Introduction
IgA nephropathy (IgAN) is characterized by the
mesangial deposit of IgA-immune complex in the kidneys of patients
(1). The dimeric
IgA-secreting-bone marrow B cells are more abundant in patients
with IgAN (2) and evidence from
animal models suggests that CD19+ B cells are essential for the
pathogenesis of IgAN (3,4). B cell activation factor (BAFF) and a
proliferation inducing ligand (APRIL) are two important factors for
B cell homeostasis (5). BAFF is a
pivotal factor for B cell survival, proliferation and maturation
(6,7). BAFF Tg mice develop an IgAN-like
nephritic syndrome, in which IgA production is essential for renal
pathology (8). APRIL controls Ig
class-switch recombination towards IgA subclass and gene
polymorphism of the APRIL gene is associated with susceptibility to
IgAN (9,10). Two different transcript variants
(NM_006573.4 and NM_001145645.2) exist for the human BAFF gene,
resulting in two isoforms of 286aa and 267aa, respectively.
Following protein translation and N-glycosylation, Isoform 1
(NP_006564.1) exists either as full-length membrane-associated
form, or as a short secreted soluble protein which is cleaved at
Arg133/Asn134 by proteinase (11,12).
However, Isoform 2 (NP_001139117.1) is not subject to cleavage
following translation (12). BAFF
and APRIL share two receptors: Transmembrane activator and calcium
modulator cyclophilin ligand interactor (TACI) and B cell
maturation antigen (BCMA), whereas BAFF also binds to its unique
receptor: BAFF receptor (BAFF-R or BR3) (13,14).
All three receptors are type III transmembrane receptors, belonging
to the superfamily of tumor necrosis factor receptors (13) and are predominantly expressed by
different subsets of B cells (14).
BAFF is closely associated with autoimmune diseases
including systemic lupus erythematosus, rheumatoid arthritis and
Sjogren's syndrome, and has become a key therapeutic target for
several autoimmune diseases (15,16).
The serum levels of BAFF are reported to be elevated in autoimmune
diseases (17–20), which cause exaggerated Ig synthesis
and later immune imbalance (21,22).
Previously, several groups have provided data of serum BAFF levels
in IgAN. Xin et al (23)
determined that serum BAFF proteins were more abundant in patients
with IgAN and were associated with kidney function and renal
histopathology. Li et al (24) identified that serum BAFF levels
were significantly higher in patients with IgAN and were associated
with Toll-like receptor (TLR)-9 mRNA levels. However, McCarthy
et al (25) determined that
BAFF levels were not elevated in patients with IgAN based on data
from two cohorts. As BAFF expression data in peripheral blood of
patients with IgAN was not consistent and the combined association
of BAFF expression with disease activity was unclear, the present
study presented detailed data on BAFF expression in peripheral
blood system of patients with IgAN from several aspects: mRNA
levels in peripheral blood mononuclear cells (PBMCs), cellular
protein levels in PBMCs and plasma protein levels. The capacity of
PBMCs to secrete BAFF proteins following the activation of TLRs and
Streptococcus pyogenes (S. pyogenes) stimulation was
also examined to explore the possible role of BAFF in IgAN.
Materials and methods
Study groups
Verified patients with IgAN and with the clinical
features of primary IgAN were enrolled in the First Affiliated
Hospital of Sun Yat-sen University (Guangzhou, China). Healthy
donors and patients with nephritis who were diagnosed as primary
minimal change disease (MCD) or primary membranous nephropathy (MN)
were also included as disease controls in the present study. All
recruited donors were from the Chinese Han population. All healthy
participants were negative for hematuria and proteinuria, and
possessed normal serum creatinine content and liver function. All
of them provided written informed consent. Patients diagnosed as
end stage renal disease were excluded from the current study. None
of the patients had been treated with steroids and/or
immunosuppressive drugs within one year of the study. All donors
were free of clinical infection symptoms 4 days before and 3 days
following the day of the blood sample test, nor did they suffer
from systemic infection within one month of the test. The present
study obtained approval from the Ethics Review Committee of the
First Affiliated Hospital of Sun Yat-sen University (Guangzhou,
China) and was conducted in accordance with the guidelines proposed
in the Declaration of Helsinki.
The renal histopathology of patients with IgAN was
categorized according to the Oxford classification (26). Renal histopathology from all
patients was scored by two renal pathologists blinded to the
clinical data for the four pathological variables: The mesangial
hypercellularity (M), the segmental glomerulosclerosis (S), the
endocapillary hypercellularity (E) and the tubular
atrophy/interstitial fibrosis (T). Global glomerulosclerosis was
also scored according to the proportion of the total number of
glomeruli that exhibited glomerulosclerosis: G0, 0–25%; G1, 26–50%;
G2, >50% (27).
Main reagents
The reverse transcription kit (cat. no. RR014A) and
Real-Time Master kit (cat. no. DRR014A) were purchased from Takara
Bio, Inc. (Otsu, Japan); DNaseI (cat. no. AM2235) from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). BAFF antibody for
western blot analysis (cat. no. ab65360) from Abcam (Cambridge,
UK); BAFF capture antibody for ELISA (cat. no. AF124) from R&D
Systems (Minneapolis, MN, USA); BAFF detection antibody for ELISA
(cat. no. RHF910B) from Antigenix America (Huntington Station, NY,
USA); lipopolysaccharide (cat. no. L2880) from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany); Pam3CSK4 (cat. no.
ALK-165-006-M002) from Enzo Life Science, Inc. (Farmingdale, NY,
USA); and, CpG2395 (cat. no. Ctrl-2395) from Thermo Fisher
Scientific, Inc.
Design of primers
The cDNA nucleotide sequences of BAFF (NCBI RefSeq
NM_006573.4), APRIL (NCBI RefSeq NM_003808.3), TACI (NCBI RefSeq
NM_012452.2), BCMA (NCBI RefSeq NM_001192.2), BAFF-R (NCBI RefSeq
NM_052945.3), TLR2 (NCBI RefSeq NM_003264.3), TLR4 (NCBI RefSeq
NM_138554.4), TLR7 (NCBI RefSeq NM_016562.3), TLR9 (NCBI
RefSeqNM_017442.3), glyceraldehyde-3-phosphate dehydrogenase
(GAPDH; NCBI RefSeq NM_002046.4) were acquired from the NCBI
database (https://www.ncbi.nlm.nih.gov). Primers for each gene
were designed by Primer 3.0 software (http://frodo.wi.mit.edu) and confirmed with
non-redundant sequence in human cDNA sequence database. BAFF,
forward 5′-CGTTCAGGGTCCAGAAGAAA-3′ and
reverse5′-GTCCCATGGCGTAGGTCTTA-3′; APRIL, forward
5′-AGCCAGGTCCTGTTTCAAGA-3′ and reverse 5′-ATGGAAGACACCTGCGCTAT-3′;
BAFF-R, forward 5′-CCCTGGACAAGGTCATCATT-3′ and reverse
5′-TCTTGGTGGTCACCAGTTCA-3′; BCMA, forward
5′-GCAGTGCTCCCAAAATGAAT-3′ and reverse 5′-GTCCCAAACAGGTCCAGAGA-3′;
TACI, forward 5′-CATCTCCTGAGGGACTGCAT-3′ and reverse
5′-TGGTACCTTCCCGAGTTGTC-3′; TLR2, forward
5′-TGATGCTGCCATTCTCATTC-3′ and reverse 5′-CGCAGCTCTCAGATTTACCC-3′;
TLR4, forward 5′-TGAGCAGTCGTGCTGGTATC-3′ and reverse
5′-CAGGGCTTTTCTGAGTCGTC-3′; TLR7, forward
5′-GATGCCTTCCAGTTGCGATA-3′ and reverse 5′-TCCGTATGGTTAACCCACCA-3′;
TLR9, forward 5′-GGAAGGGACCTCGAGTGTGA-3′ and reverse
5′-AGCCAGTTGCAGTTCACCAG-3′; GAPDH, forward
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse
5′-GACAAGCTTCCCGTTCTCAG-3′.
