
blood cells before operation and third day after operation using an
automated blood cell counter LH-750 (Beckman Coulter, Inc., Brea,
CA, USA).
Analysis of magnetic resonance imaging
(MRI)
MRI was performed using Discovery MR750 3.0T (GE
Medical Systems, Milwaukee, WI, USA) before and 12/24 months after
intra-arterial infusion with coronal T1-weighted imaging,
T2-weighted imaging and axial T1-weighted imaging, and T2-weighted
imaging. Images (3-mm) with a 0.5-mm gap were obtained using a
256×192 matrix and four excitations. The volumetric analysis of the
ONFH was assayed by Syngo via 2.0 (GE Medical Systems, Milwaukee,
WI, USA).
Surgical procedure
Under general anesthesia, each patient was placed in
the supine position, and the right femoral artery was punctured
using the Seldinger technique with a 4.0 F Cobra catheter (Terumo,
Tokyo, Japan). MSCs (10 ml) with a cell density of 5×106
to 1×107/ml were intra-arterially injected. Once the
needle was fully withdrawn, the puncture site was wrapped with
sterilized dressing. The patients remained in the supine decubitus
on the operation bed for another 30 min before being returned to
individual wards. Antibiotics were given to prevent infection.
The patients were instructed to be
non-weight-bearing for 4 weeks and partial weight-bearing for the
subsequent 6 weeks. Full weight-bearing was achieved 6 months
postoperatively (20).
Oxygen delivery index (ODI)
ODI was calculated as the ratio between the
hematocrit and systolic blood viscosity (SBV) (21,22).
ODI = hematocrit/SBV = 100H/1.4175 + 5.878H-12.98H2 +
31.964H3, where H is the volume fraction of
erythrocytes.
Treatment protocol
The following treatment protocol was used: i)
anti-platelet aggregation drugs: sarpogrelate (Mitsubishi Tanabe
Pharma Corp, Tianjin, China) 100 mg t.i.d. + clopidogrel hydrogen
sulfate (Salubris Co., Ltd., Shenzhen, China) 50 mg/day. According
to the target lesions run-off situation, dual anti-platelet drugs
should continued one year post-operation at least; ii) medications
for improving circulation: alprostadil injection (Tide
Pharmaceutical Co., Ltd., Beijing, China); and iii) perioperative
anticoagulation drugs. Low molecular weight heparin sodium
injection 4000 iu q12h hypodermic injection (Clexane; Aventis
Intercontinental, Antony, France) was also used.
Evaluation and statistical
analysis
The evaluations consisted of clinical and
radiographic analysis preoperatively and at the end of follow-up
based on the HHS. All the patients underwent clinical and
radiographic examinations at 12 and 24 months post-operation. An
excellent or good HHS was defined as ≥80 points and scores <70
were considered negative. Analyses were completed using SPSS
version 22.0 software (IBM SPSS, New York, NY, USA).
General characteristics of the ONFH
patients
The general characteristics of patients are shown in
Table I. The patients included 4
males and 5 females, with an age range of 28–51 years (mean,
41.13±3.29 years). The etiology of the osteonecrosis was
corticosteroid use in 6 hips, intemperance in 2 hips, and
idiopathic or unknown in 1 hips; 5 hips had stage II osteonecrosis
and 4 had stage IIIa osteonecrosis.
