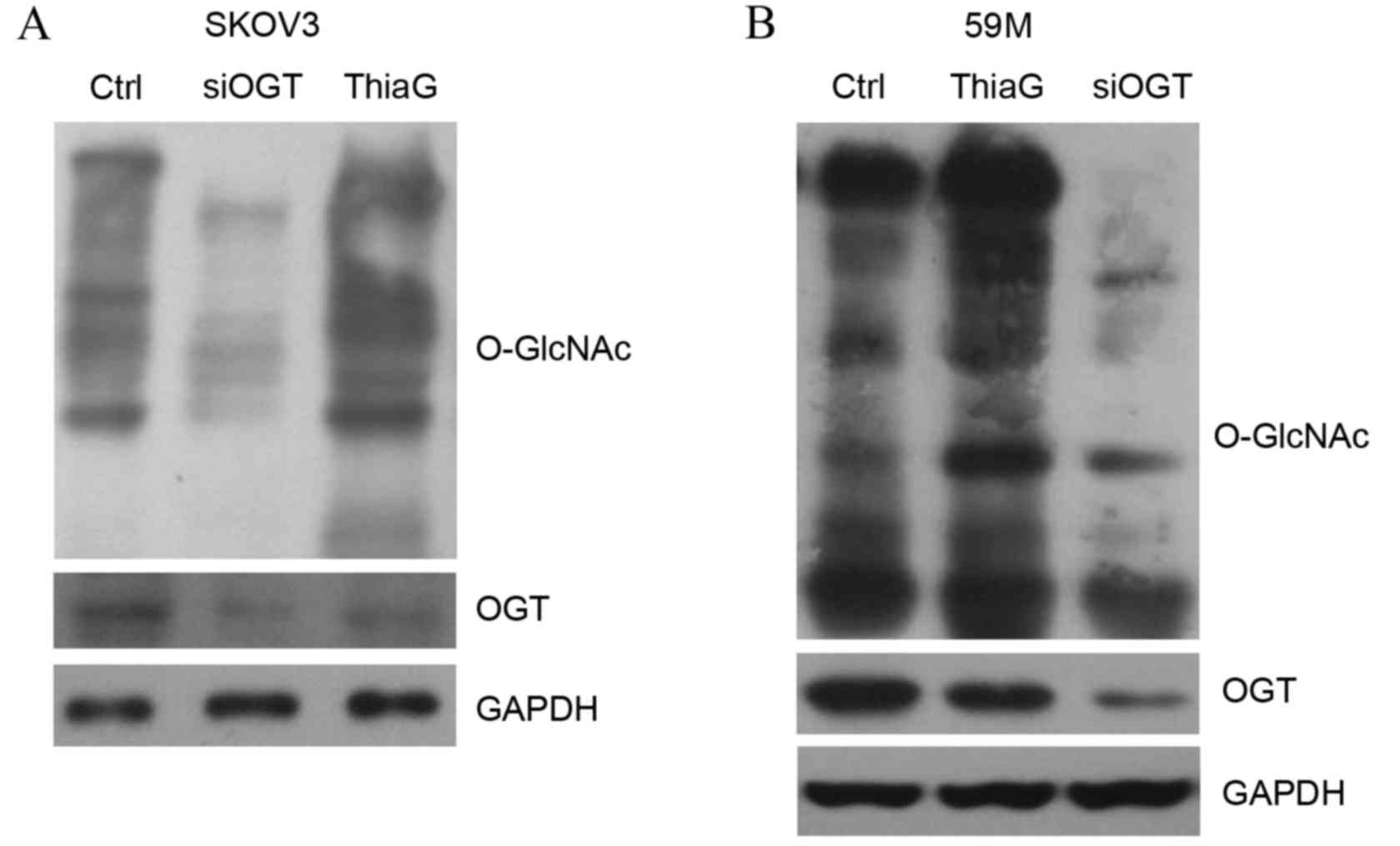
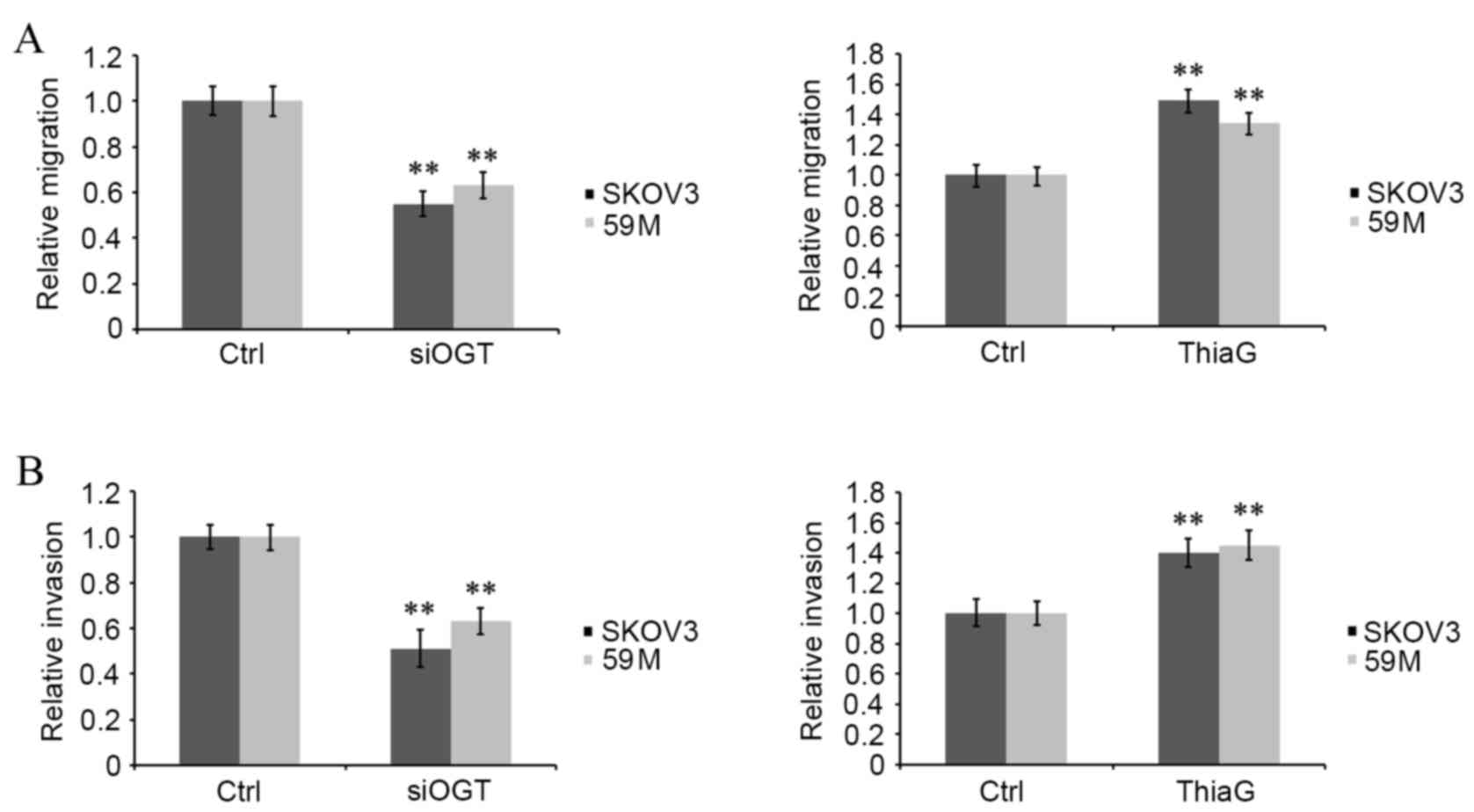
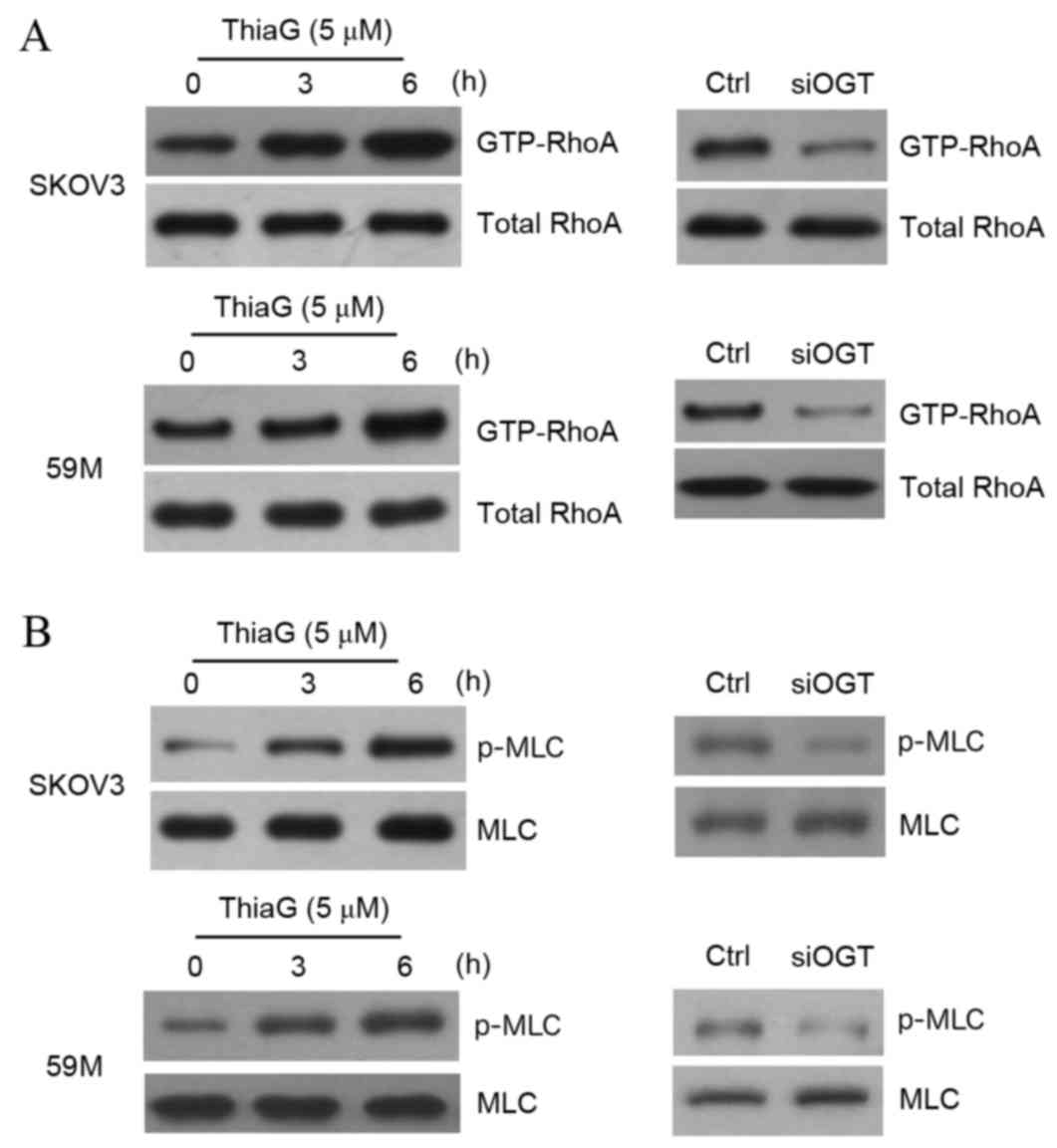
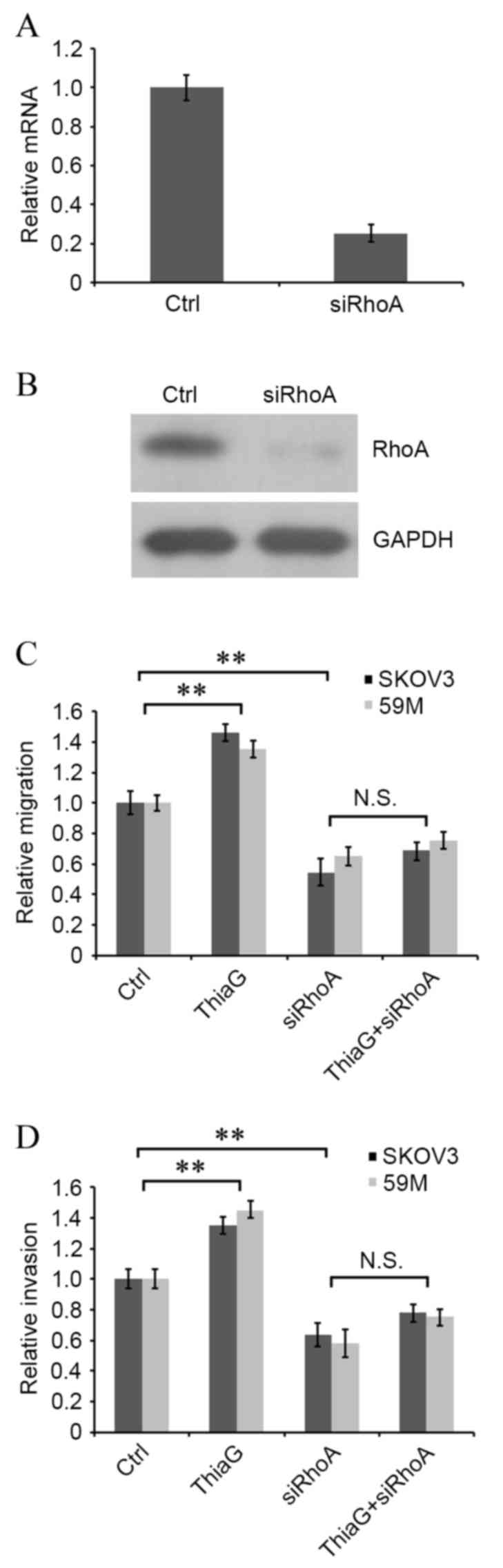
|
1
|
Torres CR and Hart GW: Topography and
polypeptide distribution of terminal N-acetylglucosamine residues
on the surfaces of intact lymphocytes. Evidence for O-linked
GlcNAc. J Biol Chem. 259:3308–3317. 1984.PubMed/NCBI
|
|
2
|
Hart GW, Slawson C, Ramirez-Correa G and
Lagerlof O: Cross talk between O-GlcNAcylation and phosphorylation:
Roles in signaling, transcription, and chronic disease. Annu Rev
Biochem. 80:825–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hart GW, Housley MP and Slawson C: Cycling
of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins.
Nature. 446:1017–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wells L, Vosseller K and Hart GW:
Glycosylation of nucleocytoplasmic proteins: Signal transduction
and O-GlcNAc. Science. 291:2376–2378. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanover JA: Glycan-dependent signaling:
O-linked N-acetylglucosamine. FASEB J. 15:1865–1876. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lazarus BD, Love DC and Hanover JA:
O-GlcNAc cycling: Implications for neurodegenerative disorders. Int
J Biochem Cell Biol. 41:2134–2146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X, Ongusaha PP, Miles PD, Havstad JC,
Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ and
Evans RM: Phosphoinositide signalling links O-GlcNAc transferase to
insulin resistance. Nature. 451:964–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slawson C and Hart GW:
O-GlcNAc-signalling: Implications for cancer cell biology. Nat Rev
Cancer. 11:678–684. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zachara NE and Hart GW: Cell signaling,
the essential role of O-GlcNAc. Biochim Biophys Acta. 1761:599–617.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chou TY, Hart GW and Dang CV: C-Myc is
glycosylated at threonine 58, a known phosphorylation site and a
mutational hot spot in lymphomas. J Biol Chem. 270:18961–18965.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kamemura K, Hayes BK, Comer FI and Hart
GW: Dynamic interplay between O-glycosylation and O-phosphorylation
of nucleocytoplasmic proteins. Alternative
glycosylation/phosphorylation of Thr-58, a known mutational hot
spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem.
277:19229–19235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C,
Yang J, Han F, Lu X and Yu W: GlcNAcylation plays an essential role
in breast cancer metastasis. Cancer Res. 70:6344–6351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X,
Cong Q and Yu W: O-GlcNAcylationis a novel regulator of lung and
colon cancer malignancy. Biochim Biophys Acta. 1812:514–519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yehezkel G, Cohen L, Kliger A, Manor E and
Khalaila I: O-linked β-N-acetylglucosaminylation (O-GlcNAcylation)
in primary and metastatic colorectal cancer clones and effect of
N-acetyl-beta-D-glucosaminidase silencing on cell phenotype and
transcriptome. J Biol Chem. 287:28755–28769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma Z, Vocadlo DJ and Vosseller K:
Hyper-OglcNAcylation is anti-apoptotic and maintains constitutive
NF-κB activity in pancreatic cancer cells. J Biol Chem.
288:15121–15130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lynch TP, Ferrer CM, Jackson SR, Shahriari
KS, Vosseller K and Reginato MJ: Critical role of O-Linked
β-N-acetylglucosamine transferase in prostate cancer invasion,
angiogenesis, and metastasis. J Biol Chem. 287:11070–11081. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: GLOBOCAN 2008v1.2. Cancer incidence,
mortality and prevalence worldwideIARC Cancer Base No 10
[Internet]. Lyon, France: IARC Press; 2010
|
|
18
|
Treating advanced ovarian cancer.
https://www.cancerresearchuk.orgMay
16–2015.
|
|
19
|
Ruddon RW: Cancer biology. 4th edition.