Preparation of PBMCs and gene
expression analysis
PBMCs from venous blood with anticoagulant EDTA-K2
were enriched by Ficoll-paque density centrifugation (700 × g for
20 min). Following centrifugation, cells were washed four times
with PBS. Their viability was above 95% as observed with eosin red
staining. Total RNA of PBMCs was extracted using TRIzol reagent
following the manufacturer's protocols, then treated with DNase I
digestion. A total of 200 ng of RNA sample was subjected to reverse
transcriptase reaction for 30 min incubation at 37°C with the cDNA
synthesis kit DRR037A (Takara Bio, Inc., Otsu, Japan). The
quantitative reaction system was prepared following the protocol of
the quantitative polymerase chain reaction (PCR) kit and the
reaction was performed for 40 cycles of 95°C for 30 sec, 58°C for
30 sec and 72°C for 30 sec in an ABI7900 instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The data were analyzed
using SDS2.4 (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and Microsoft Excel software (Microsoft, Redmond, WA, USA). The
relative gene expression was calculated with the 2-∆∆Cq
method (28), using GAPDH as the
internal control.
Detection of cellular BAFF protein in
western blot analysis and plasma BAFF protein in ELISA
Proteins of PBMCs were extracted using RIPA buffer
and lysates were separated in SDS-PAGE. The proteins were
transferred onto nitrocellulose membranes, blocked with 5% dried
milk and then incubated with anti-human BAFF antibody (1:200; cat
no. Ab65360; Abcam) and secondary antibody (1:1,000; cat no.
711-035-152; Jackson Immuno Research Laboratories Inc., West Grove,
PA, USA) for chemiluminescence development.
Plasma samples were diluted 10 times
for BAFF ELISA
Briefly, 96-well plates coated with BAFF antibody (1
µg/ml; cat no. AF124, R&D Systems, Inc.) were blocked with 1%
BSA+1% FBS/PBS for 4 h at 37°C. The wells were washed with PBS
containing 0.05% Tween-20 (PBST) and incubated with the diluted
sample or standard BAFF proteins at 4°C overnight. Following
washing with PBST, the plates were then incubated sequentially with
100 µl biotinylated anti-human BAFF antibody (0.1 µg/ml; cat no.
RH910B; Antigenix America) at 37°C for 3 h, then
peroxidase-conjugated streptavidin in at 37°C for 60 min. The color
was developed with tetramethylbenzidine and stopped by adding 100
µl 2 N HCl. The optical density of each well was measured by
spectrophotometer (Spectra Max M5; Molecular Devices, LLC,
Sunnyvale, CA, USA) at OD450 nm.
Secretion of BAFF proteins from PBMCs
upon TLR ligands and S. pyogenes stimulation ex vivo
PBMCs isolated from Ficoll-paque density
centrifugation were suspended in complete 1640 medium (RPMI-1640
medium, 10% FBS, 100 µg/ml penicillin and streptomycin, 2 mM
L-glutamine, 100 mM sodium pyruvate, 55 mM β-mercaptoethanol; all
supplied by Thermo Fisher Scientific, Inc.) and then cultured at
37°C in a 5% CO2 incubator at a density of 2.5×105
PBMCs/well in a 96-well plate. The cells were stimulated with
different TLR ligands (TLR2-Pam3CSk4, 1 µg/ml; TLR4-LPS, 1 µg/ml;
and TLR9-CpG, 5 µg/ml) or heat-inactivated S. pyogenes
(2×107 CFU/ml). Following incubation for 72 h, the supernatant of
the cell culture was harvested and concentrated for BAFF ELISA.
Stimulated PBMCs were also collected for analysis of gene
expression in quantitative PCR. For preparation of S.
pyogenes, a single colony was cultured in tryptone yeast medium
plus 0.2% glucose overnight, then subcultured for another 48 h in
5% CO2 incubator at 37°C. The specificity of S.
pyogenes was additionally confirmed with S. pyogenes
specific primers in PCR as previously performed by Liu et al
(29). The CFU of bacteria was
counted on a blood agar plate. The harvested bacteria were
inactivated at 70°C for 1 h then stored at −80°C for later use.
Statistical analysis
Data were expressed as the mean ± standard error and
tested with Student's t-test. For data where three and more groups
were involved, a one-way analysis of variance analysis was
performed and additional post hoc tests were conducted when
P<0.05. The correlation was tested with Pearson's correlation
coefficients and presented with scatter plot. Statistical analyses
were performed with SPSS software, version 18.0 (SPSS, Inc.,
Chicago, IL, USA). All statistical assessments were two-sided using
a significance value of P<0.05, which was considered to indicate
a statistically significant difference.
Results
The mRNA levels of BAFF, APRIL and
their receptors in PBMCs of patients with IgAN
For mRNA level analysis, 51 healthy donors, 44
patients with IgAN and 22 disease controls (14 cases of MN, 8 cases
of MCD) were enrolled. The demographic and clinical parameters are
presented in Table I. The mRNA
levels of BAFF, APRIL, BCMA, TACI and BAFF-R in PBMCs were analyzed
by quantitative PCR as presented in Fig. 1A. The relative BAFF mRNA levels
demonstrated a small but not significant change in patients with
IgAN compared with healthy controls (0.044±0.002 vs. 0.040±0.002,
P=0.063) and disease controls (0.044±0.002 vs. 0.040±0.002,
P=0.16). The mRNA levels of APRIL were not significantly altered in
patients with IgAN, as compared with healthy or disease controls.
Notably, mRNA levels of BAFF-R, TACI and BCMA were all increased in
the individuals with IgAN, MCD and MN, as compared with the healthy
controls. Expression of BAFF-R was increased by 42.6% (P=0.096) in
patients with IgAN and by 86.3% (P<0.0001) in disease controls.
Expression of TACI was increased by 25.9% (P=0.083) in patients
with IgAN and by 52.4% (P=0.007) in disease controls. Expression of
BCMA was increased by 26.7% (P=0.273) in patients with IgAN and by
85.4% (P<0.0001) in disease controls.