Results
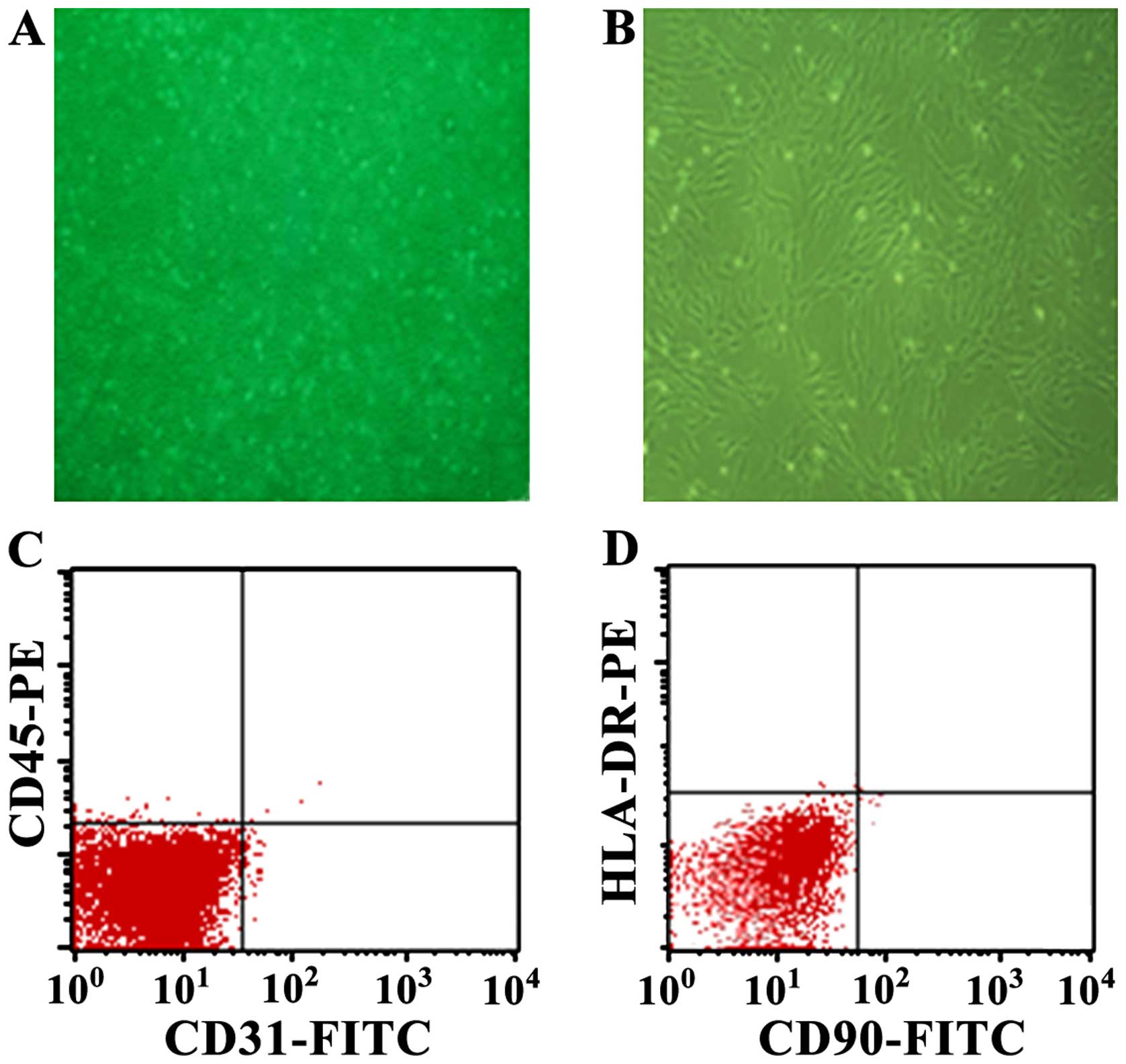
Evaluation of hUC-MSCs
Cells derived from UC were observed 24 h after they
were seeded (Fig. 1A), when part
of the round mononuclear cells was adherent. Three days after
inoculation, small colonies of the adherent cells with typical
fibroblast-shaped morphology were obtained (Fig. 1B). These primary cells reached
monolayer confluence, after planting for 5–6 days, when passaged
for the first time. Fifth-passaged cells were analyzed by flow
cytometry, and were strongly positive for CD31 (98.95%) and CD90
(97.77%), but negative for CD45 (0.55%) and HLA-DR (0.14%)
(Fig. 1C and D).