Oxford University Press; Oxford: pp. 2232007
|
|
20
|
Basile JR, Gavard J and Gutkind JS:
Plexin-B1 utilizes RhoA and Rho kinase to promote the
integrin-dependent activation of Akt and ERK and endothelial cell
motility. J Biol Chem. 282:34888–34895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nikonova E, Tsyganov MA, Kolch W, Fey D
and Kholodenko BN: Control of the G-protein cascade dynamics by GDP
dissociation inhibitors. Mol Biosyst. 9:2454–2462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Samuel MS, Lopez JI, McGhee EJ, Daniel R,
Croft DR, Strachan D, Timpson P, Munro J, Schröder E, Zhou J, et
al: Actomyosin-mediated cellular tension drives increased tissue
stiffness and β-catenin activation to induce epidermal hyper-plasia
and tumor growth. Cancer Cell. 19:776–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rösel D, Brábek J, Tolde O, Mierke CT,
Zitterbart DP, Raupach C, Bicanová K, Kollmannsberger P, Panková D,
Vesely P, et al: Up-regulation of Rho/ROCK signaling in sarcoma
cells drives invasion and increased generation of protrusive
forces. Mol Cancer Res. 6:1410–1420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gadea G, De Toledo M, Anguille C and Roux
P: Loss of p53 promotes RhoA-ROCK-dependent cell migration and
invasion in 3D matrices. J Cell Biol. 178:23–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amano M, Ito M, Kimura K, Fukata Y,
Chihara K, Nakano T, Matsuura Y and Kaibuchi K: Phosphorylation and
activation of myosin by Rho-associated kinase (Rho-kinase). J Biol
Chem. 271:20246–20249. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Riento K and Ridley AJ: Rocks:
Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kolodney MS and Elson EL: Contraction due
to microtubule disruption is associated with increased
phosphorylation of myosin regulatory light chain. Proc Natl Acad
Sci USA. 92:10252–10256. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.PubMed/NCBI
|
|
29
|
Pan Y, Bi F, Liu N, Xue Y, Yao X, Zheng Y
and Fan D: Expression of seven main Rho family members in gastric
carcinoma. Biochem Biophys Res Commun. 315:686–691. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang WG, Watkins G, Lane J, Cunnick GH,
Douglas-Jones A, Mokbel K and Mansel RE: Prognostic value of rho
GTPases and rho guanine nucleotide dissociation inhibitors in human
breast cancers. Clin Cancer Res. 9:6432–6440. 2003.PubMed/NCBI
|
|
31
|
Kamai T, Yamanishi T, Shirataki H, Takagi
K, Asami H, Ito Y and Yoshida K: Overexpression of RhoA, Rac1, and
CDC42 GTPases is associated with progression in testicular cancer.
Clin Cancer Res. 10:4799–4805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horiuchi A, Imai T, Wang C, Ohira S, Feng
Y, Nikaido T and Konishi I: Up-regulation of small GTPases, RhoA
and RhoC, is associated with tumor progression in ovarian
carcinoma. Lab Invest. 83:861–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao XQ and Lu X: Expression of RhoA and
ROCK in breast carcinomas and their significance. J Radioimmunol.
4:439–442. 2012.
|
|
34
|
Wang X, Jiang W, Kang J, Liu Q and Nie M:
Knockdown of RhoA expression alters ovarian cancer biological
behavior in vitro and in nude mice. Oncol Rep. 34:891–899.
2015.PubMed/NCBI
|
|
35
|
Slack JL, Bi W, Livak KJ, Beaubier N, Yu
M, Clark M, Kim SH, Gallagher RE and Willman CL: Pre-clinical
validation of a novel, highly sensitive assay to detect
PML-RARalpha mRNA using real-time reverse-transcription polymerase
chain reaction. J Mol Diagn. 3:141–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu Y, Zhang J, Mi W, Yang J, Han F, Lu X
and Yu W: Silencing of GM3 synthase suppresses lung metastasis of
murine breast cancer cells. Breast Cancer Res. 10:R12008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yanagisawa M and Anastasiadis PZ: p120
catenin is essential for mesenchymal cadherin-mediated regulation
of cell motility and invasiveness. J Cell Biol. 174:1087–1096.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Uehata M, Ishizaki T, Satoh H, Ono T,
Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M
and Narumiya S: Calcium sensitization of smooth muscle mediated by
a Rho-associated protein kinase in hypertension. Nature.