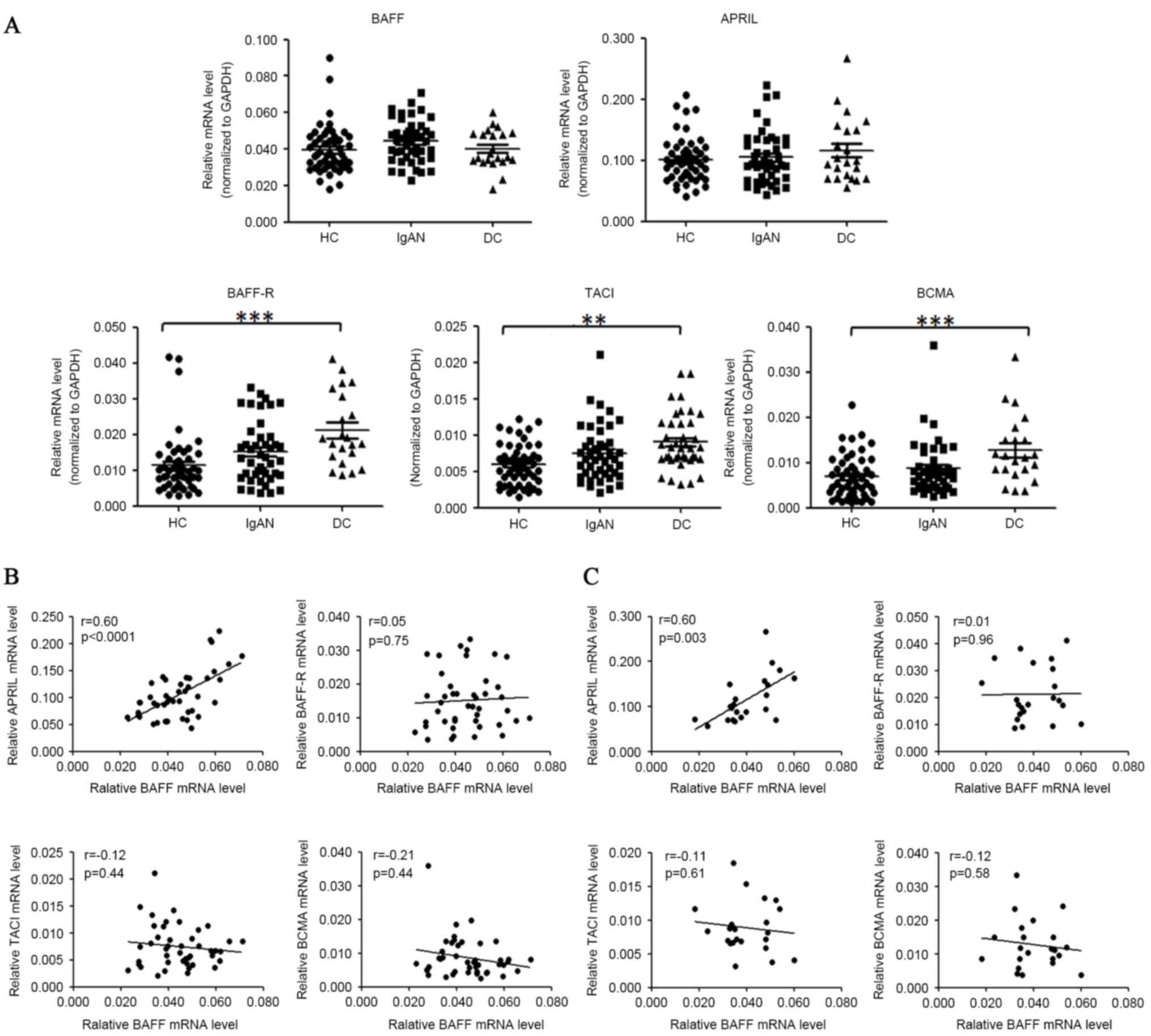 | Figure 1.BAFF mRNA levels were not
significantly elevated in the PBMCs of patients with IgAN. (A)
PBMCs of donors were prepared by Ficoll-paque density
centrifugation and subjected to quantitative PCR analysis for
target gene analysis. The horizontal line represents the mean value
of each group. HC, healthy controls; IgAN, patients with IgAN; DC,
disease controls (MCD/MN). (B) Correlation of BAFF mRNA levels with
APRIL, BAFF-R, TACI, BCMA mRNA levels in patients with IgAN. (C)
Correlation of BAFF mRNA levels with APRIL, BAFF-R, TACI, BCMA mRNA
levels in disease controls. *P<0.05, **P<0.01, ***P<0.001.
BAFF, B cell activation factor; PBMCs, peripheral blood cells;
IgAN, IgA nephropathy; PCR, polymerase chain reaction; MCD, minimal
change disease; MN, membranous nephropathy; APRIL, a proliferation
inducing ligand; BAFF-R, BAFF receptor; TACI, transmembrane
activator and calcium modulator cyclophilin ligand interactor;
BCMA, B cell maturation antigen; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
 | Table I.Clinical and demographic features of
patients with IgAN and disease controls. |
Table I.
Clinical and demographic features of
patients with IgAN and disease controls.
|
| BAFF mRNA
levels | BAFF protein levels
in plasma |
|---|
|
|
|
|
|---|
| Feature | Healthy
controls | IgAN | Disease
controls | Healthy
controls | IgAN | Disease
controls |
|---|
| Gender |
|
Male | 24 | 19 | 13 | 25 | 39 | 24 |
|
Female | 27 | 25 | 9 | 42 | 37 | 22 |
| Age (year) | 33.1±8.7 | 34.2±9.3 | 37.3±12.5 | 34.3±9.1 | 33.3±9.9 | 79.0±31.9 |
| Serum creatinine
(µM) |
| 87.8±41.0 | 70.6±18.1 |
| 104.5±61.9 | 79.0±31.9 |
| 24 h-proteinuria
(g/24 h) |
| 1.16±1.4 | 5.12±4.78 |
| 0.95±0.97 | 4.06±4.13 |
| Serum uric acid
(µM) |
| 362.7±115.5 | 423.9±80.6 |
| 404.7±123.0 | 380.4±91.3 |
| CKD I |
| 33 | 18 |
| 45 | 34 |
| CKD II |
| 5 | 3 |
| 10 | 8 |
| CKD III |
| 5 | 1 |
| 13 | 3 |
| CKD IV |
| 1 | 0 |
| 8 | 1 |
The association among mRNA levels of
BAFF, APRIL, their receptors and TLRs
Among the mRNA levels of BAFF, APRIL, BAFF-R, BCMA
and TACI in patients with IgAN, a marked positive association
existed between BAFF and APRIL mRNA levels (r=0.60, P<0.0001).
The mRNA abundance of BAFF was not significantly correlated with
those of its receptors (BAFF-R, BCMA and TACI) in either patients
with IgAN or disease controls (Fig.
1B). The positive association between mRNA levels of BAFF and
APRIL was also observed in disease controls (r=0.60, P=0.003,
Fig. 1C), however not in healthy
controls (r=0.084, P=0.54).
For correlation among mRNA levels of BAFF and those
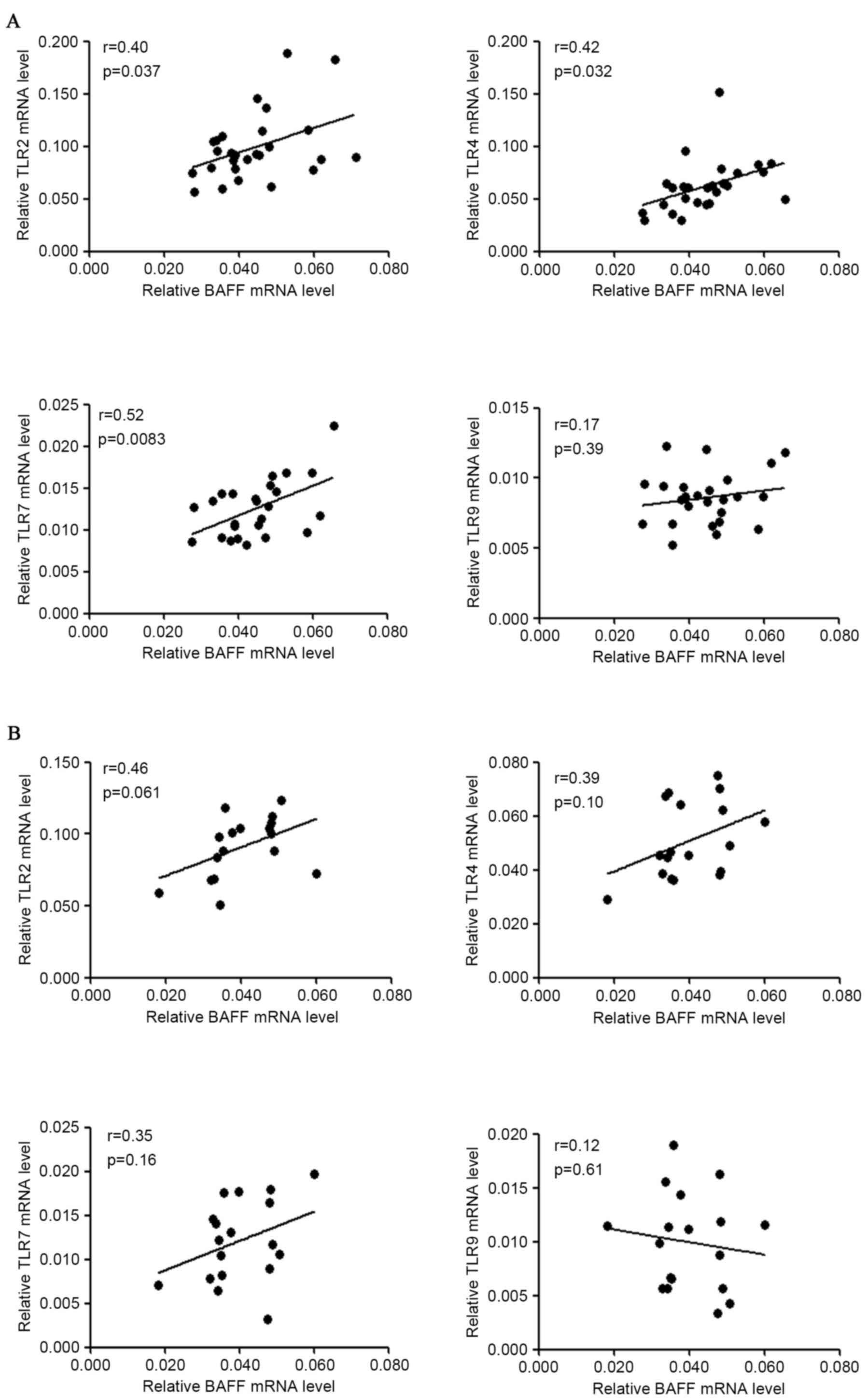
of TLRs, it was identified that BAFF mRNA levels were positively
associated with TLR2 (r=0.40, P=0.037), TLR4 (r=0.42, P=0.032) and
TLR7 (r=0.52, P=0.083) mRNA levels in patients with IgAN, but not
with TLR9 mRNA levels in patients with IgAN (Fig. 2A). BAFF mRNA levels were not
significantly associated with TLR2/4/7/9 mRNA levels in disease
controls (Fig. 2B). However, the
small sample size (n<20) may underestimate the possible
correlation of BAFF mRNA with TLRs mRNA in disease controls.