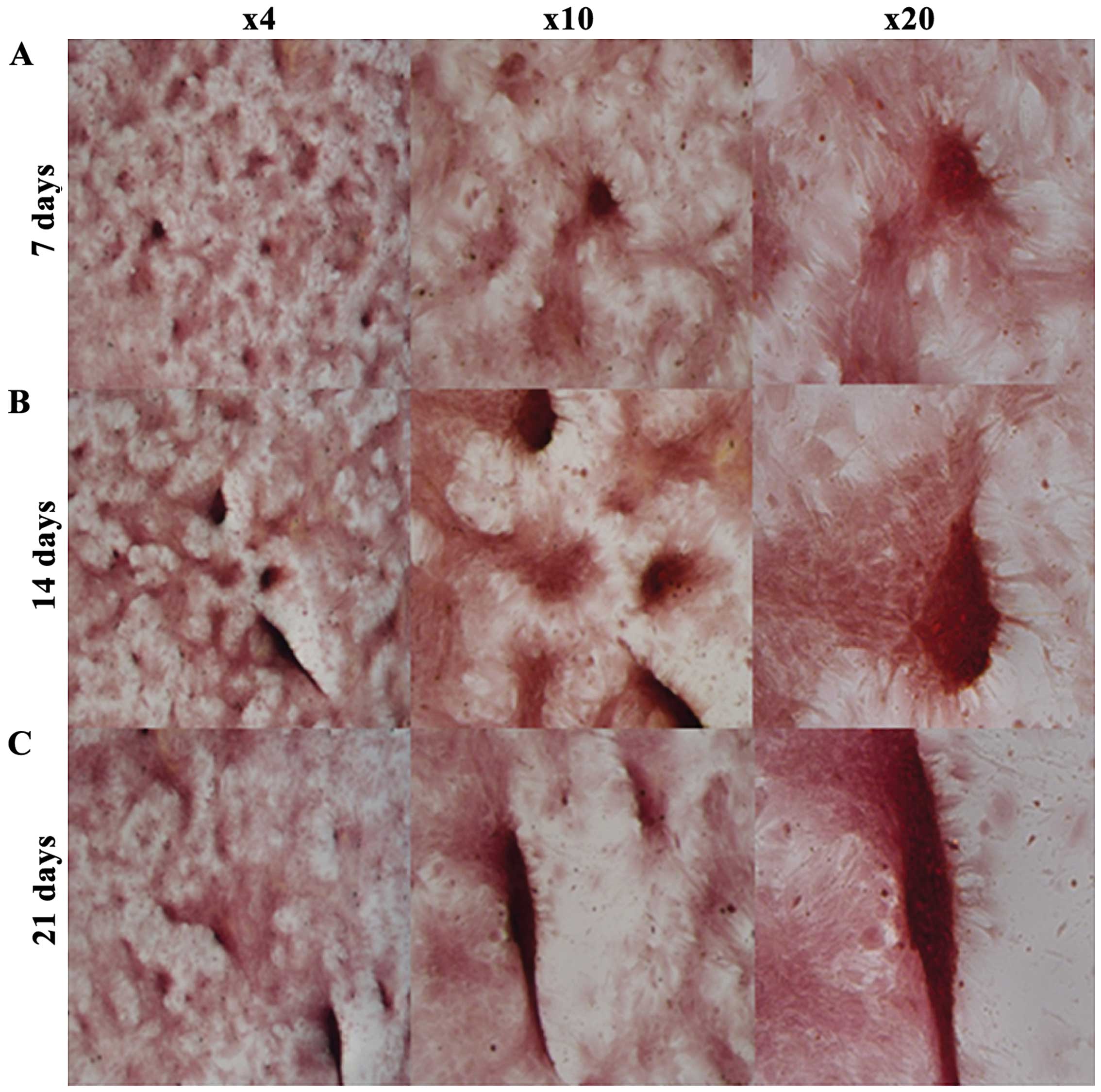
hUC-MSC osteogenic mineralization
ARS staining was used to examine osteogenic
mineralization. The purpose of this method was to confirm that
hUC-MSCs cultured in osteogenic media were able to produce
minerals. ARS stained calcium minerals into a red color (Fig. 2), while no red staining was found
in hUC-MSCs cultured in tissue culture media, which served as a
control (data not shown). hUC-MSCs in osteogenic media showed
significant mineral staining from the 7th day (Fig. 2). Red staining became progressively
thicker and darker at 14 and 21 days.
Patient-specific extent-related
parameters of red blood cells and platelet data
The pre-operation red cell count, Hb and Hct levels
were significantly reduced three days after the operation. The same
results were observed in the mean cell volume, mean cell Hb
concentration, mean cell Hb and red cell distribution width.