389:990–994. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Uehata M: Y-27632. Selective probe of
ROCK/Rho-kinase. JikkenIgaku. 17:850–855. 1999.
|
|
40
|
Magtibay PM, Adams PB, Silverman MB, Cha
SS and Podratz KC: Splenectomy as part of cytoreductive surgery in
ovarian cancer. Gynecol Oncol. 102:369–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lim MC, Kang S, Lee KS, Han SS, Park SJ,
Seo SS and Park SY: The clinical significance of hepatic
parenchymal metastasis in patients with primary epithelial ovarian
cancer. Gynecol Oncol. 112:28–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Braun S, Schindlbeck C, Hepp F, Janni W,
Kentenich C, Riethmüller G and Pantel K: Occult tumor cells in bone
marrow of patients with locoregionally restricted ovarian cancer
predict early distant metastatic relapse. J ClinOncol. 19:368–375.
2001. View Article : Google Scholar
|
|
43
|
Schmidt LJ, Duncan K, Yadav N, Regan KM,
Verone AR, Lohse CM, Pop EA, Attwood K, Wilding G, Mohler JL, et
al: RhoA as a mediator of clinically relevant androgen action in
prostate cancer cells. Mol Endocrinol. 26:716–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zohrabian VM, Forzani B, Chau Z, Murali R
and Jhanwar-Uniyal M: Rho/ROCK and MAPK signaling pathways are
involved in glioblastoma cell migration and proliferation.
Anticancer Res. 29:119–123. 2009.PubMed/NCBI
|
|
45
|
Somlyo AV, Bradshaw D, Ramos S, Murphy C,
Myers CE and Somlyo AP: Rho-kinase inhibitor retards migration and
in vivo dissemination of human prostate cancer cells. Biochem
Biophys Res Commun. 269:652–659. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ying H, Biroc SL, Li WW, Alicke B, Xuan
JA, Pagila R, Ohashi Y, Okada T, Kamata Y and Dinter H: The Rho
kinase inhibitor fasudil inhibits tumor progression in human and
rat tumor models. Mol Cancer Ther. 5:2158–2164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nakajima M, Katayama K, Tamechika I,
Hayashi K, Amano Y, Uehata M and Kondo T: WF-536 inhibits
metastatic invasion by enhancing the host cell barrier and
inhibiting tumour cell motility. Clin Exp Pharmacol Physiol.
30:457–463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wong CC, Wong CM, Tung EK, Man K and Ng
IO: Rho-kinase 2 is frequently overexpressed in hepatocellular
carcinoma and involved in tumor invasion. Hepatology. 49:1583–1594.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nakajima M, Hayashi K, Egi Y, Katayama K,
Amano Y, Uehata M, Ohtsuki M, Fujii A, Oshita K and Kataoka H:
Effect of Wf-536, a novel ROCK inhibitor, against metastasis of B16
melanoma. Cancer Chemother Pharmacol. 52:319–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sahai E, Ishizaki T, Narumiya S and
Treisman R: Transformation mediated by RhoA requires activity of
ROCK kinases. Curr Biol. 9:136–145. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xue F, Takahara T, Yata Y, Xia Q, Nonome
K, Shinno E, Kanayama M, Takahara S and Sugiyama T: Blockade of
Rho/Rho-associated coiled coil-forming kinase signaling can prevent
progression of hepatocellular carcinoma in matrix
metalloproteinase-dependent manner. Hepatol Res. 38:810–817. 2006.
View Article : Google Scholar
|
|
52
|
Larrea MD, Wander SA and Slingerland JM:
p27 as Jekyll and Hyde: Regulation of cell cycle and cell motility.
Cell Cycle. 8:3455–3461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hoshino D, Tomari T, Nagano M, Koshikawa N
and Seiki M: A novel protein associated with membrane-type 1 matrix
metalloproteinase binds p27(kip1) and regulates RhoA activation,
actin remodeling and matrigel invasion. J Biol Chem.
284:27315–27326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chang YC, Nalbant P, Birkenfeld J, Chang
ZF and Bokoch GM: GEF-H1 couples nocodazole-induced microtubule
disassembly to cell contractility via RhoA. Mol Biol Cell.
19:2147–2153. 2008. View Article : Google Scholar : PubMed/NCBI
|