To analyze the possible association between BAFF
expression and clinical parameters, 10 parameters representing
renal function and immunological response were included for
statistical analysis. Statistical data demonstrated that there is
no significant correlation between BAFF mRNA levels and age, serum
creatinine, 24-h proteinuria, serum uric acid, C-reactive protein,
serum C3 protein, serum amyloid A, anti-streptolysin O, anti-DNase
B titer, serum IgA or serum IgG in patients with IgAN (Table II).
 | Table II.Association of BAFF expression levels
with clinical parameters in patients with IgAN. |
Table II.
Association of BAFF expression levels
with clinical parameters in patients with IgAN.
|
| BAFF mRNA levels in
PBMCs | BAFF protein levels
in plasma |
|---|
|
|
|
|
|---|
| Feature | Pearson r | P-value | Pearson r | P-value |
|---|
| Age (year) | −0.16 | 0.30 |
0.019 | 0.87 |
| Serum creatinine
(µMol/l) |
−0.014 | 0.93 | 0.25 | 0.03a |
| 24 h-proteinuria
(g/24 h) | −0.02 | 0.90 | 0.25 | 0.03a |
| Serum uric acid
(µMol/l) | −0.28 | 0.10 | 0.27 | 0.02a |
| Serum IgA
(g/l) | −0.05 | 0.76 | −0.17 | 0.18 |
| Serum IgG
(g/l) | −0.06 | 0.72 | −0.20 | 0.11 |
| Serum C3 (g/l) | −0.11 | 0.53 | −0.06 | 0.63 |
| CRP (mg/l) | −0.22 | 0.20 | −0.11 | 0.37 |
| SAA (mg/l) | −0.12 | 0.48 | −0.17 | 0.17 |
| ASO (kU/l) | −0.22 | 0.18 | −0.03 | 0.81 |
| Anti-DNase B
(IU/ml) | 0.17 | 0.31 | 0.25 | 0.04a |
BAFF protein levels in PBMCs and in
plasma of patients with IgAN
For the detection of cellular BAFF proteins in
PBMCs, 23 healthy controls and 21 patients with IgAN were enrolled
for this assay. Proteins extracted from PBMCs were analyzed by
western blot analysis to detect the cellular BAFF proteins. Two
clear bands at ~35 kDa for BAFF protein in western blot analysis
were detected (Fig. 3A). The
detected protein larger in size than predicted was possibly due to
N-glycosylation of BAFF protein as previously reported (30,31).
When normalized to GAPDH, the abundance of BAFF protein was not
significantly altered in the PBMCs of patients with IgAN when
compared with healthy controls (Fig.
3B).
 | Figure 3.Detection of BAFF proteins in PBMCs
and plasma of patients with IgAN. (A) Cellular BAFF proteins
detected in PBMCs of patients with IgAN. Proteins were isolated
from PBMCs of patients with IgAN and subjected to western blot
analysis. (B) Statistical analysis of BAFF protein content
normalized to GAPDH protein content in PBMCs. (C) Concentrations of
soluble BAFF protein in plasma were downregulated in patients with
IgAN. Diluted plasma samples were detected by ELISA for different
groups. (D) The correlation of BAFF mRNA levels with plasma BAFF
protein levels. HC, healthy controls; IgAN, patients with IgAN; DC,
disease controls (MCD/MN); LN, lupus nephritis. *P<0.05,
**P<0.01, ***P<0.001. BAFF, B cell activation factor; PBMCs,
peripheral blood mononuclear cells; IgAN, IgA nephropathy; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; MCD, minimal change
disease; MN, membranous nephropathy. |
For the detection of BAFF proteins in plasma, 67
healthy donors, 76 patients with IgAN, 48 disease controls and 20
patients with lupus nephritis (LN) were recruited. Each plasma
sample were repeated at least twice in ELISA. Serum BAFF levels
have been reported to be remarkably enhanced in patients with LN
(32,33), thus these samples were used as the
positive control (3,291±309 pg/ml) in the present study. However,
plasma BAFF proteins in patients with IgAN were significantly fewer
when compared with those in healthy controls (2,049±32.9 pg/ml vs.
2,422±54.2 pg/ml, P<0.0001) and in disease controls (2,049±32.9
pg/ml vs. 2,220±70.4 pg/ml, P=0.014; Fig. 3C). Meanwhile, the plasma BAFF
levels were not associated with BAFF mRNA levels in patients with
IgAN (n=27; Fig. 3D). Plasma BAFF
protein concentrations in female and male patients with IgAN were
not significantly different (2,049±52.2 pg/ml vs. 2,057±41.5 pg/ml,
P=0.91).
Despite the evidence that plasma levels of BAFF were
decreased in patients with IgAN, plasma BAFF levels were positively
associated with serum creatinine (r=0.25, P=0.03), 24-hproteinuria
(r=0.25, P=0.03), serum uric acid (r=0.27, P=0.02) and anti-DNase B
titer (r=0.25, P=0.04) in patients with IgAN (Table II), which was not observed in
disease controls (data not shown). Anti-DNase B titer is an index
for group A streptococcus (GAS) infection. S.
pyogenes is an important species of gram-positive bacterium
that can cause bacterial pharyngitis and the development of
post-streptococcal infection including acute glomerulonephritis,
rheumatic fever and reactive arthritis (34). The association of plasma BAFF
concentrations and renal histopathology in patients with IgAN was
also analyzed (Fig. 4). It was
determined that plasma BAFF protein levels were significantly
higher in patients with a high score in tubular
atrophy/interstitial fibrosis and global glomerulosclerosis. A
significant association in plasma BAFF levels and
glomerulosclerosis percentile (r=0.32, P=0.005) in all recruited
patients with IgAN was also identified. Plasma BAFF protein levels
were not associated with the grade of mesangial hypercellularity,
endocapillary hypercellularity or segmental glomerulosclerosis in
the kidney samples of patients with IgAN.