Related parameters of platelet remained unchanged at all time
points, as anti-platelet aggregation drugs were used before and
after the operation (Table
II).
 | Table II.Changes in parameters related to red
blood cells and platelet before and after the operation. |
Table II.
Changes in parameters related to red
blood cells and platelet before and after the operation.
| Variables | count
(×1012/l) | Hb (g/dl) | Hct (%) | Mean cell volume
(fl) | Mean cell Hb
(pg) | Mean cell Hb
concentration (g/dl) | Red cell distribution
width (%) | Platelet count
(×109/l) | Platelet volume
(fl) | Mean platelet volume
(fl) | Platelet
distribution width (%) |
|---|
| Preoperation | 4.15±0.09 | 131.43±2.62 | 40.15±1.03 | 94.35±1.36 | 31.61±1.24 | 333.16±19.23 | 12.81±1.25 | 231.54±29.27 | 0.28±0.01 | 11.42±1.56 | 12.02±2.19 |
| 3 days
postoperation |
3.95±0.12a |
120.02±3.55a |
37.05±1.63a | 93.58±2.62 | 32. 81±1.32 |
344.65±23.16a | 11.93±1.09 | 224.28±28.35 | 0.26±0.02 | 11.17±2.01 | 11.96±1.35 |
ODI changes
As shown in Table
III, after operation, ODI in all the patients increased by
about 5% compared to pre-operation readings. This increase was
especially more obvious in the ARCO stage II patients. These
results consisted of Hct reduction, which suggested a more
efficient oxygen transport (23).
 | Table III.ODI changes after operation in 3
days. |
Table III.
ODI changes after operation in 3
days.
| Variables | ARCO stage II | ARCO stage
IIIa |
|---|
| ODI |
|
|
| BO | 10.31±0.26 | 10.03±0.27 |
| PO |
10.86±0.34a |
10.53±0.09a |
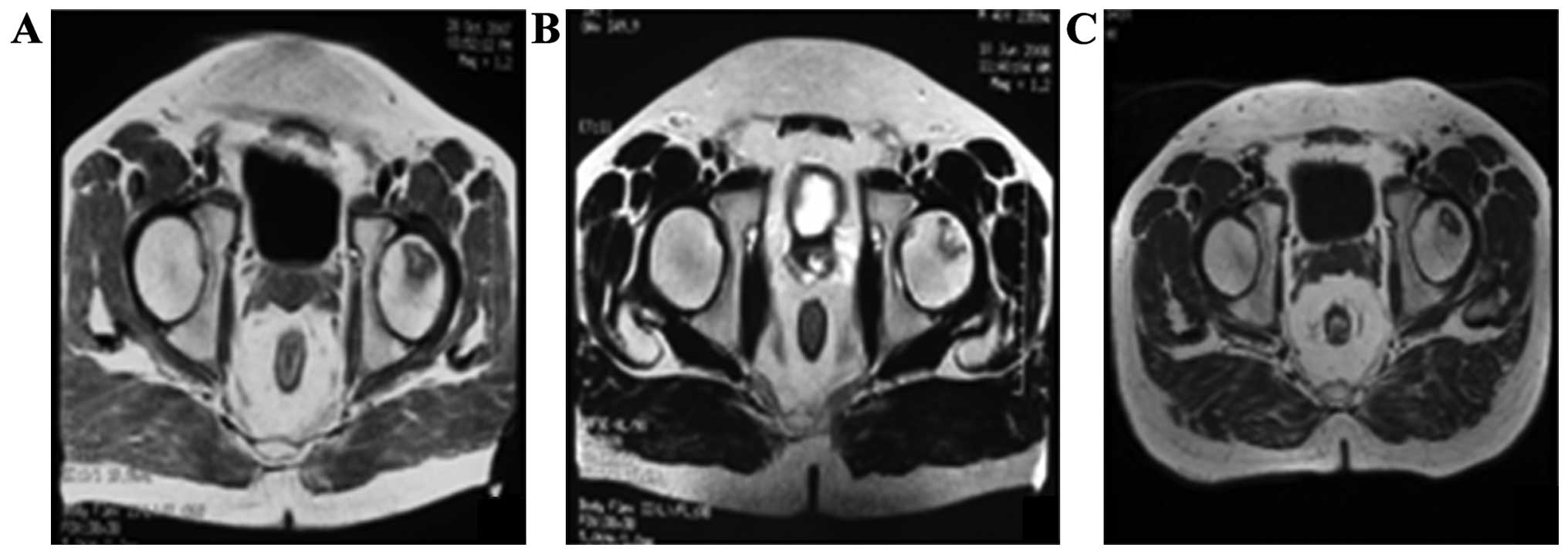
Regression of a necrotic lesion after
intra-arterial infusion on MRI
MRI was performed to determine conditions of ONFH
after 12 months. The patients showed sporadic low signal intensity
on T1W, but no obvious abnormal signal intensity on T2W R, profile
rules, and comparatively smooth edge (Fig. 3B). The result from the volumetric
analysis showed that the necrotic volume of femoral heads was
7.16±0.73 cm3; whereas, the necrosis volume at 24 months
decreased to 5.86±1.67 cm3 (p<0.05) (Fig. 3C).