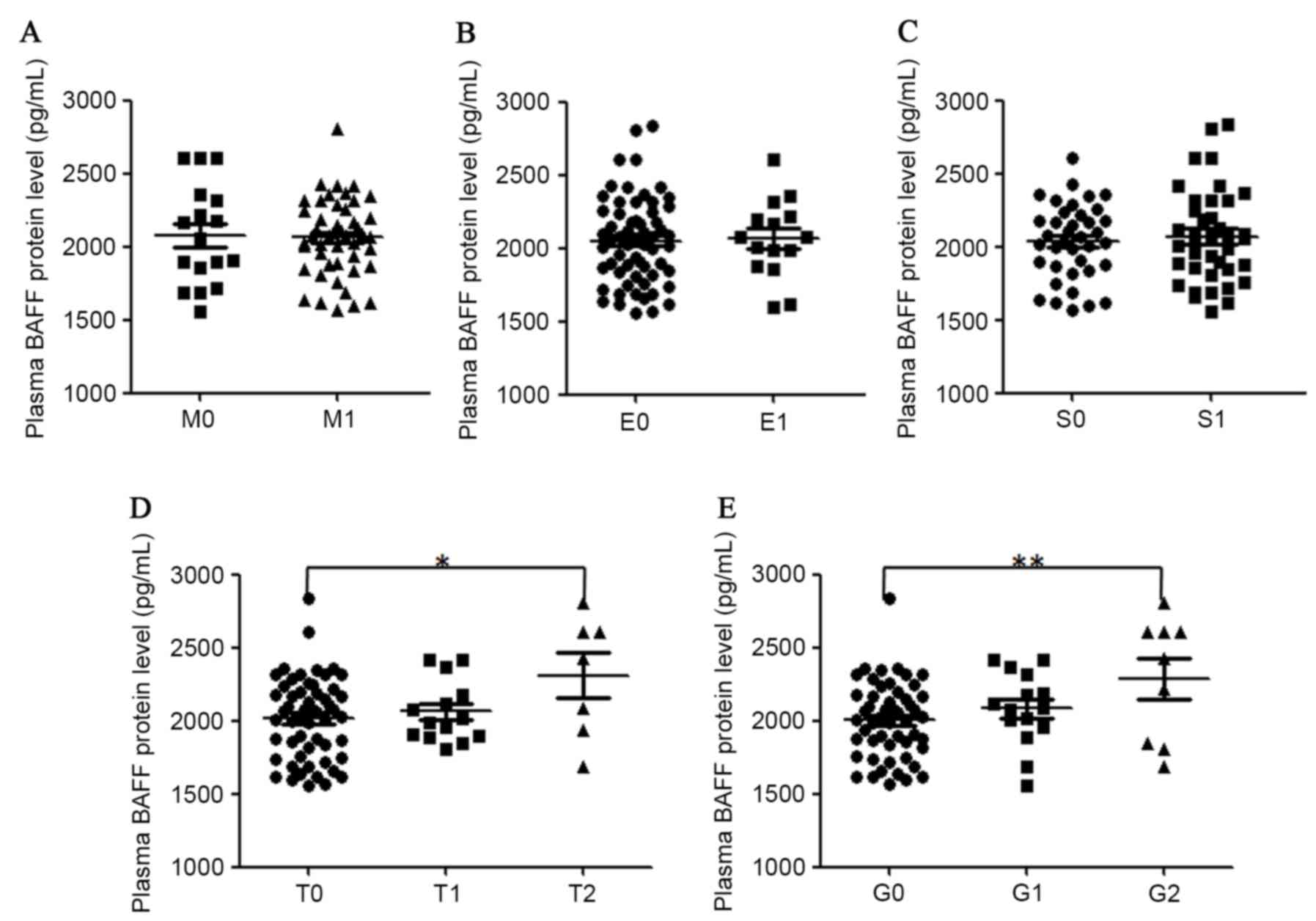 | Figure 4.Correlation of plasma levels of BAFF
protein with different renal histopathological parameters in
patients with IgAN. Plasma BAFF concentrations were classified
according to grades (A) M, (B) E, (C) S, (D) T and (E) G.
Statistical significance in different groups was analyzed with SPSS
software version 18. BAFF, B cell activation factor; IgAN, IgA
nephropathy; M, mesangial hypercellularity; S, segmental
glomerulosclerosis; E, endocapillary hypercellularity; T, tubular
atrophy/interstitial fibrosis; G, portion of global
glomerulosclerosis. |
The secretion of BAFF proteins from
PBMCs of patients with IgAN upon TLRs or S. pyogenes stimulation ex
vivo
As the mRNA levels of BAFF were closely associated
with those of TLRs, and plasma BAFF concentrations were associated
with S. pyogenes infection in patients with IgAN, the
secretion of BAFF proteins from PBMCs in the presence of TLRs
ligands or S. pyogenes stimulation ex vivo was
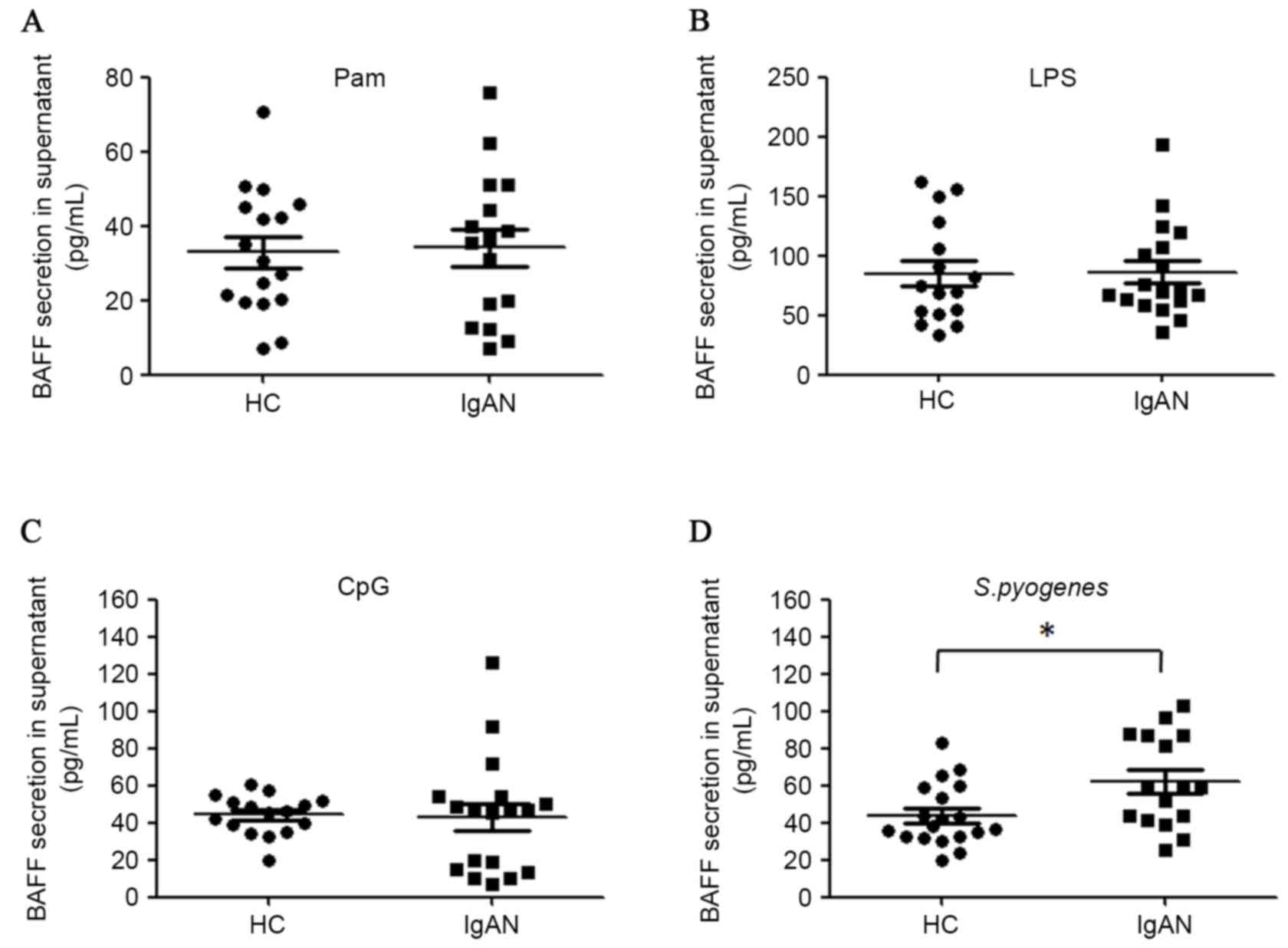
analyzed. TLR7/8 ligand R848 demonstrated very weak stimulatory
effect on BAFF secretion in the culture system (data not shown) and
therefore was not further investigated in the present study.