HHS evaluation
The mean preoperative HSS was 39.19±5.06 points
pre-operation (Table IV). HHS
points increased obviously at the end of the 12 month
postoperation, but these points decreased 10% by the end of the
24-month post-operation.
 | Table IV.Clinical results of survival
hips. |
Table IV.
Clinical results of survival
hips.
|
| ARCO stage II | ARCO stage
IIIa |
|---|
| HHS
(preoperative) | 39.19±5.06 | 33.25±6.37 |
| HHS (12 months
postoperation) | 89.46±9.11 | 83.13±11.66 |
| Survival (12 months
postoperation), n | 5 | 3 |
| EGR (12 months
postoperation), n, % | 5, 100 | 3, 75 |
| HHS (24 months
postoperation) | 80.09±10.16 | 71.52±9.23 |
| Survival (24 months
postoperation), n | 4 | 2 |
| EGR (24 months
postoperation), n, % | 4, 80 | 2, 50 |
Discussion
We treated the ONFH patients with hUC-MSCs by
intra-arterial infusion, and assessed the efficacy ischemia
reperfusion with ODI in the acute phase postoperative and imaging
evaluation in the following 24 months. To the best of our
knowledge, this is the first study on the subject of predicting the
efficacy of ONFH treatment with ODI index. We also showed that the
migration of hUC-MSCs promoted bone formation in the ischemia area
in ONFH patients. Our experimental results supported cell therapy
for ONFH. The hUC-MSCs used in the current study meet the criterion
of the International Society for Cell Therapy (24). The grafted hUC-MSCs differentiated
into osteoblasts in vitro. In this experiment, we compared
the postoperation red blood cell-related parameters with
preoperative results and ODI value. The number of red blood cells
and HCV values were all reduced postoperatively. This was
advantageous to the red blood cells through newborn capillaries to
carry oxygen. The HHS points increased by the end of the 3rd month
post-operation. Two patients of ARCO II stage had over 80 HHS at
the end of the 24th month. Additionally, sporadic low signal
intensity on T1W, and no obvious abnormal signal intensity on T2W
was observed by MRI scan at the end of 12 months, and comparatively
smooth edge after 24 months of treatments. The rate of radiological
progression on the necrotic lesion after hUC-MSCs treatment was
statistically lower than pre-operation. In addition, the results
from the volumetric analysis revealed the necrotic volume of
femoral heads decreased. Therefore, we considered that
intra-arterial infused hUC-MSCs in ONFH could migrate into the
necrotic field of femoral head, multiply and differentiate into
osteoblasts. Taken together, these results indicated that hUC-MSCs
with intra-arterial infusion obviously improved trabecular bone
shape, and decreased empty lacunae during the early ONFH.
Of note, no obvious abnormalities on the mental
condition, body temperature, and body weight were observed and no
significant difference on routine blood tests was detected. Blood
tests included WBC-related parameters (data not shown) of
preoperation and postoperation. PLT-related parameters, including
platelet count, volume, mean volume and distribution width
decreased post-operation compared to pre-operation. Similar results
on safety have been reported in our previous study, in which we
injected hUC-MSCs into rats and found hUC-MSCs located in the rat
non-union area that differentiated into osteoblasts, as well as no
local or systematic manifestations of toxic reactions and graft vs.
host disease during and after transplantation (11,12).