Activation of TLR2, TLR4 and TLR9 was able to induce BAFF synthesis
in PBMCs ex vivo. However, activation of these three TLRs by
ligands did not lead to more potent BAFF secretion in patients with
IgAN compared with healthy controls (Fig. 5A-C). However, it was demonstrated
that heat-inactivated S. pyogenes induced more BAFF
production in the PBMCs of patients with IgAN compared with healthy
controls (62.0 pg/ml vs. 43.9 pg/ml, P=0.015; Fig. 5D). Furthermore, mRNA levels of BAFF
and associated genes were analyzed following stimulation and the
result demonstrated that BAFF mRNA level was positively associated
with those of BAFF-R (r=0.50, P=0.03), TLR2 (r=0.74, P=0.0002),
TLR4 (r=0.70, P=0.001) and TLR7 (r=0.54, P=0.02) in S.
pyogenes-stimulated PBMCs from patients with IgAN (Fig. 6). A marginal but non-significant
correlation between the mRNA levels of BAFF and APRIL (r=0.44,
P=0.076) was also observed for patients with IgAN following S.
pyogenes stimulation.
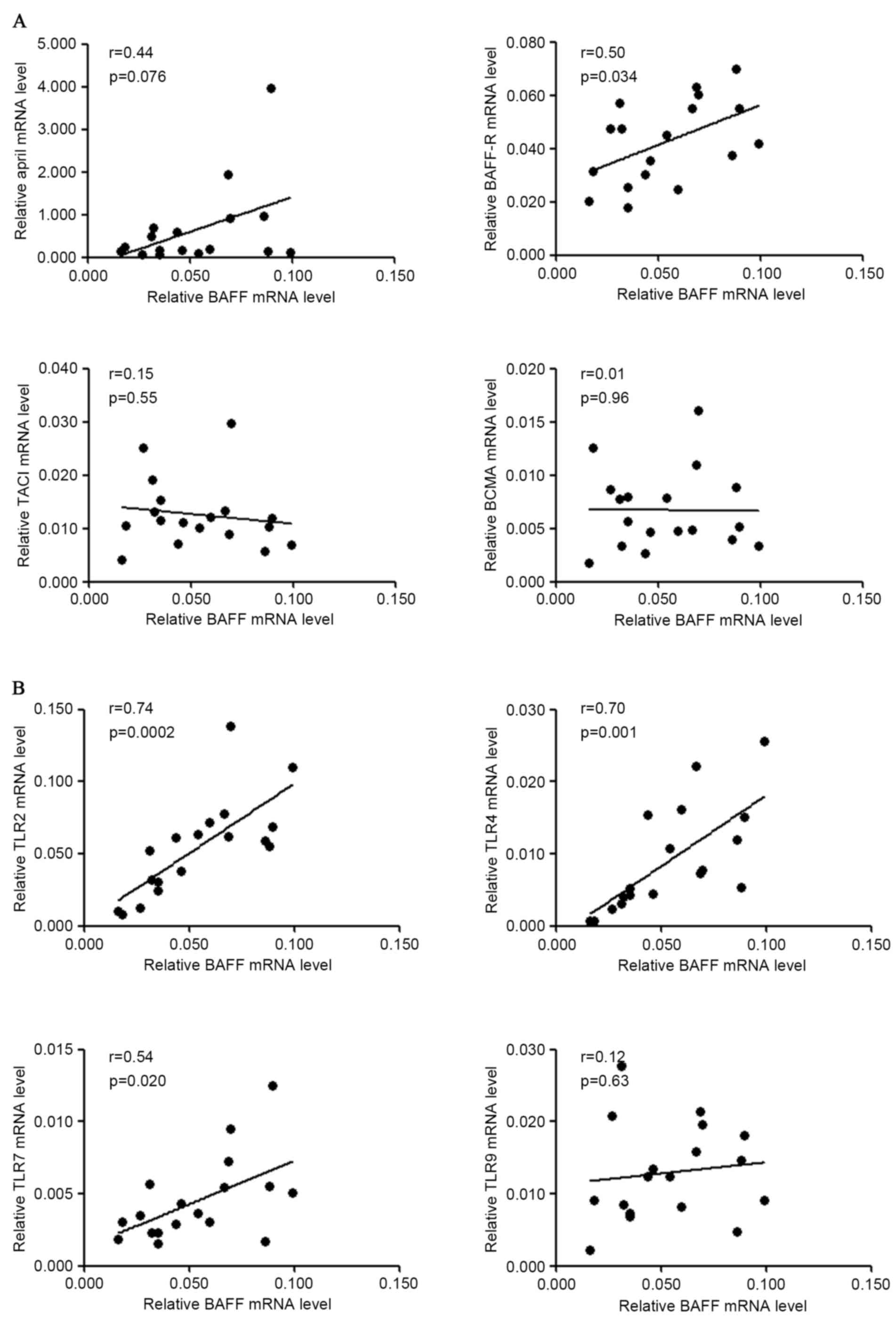 | Figure 6.Correlation of gene expression of
BAFF and other molecules in PBMCs following S. pyogenes
stimulation. PBMCs from patients with IgAN were cultured with
heat-inactivated S. pyogenes for 72 h and RNA was extracted
for gene expression analysis. (A) Correlation of BAFF mRNA levels
with APRIL, BAFF-R, TACI, BCMA mRNA levels in patients with IgAN.
(B) Correlation of BAFF mRNA levels with TLR2, TLR4, TLR7, TLR9
mRNA levels in patients with IgAN. BAFF, B cell activation factor;
PBMCs, peripheral blood mononuclear cells; IgAN, IgA nephropathy;
APRIL, a proliferation inducing ligand; BAFF-R, BAFF receptor;
TACI, transmembrane activator and calcium modulator cyclophilin
ligand interactor; BCMA, B cell maturation antigen; TLR, Toll-like
receptor. |
Discussion
The present study provided detailed data on the
abundance of plasma BAFF protein, in addition to BAFF mRNA levels
and cellular BAFF protein contents in peripheral blood of patients
with IgAN. The data demonstrated that, although BAFF abundance was
not higher in the peripheral blood systems of IgAN patients as
compared with healthy controls and disease controls, BAFF
concentration was closely associated with inflammatory factors,
kidney function and renal pathology. The previous data of Xin et
al (23) also demonstrated
that serum BAFF levels were significantly associated with clinical
features and glomerular histopathology in patients with IgAN.
However, they identified that BAFF serum levels were elevated in
Chinese patients with IgAN (23,24).