Based on the above results, this approach is relatively safe.
Currently, there are three methods to transplant
MSCs, including local injection (25,26),
intra-venous delivery (27,28),
and targeted intra-arterial injection (29). It has been shown that the delivery
of MSCs via the targeted artery can significantly increase the
number of cells homing into the injured tissue and improve function
compared with injection via the intravenous route (30). In the present study, we
investigated the fate and distribution of hUC-MSCs with
intra-arterial infusion in femoral head to treat ONFH patients and
assess the feasibility and safety thereof. To improve the survival
of the hip, further research should be considered to combine this
method with core decompression. We did not supervise the
histopathology in this study, and the potential mechanism
underlying this approach requires further investigation.
Consequently, the results have shown that hUC-MSCs
with intra-arterial infusion were inclined to commit to repair and
regeneration in the condition of bone necrosis. An important
mechanism may be to improve the abovementioned signs of ONFH in
imaging. We considered hUC-MSCs with intra-arterial infusion a
feasible and relatively safe avenue for the treatment of ONFH. In
addition, the long-term safety of hUC-MSCs with intra-arterial
infusion needs further evaluation.
Acknowledgements
This study has been supported by the National High
Technology Research and Development Program of (863 Program), nos.
2011AA020101 and 2012A020905.
References
|
1
|
Kaushik AP, Das A and Cui Q: Osteonecrosis
of the femoral head: an update in year 2012. World J Orthop.
3:49–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang C, Wang Y, Meng HY, Yuan XL, Xu XL,
Wang AY, Guo QY, Peng J and Lu SB: Application of bone marrow
mesenchymal stem cells to the treatment of osteonecrosis of the
femoral head. Int J Clin Exp Med. 8:3127–3135. 2015.PubMed/NCBI
|
|
3
|
Ince A, Lermann J, Göbel S, Wollmerstedt N
and Hendrich C: No increased stem subsidence after arthroplasty in
young patients with femoral head osteonecrosis: 41 patients
followed for 1–9 years. Acta Orthop. 77:866–870. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YH, Choi Y and Kim JS: Cementless
total hip arthroplasty with alumina-on-highly cross-linked
polyethylene bearing in young patients with femoral head
osteonecrosis. J Arthroplasty. 26:218–223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Persiani P, De Cristo C, Graci J, Noia G,
Gurzì M and Villani C: Stage-related results in treatment of hip
osteonecrosis with core-decompression and autologous mesenchymal
stem cells. Acta Orthop Belg. 81:406–412. 2015.PubMed/NCBI
|
|
6
|
Tabatabaee RM, Saberi S, Parvizi J,
Mortazavi SM and Farzan M: Combining concentrated autologous bone
marrow stem cells injection with core decompression improves
outcome for patients with Early-Stage osteonecrosis of the femoral
head: a comparative study. J Arthroplasty 30 (Suppl 9). 11–15.
2015. View Article : Google Scholar
|
|
7
|
Sonoda K, Yamamoto T, Motomura G,
Nakashima Y, Yamaguchi R and Iwamoto Y: Outcome of
transtrochanteric rotational osteotomy for posttraumatic
osteonecrosis of the femoral head with a mean follow-up of 12.3
years. Arch Orthop Trauma Surg. 135:1257–1263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao D, Liu B, Wang B, Yang L, Xie H,
Huang S, Zhang Y and Wei X: Autologous bone marrow mesenchymal stem
cells associated with tantalum rod implantation and vascularized
iliac grafting for the treatment of end-stage osteonecrosis of the
femoral head. Biomed Res Int. 2015:2405062015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin H, Xu T, Chen Q, Wu C, Wang P, Mao Q,
Zhang S, Shen J and Tong P: The fate and distribution of autologous
bone marrow mesenchymal stem cells with intra-arterial infusion in
osteonecrosis of the femoral head in dogs. Stem Cells Int.