One possible reason for this discrepancy is that the present study
emphasized that all recruited donors had no clinical symptoms of
infection, since infection strongly evokes BAFF expression
(31,35) and PBMCs react quickly towards
infection. In the present study, all the donors were clear of any
symptoms of infection for at least 4 days prior to obtaining the
blood samples. Additionally, all donors were followed up for at
least 3 days to ensure their steady status. The exclusion of active
infection allowed the present study to investigate the basal
expression levels of BAFF and associated genes in PBMCs in patients
with IgAN. Evidence supporting this is that McCarthy et al
(8) tested serum BAFF levels in
two cohorts (Toronto and Alabama) and they did not observe
significant elevation of serum BAFF levels in patients with IgAN
when compared with healthy controls.
In the present study, soluble plasma BAFF protein
levels were significantly decreased in patients with IgAN while
BAFF mRNA levels and cellular BAFF protein levels in PBMCs were not
decreased. When interpreting these data, the transcription and
later processing of the BAFF gene should be considered. The BAFF
gene is translated into two isoforms with distinct functions, in
which isoform 1 can exists as a full-length membrane-associated
protein or a cleaved short soluble BAFF protein (11,12,31).
In quantitative PCR and western blot analysis, all transcript
variants/isoforms were targeted for measurement, while in ELISA,
only secreted BAFF proteins derived from isoform 1 of the BAFF
protein were detected. Another possible explanation is that BAFF
proteins are produced by numerous cells throughout the body
(14,36), not just peripheral blood cells. The
decreased plasma BAFF levels reflect fluctuation within the whole
body, not just one local site. Also, the binding of BAFF protein
(trimer or 60mers) to its receptors resulted in decreased free BAFF
proteins in circulation (13),
which possibly happened in IgAN since three receptors of BAFF were
all upregulated.
It was identified that BAFF mRNA levels were
strongly associated with APRIL mRNA levels in patients with IgAN,
in addition to patients with MCD and MN, however not in healthy
controls. This result indicated that certain common factors in
different types of nephritis regulated the BAFF and APRIL
expression with a similar mechanism. Upregulated gene expression of
BAFF-R, TACI and BCMA in patients with IgAN, MCD and MN was also
detected, which was a new evidence for nephritis. Another novel
finding was that in patients with IgAN, gene expression of BAFF was
closely associated with those of TLR2, 4 and 7 in PBMCs whether
freshly isolated or stimulated by group A Streptococcus,
which indicated that BAFF synthesis was closely associated with
TLRs abundance in IgAN. He et al (37) identified that TLR3 activation in
tonsillar monocytes of patients with IgAN led to enhanced secretion
of BAFF and later augmented IgA synthesis.
The connection between IgAN and GAS infection has
been reported. The pathogenic antigen of group A
Streptococcus-M protein, was detected as deposits in the
kidney biopsies of patients with IgAN (38) and provoked the proliferation of
IgA-positive B cells in vitro (39). The evidence that
Streptococci promoted IgA class-switch recombination in
patients with IgAN (40)
additionally supported the hypothesis that it was closely
associated with B cell dysfunction in IgAN. The present study
identified that plasma levels of BAFF protein were positively
associated with anti-DNase B titer in patients with IgAN, however
not in disease controls (r=−0.16, P=0.28). More notably, secretion
of soluble BAFF proteins was enhanced in the PBMCs of patients with
IgAN upon heat-inactivated stimulation with S. pyogenes,
compared with healthy controls. This result indicated that BAFF
synthesis was sensitively controlled by the action of GAS in IgAN,
which suggested a novel possible mechanism of IgAN pathogenesis.
S. pyogenes usually launches its first attack on the upper
respiratory mucosal system, therefore it is possible that the upper
respiratory immune system is crucial in regulating BAFF homeostasis
in IgAN.
BAFF is expressed by a wide range of cells and
organs within the body and its expression is regulated by multiple
cytokines and pathogen antigens, in addition to the local
inflammatory environment (22,35,41,42).
BAFF expression in the PBMCs was a part of the complicated BAFF
production system, concurrent with its production in local
inflammation sites (43). The
present study identified that plasma BAFF levels were positively
associated with index of S. pyogenes infection and that the
ability of producing BAFF was augmented in PBMCs of patients with
IgAN upon being activated by S. pyogenes ex vivo. However,
the total plasma BAFF levels were significantly decreased in
patients with IgAN, which remains to be elucidated. In the case of
patients with lupus, the serum BAFF levels were not associated with
BAFF mRNA levels in PBMCs, but rather associated with CRP levels
(17,44), which also indicated that the origin
and regulation of BAFF production was rather complex. To solve this
puzzle, the production of BAFF within the whole body and the
downstream consumption of BAFF requires careful dissection.
However, the way in which the immune system responds to GAS
infection remains important for demonstrating the regulation of
BAFF synthesis in patients with IgAN.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31200664) and the
Young Researcher Foundation of Sun Yat-sen University, China (grant
no. 14YKPY17).
Glossary
Abbreviations
Abbreviations:
|
IgAN
|
IgA nephropathy
|
|
BAFF
|
B cell activation factor
|
|
APRIL
|
a proliferation inducing ligand
|
|
TACI
|
transmembrane activator and calcium
modulator cyclophilin ligand interactor
|
|
BCMA
|
B cell maturation antigen
|
|
BAFF-R
|
BAFF receptor
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
MCD
|
minimal change disease
|
|
MN
|
membranous nephropathy
|
|
LN
|
lupus nephritis
|
|
TLR
|
Toll-like receptor
|
References
|
1
|
Barratt J and Feehally J: Primary IgA
nephropathy: New insights into pathogenesis. Semin Nephrol.
31:349–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harper SJ, Allen AC, Pringle JH and
Feehally J: Increased dimeric IgA producing B cells in the bone
marrow in IgA nephropathy determined by in situ hybridisation for J
chain mRNA. J Clin Pathol. 49:38–42. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boyd JK, Cheung CK, Molyneux K, Feehally J
and Barratt J: An update on the pathogenesis and treatment of IgA
nephropathy. Kidney Int. 81:833–843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakata J, Suzuki Y, Suzuki H, Sato D, Kano
T, Horikoshi S, Novak J and Tomino Y: Experimental evidence of cell
dissemination playing a role in pathogenesis of IgA nephropathy in
multiple lymphoid organs. Nephrol Dial Transplant. 28:320–326.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mackay F and Ambrose C: The TNF family
members BAFF and APRIL: The growing complexity. Cytokine Growth
Factor Rev. 14:311–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rolink AG and Melchers F: BAFFled B cells
survive and thrive: Roles of BAFF in B-cell development. Curr Opin
Immunol. 14:266–275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang M, Hase H, Legarda-Addison D,
Varughese L, Seed B and Ting AT: B cell maturation antigen, the
receptor for a proliferation-inducing ligand and B cell-activating
factor of the TNF family, induces antigen presentation in B cells.
J Immunol. 175:2814–2824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mccarthy DD, Kujawa J, Wilson C, Papandile
A, Poreci U, Porfilio EA, Ward L, Lawson MA, Macpherson AJ, McCoy
KD, et al: Mice overexpressing BAFF develop a commensal
flora-dependent, IgA-associated nephropathy. J Clin Invest.
121:3991–4002. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castigli E, Scott S, Dedeoglu F, Bryce P,
Jabara H, Bhan AK, Mizoguchi E and Geha RS: Impaired IgA class
switching in APRIL-deficient mice. Proc Natl Acad Sci USA.
101:3903–3908. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu XQ, Li M, Zhang H, Low HQ, Wei X, Wang
JQ, Sun LD, Sim KS, Li Y, Foo JN, et al: A genome-wide association
study in Han Chinese identifies multiple susceptibility loci for
IgA nephropathy. Nat Genet. 44:178–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schneider P, Mackay F, Steiner V, Hofmann
K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea
H, et al: BAFF, a novel ligand of the tumor necrosis factor family,
stimulates B cell growth. J Exp Med. 189:1747–1756. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gavin AL, Ait-Azzouzene D, Ware CF and
Nemazee D: DeltaBAFF, an alternate splice isoform that regulates
receptor binding and biopresentation of the B cell survival
cytokine, BAFF. J Biol Chem. 278:38220–38228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bossen C and Schneider P: BAFF, APRIL and
their receptors: Structure, function and signaling. Semin Immunol.