2016:86161432016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park HW, Chang JW, Yang YS, Oh W, Hwang
JH, Kim DG and Paek SH: The effect of donor-dependent
administration of human umbilical cord blood-derived mesenchymal
stem cells following focal cerebral ischemia in rats. Exp
Neurobiol. 24:358–365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu Z, Guo S, Fang G, Cui Z and Liu Y: AKT
pathway affects bone regeneration in nonunion treated with
umbilical cord-derived mesenchymal stem cells. Cell Biochem
Biophys. 2014.
|
|
12
|
Qu Z, Guo L, Fang G, Cui Z, Guo S and Liu
Y: Biological characteristics and effect of human umbilical cord
mesenchymal stem cells (hUC-MSCs) grafting with blood plasma on
bone regeneration in rats. Cell Biochem Biophys. 63:171–181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qu Z, Fang G, Cui Z and Liu Y: Cell
therapy for bone nonunion: a retrospective study. Minerva Med.
106:315–321. 2015.PubMed/NCBI
|
|
14
|
Klimczak A and Kozlowska U: Mesenchymal
stromal cells and tissue-specific progenitor cells: their role in
tissue homeostasis. Stem Cells Int. 2016:42852152016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mattar P and Bieback K: Comparing the
immunomodulatory properties of bone marrow, adipose tissue, and
birth-associated tissue mesenchymal stromal cells. Front Immunol.
6:5602015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang
L, Liu B and Yu X: Treatment of early stage osteonecrosis of the
femoral head with autologous implantation of bone marrow-derived
and cultured mesenchymal stem cells. Bone. 50:325–330. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mao Q, Jin H, Liao F, Xiao L, Chen D and
Tong P: The efficacy of targeted intraarterial delivery of
concentrated autologous bone marrow containing mononuclear cells in
the treatment of osteonecrosis of the femoral head: a five year
follow-up study. Bone. 57:509–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Association Research Circulation Osseous,
. Committee on terminology and classification. ARCO News. 4:41–46.
1992.
|
|
19
|
Harris WH: Traumatic arthritis of the hip
after dislocation and acetabular fractures: treatment by mold
arthroplasty. An end-result study using a new method of result
evaluation. J Bone Joint Surg Am. 51:737–755. 1969.PubMed/NCBI
|
|
20
|
Zhao D, Zhang Y, Wang W, Liu Y, Li Z, Wang
B and Yu X: Tantalum rod implantation and vascularized iliac
grafting for osteonecrosis of the femoral head. Orthopedics.
36:789–795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kameneva MV, Watach MJ and Borovetz HS:
Gender difference in oxygen delivery index: Potential link to
development of cardiovascular diseases. Appl Cardiopulm
Pathophysiol. 9:382–387. 2000.
|
|
22
|
Cho YI and Cho DJ: Hemorheology and
microvascular disorders. Korean Circ J. 41:287–295. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai AG, Hofmann A, Cabrales P and
Intaglietta M: Perfusion vs. oxygen delivery in transfusion with
‘fresh’ and ‘old’ red blood cells: the experimental evidence.
Transfus Apheresis Sci. 43:69–78. 2010. View Article : Google Scholar
|
|
24
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
et al: Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu KH, Han ZC, Mo XM and Zhou B: Cell
delivery in cardiac regenerative therapy. Ageing Res Rev. 11:32–40.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barbash IM, Chouraqui P, Baron J, Feinberg
MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, et
al: Systemic delivery of bone marrow-derived mesenchymal stem cells
to the infarcted myocardium: feasibility, cell migration, and body
distribution. Circulation. 108:863–868. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Fang J, Su H, Yang M, Lai W, Mai
Y and Wu Y: Bone marrow mesenchymal stem cells attenuate lung
inflammation of hyperoxic newborn rats. Pediatr Transplant.
16:589–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Molina EJ, Palma J, Gupta D, Torres D,
Gaughan JP, Houser S and Macha M: Improvement in hemodynamic
performance, exercise capacity, inflammatory profile, and left
ventricular reverse remodeling after intracoronary delivery of
mesenchymal stem cells in an experimental model of pressure
overload hypertrophy. J Thorac Cardiovasc Surg. 135:292–299,
299.e1. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Freyman T, Polin G, Osman H, Crary J, Lu
M, Cheng L, Palasis M and Wilensky RL: A quantitative, randomized
study evaluating three methods of mesenchymal stem cell delivery
following myocardial infarction. Eur Heart J. 27:1114–1122. 2006.
View Article : Google Scholar : PubMed/NCBI
|