18:263–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mackay F and Schneider P: Cracking the
BAFF code. Nat Rev Immunol. 9:491–502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vincent FB, Morand EF and Mackay F: BAFF
and innate immunity: New therapeutic targets for systemic lupus
erythematosus. Immunol Cell Biol. 90:293–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsushita T and Sato S: The role of BAFF
in autoimmune diseases. Nihon Rinsho Meneki Gakkai Kaishi.
28:333–342. 2005.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eilertsen GO, Van Ghelue M, Strand H and
Nossent JC: Increased levels of BAFF in patients with systemic
lupus erythematosus are associated with acute-phase reactants,
independent of BAFF genetics: A case-control study. Rheumatology
(Oxford). 50:2197–2205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsushita T, Hasegawa M, Yanaba K, Kodera
M, Takehara K and Sato S: Elevated serum BAFF levels in patients
with systemic sclerosis: Enhanced BAFF signaling in systemic
sclerosis B lymphocytes. Arthritis Rheum. 54:192–201. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bosello S, Youinou P, Daridon C, Tolusso
B, Bendaoud B, Pietrapertosa D, Morelli A and Ferraccioli G:
Concentrations of BAFF correlate with autoantibody levels, clinical
disease activity, and response to treatment in early rheumatoid
arthritis. J Rheumatol. 35:1256–1264. 2008.PubMed/NCBI
|
|
20
|
Mariette X, Roux S, Zhang J, Bengoufa D,
Lavie F, Zhou T and Kimberly R: The level of BLyS (BAFF) correlates
with the titre of autoantibodies in human Sjögren's syndrome. Ann
Rheum Dis. 62:168–171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pers JO, Daridon C, Devauchelle V, Jousse
S, Saraux A, Jamin C and Youinou P: BAFF overexpression is
associated with autoantibody production in autoimmune diseases. Ann
N Y Acad Sci. 1050:34–39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moisini I and Davidson A: BAFF: A local
and systemic target in autoimmune diseases. Clin Exp Immunol.
158:155–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xin G, Shi W, Xu LX, Su Y, Yan LJ and Li
KS: Serum BAFF is elevated in patients with IgA nephropathy and
associated with clinical and histopathological features. J Nephrol.
26:683–690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li W, Peng X, Liu Y, Liu H, Liu F, He L,
Liu Y, Zhang F, Guo C, Chen G, et al: TLR9 and BAFF: Their
expression in patients with IgA nephropathy. Mol Med Rep.
10:1469–1474. 2014.PubMed/NCBI
|
|
25
|
Mccarthy DD, Chiu S, Gao Y, Summers-Deluca
LE and Gommerman JL: BAFF induces a hyper-IgA syndrome in the
intestinal lamina propria concomitant with IgA deposition in the
kidney independent of LIGHT. Cell Immunol. 241:85–94. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Working Group of the International IgA
Nephropathy Network and the Renal Pathology Society, ; Cattran DC,
Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE,
Amore A, Barratt J, et al: The Oxford classification of IgA
nephropathy: Rationale, clinicopathological correlations, and
classification. Kidney Int. 76:534–545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kataoka H, Ohara M, Shibui K, Sato M,
Suzuki T, Amemiya N, Watanabe Y, Honda K, Mochizuki T and Nitta K:
Overweight and obesity accelerate the progression of IgA
nephropathy: Prognostic utility of a combination of BMI and
histopathological parameters. Clin Exp Nephrol. 16:706–712. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu D, Hollingshead S, Swiatlo E, Lawrence
ML and Austin FW: Rapid identification of Streptococcus pyogenes
with PCR primers from a putative transcriptional regulator gene.
Res Microbiol. 156:564–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parameswaran R, Muschen M, Kim YM, Groffen
J and Heisterkamp N: A functional receptor for B-cell-activating
factor is expressed on human acute lymphoblastic leukemias. Cancer
Res. 70:4346–4356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nardelli B, Belvedere O, Roschke V, Moore
PA, Olsen HS, Migone TS, Sosnovtseva S, Carrell JA, Feng P, Giri JG
and Hilbert DM: Synthesis and release of B-lymphocyte stimulator
from myeloid cells. Blood. 97:198–204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang XF, Yuan SL, Jiang L, Zhang XL, Li
SF, Guo Y, Wu CL and Chen JJ: Changes of serum BAFF and IL-21
levels in patients with systemic lupus erythematosus and their
clinical significance. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
23:1041–1042. 2007.(In Chinese). PubMed/NCBI
|
|
33
|
Vincent FB, Northcott M, Hoi A, Mackay F
and Morand EF: Association of serum B cell activating factor from
the tumour necrosis factor family (BAFF) and a
proliferation-inducing ligand (APRIL) with central nervous system
and renal disease in systemic lupus erythematosus. Lupus.
22:873–884. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cunningham MW: Pathogenesis of group A
streptococcal infections. Clin Microbiol Rev. 13:470–511. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huard B, Arlettaz L, Ambrose C, Kindler V,
Mauri D, Roosnek E, Tschopp J, Schneider P and French LE: BAFF
production by antigen-presenting cells provides T cell
co-stimulation. Int Immunol. 16:467–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schneider P: The role of APRIL and BAFF in
lymphocyte activation. Curr Opin Immunol. 17:282–289. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He L, Peng X, Wang J, Tang C, Zhou X, Liu
H, Liu F, Sun L and Peng Y: Synthetic double-stranded RNA Poly
(I:C) aggravates IgA nephropathy by triggering IgA class switching
recombination through the TLR3-BAFF axis. Am J Nephrol. 42:185–197.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schmitt R, Carlsson F, Mörgelin M, Tati R,
Lindahl G and Karpman D: Tissue deposits of IgA-binding
streptococcal M proteins in IgA nephropathy and Henoch-Schonlein
purpura. Am J Pathol. 176:608–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nishikawa Y, Shibata R, Ozono Y, Ichinose
H, Miyazaki M, Harada T and Kohno S: Streptococcal M protein
enhances TGF-beta production and increases surface IgA-positive B
cells in vitro in IgA nephropathy. Nephrol Dial Transplant.
15:772–777. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu H, Peng Y, Liu F, Xiao W, Zhang Y and
Li W: Expression of IgA class switching gene in tonsillar
mononuclear cells in patients with IgA nephropathy. Inflamm Res.
60:869–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chu VT, Enghard P, Schurer S, Steinhauser
G, Rudolph B, Riemekasten G and Berek C: Systemic activation of the
immune system induces aberrant BAFF and APRIL expression in B cells
in patients with systemic lupus erythematosus. Arthritis Rheum.
60:2083–2093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Y, Enoksson S Lind, Johansson C,
Karlsson MA, Lundeberg L, Nilsson G, Scheynius A and Karlsson MC:
The expression of BAFF, APRIL and TWEAK is altered in eczema skin
but not in the circulation of atopic and seborrheic eczema
patients. PLoS One. 6:e222022011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nakajima K, Itoh K, Nagatani K,
Okawa-Takatsuji M, Fujii T, Kuroki H, Katsuragawa Y, Aotsuka S and
Mimori A: Expression of BAFF and BAFF-R in the synovial tissue of
patients with rheumatoid arthritis. Scand J Rheumatol. 36:365–372.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stohl W, Metyas S, Tan SM, Cheema GS,
Oamar B, Xu D, Roschke V, Wu Y, Baker KP and Hilbert DM: B
lymphocyte stimulator overexpression in patients with systemic
lupus erythematosus: Longitudinal observations. Arthritis Rheum.
48:3475–3486. 2003. View Article : Google Scholar : PubMed/NCBI
|