Introduction
Mesenchymal stem cells (MSCs) migrate to the injury
site following a fracture and differentiate into osteoblasts, which
produce the bone matrix and repair the fractured bone (1). Multiple signals, including the potent
osteoinductive signal induced by bone morphogenetic proteins (BMPs)
regulate this process (2–4). It has been reported that BMP-2, 4, 6,
7 and 9 induce osteogenic differentiation of MSCs in vitro
and in vivo (5–11). By binding to type 1 and 2 receptors
on the surface of cells, BMPs exert their signals through the
activation of intracellular Smad proteins. Then, by interacting
with different transcription factors, the activated Smad protein
complex regulates the expression of osteoblastic genes (12–14).
BMP-mediated osteogenic differentiation is mediated by
extracellular BMP antagonists, including noggin, gremlin-1, chordin
and follistatin (15–18).
The function of gremlin-1 in BMP-regulated
osteogenic differentiation has been previously investigated in
rodent cells and animal models and the results have indicated that
gremlin-1 is an inhibitor of osteogenesis. Gremlin-1 is a highly
conserved glycoprotein with a molecular weight of 27 kDa belonging
to the DAN/Cerberus family and was first isolated from
Xenopus (19). Gremlin-1 is
primarily distributed in the extracellular matrix, but a small
amount of gremlin-1 is also distributed in the endoplasmic
reticulum (20). Gremlin-1
combines with BMP-2, 4 and 7 and inhibits their association with
the BMP receptors on the cell membrane (20). The expression of gremlin-1 was
demonstrated to be reduced in tumor cells and contributed to the
growth of tumor cells (21).
Gremlin-1 is involved in the development of bone and kidney organs,
and gremlin-1 mutant mice develop severe limb skeletal deformities
(22–25). Mouse bone tissue-specific
overexpression of gremlin-1 results in severe symptoms of
osteoporosis; however, conditional knockout of gremlin-1 in bone
tissue increases bone formation and trabecular bone volume
(26). In osteoblasts,
overexpression of gremlin-1 reduces the biological activity of
BMP-2 and the use of RNA interference to reduce gremlin-1
expression in osteoblasts increases the biological activity of
BMP-2 (26). Collectively, these
studies demonstrate that the effects of gremlin-1 on BMP-induced
osteogenesis of human MSCs remain elusive and further investigation
into the involvement of gremlin-1 in osteogenesis of human MSCs is
warranted. The present study was conducted to examine the effects
of gremlin-1 suppression on cell viability and BMP-2-induced
osteogenic differentiation of primary human MSCs in
vitro.
Materials and methods
Isolation and culture of human
MSCs
Bone marrow samples were obtained from 5 donor
patients who underwent orthopedic surgery; written informed consent
were obtained from all patients. The present study was approved by
the Research Ethics Committee of The First Affiliated Hospital of
Anhui Medical University (Hefei, China). Mononuclear cells from the
bone marrow samples of the 5 patients were separated by
centrifugation at 400 × g for 25 min at room temperature and
Ficoll-Paque medium (GE Healthcare Life Sciences, Chalfont, UK) and
then inoculated in MSC growth medium (MGM; high glucose Dulbecco's
modified Eagle's medium [DMEM] containing 10% fetal bovine serum
[FBS], 0.29 mg/ml GlutaMAX, 100 mg/ml streptomycin, 100 U/ml
penicillin; all from Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and 4 ng/ml basic fibroblast growth factor
(Merck KGaA, Darmstadt, Germany) at a density of 4×105 cells/cm2.
Following incubation for 3 days, non-adherent cells were discarded
and adherent cells were washed twice using phosphate buffered
saline (PBS; Invitrogen; Thermo Fisher Scientific, Inc.) and then
cultured in MGM. The cells of each patient were either cultured for
further examinations or frozen in liquid nitrogen in 1 ml aliquots
7 days later. Cells were cultured in a 5% CO2 and
incubated at 37°C for all experiments. For each patient, all
experiments were conducted in either triplicate or quadruplicate on
individual cell cultures. The data generated from the individual
cell cultures of these patients were collected and presented as
combined data in the present study (mean ± standard deviation of
n=5).
BMP-2 treatment
In order to determine whether BMP-2 induces
gremlin-1 mRNA expression in human MSCs, dose-response and
time-response studies were conducted. For the dose-response study,
human MSCs were inoculated in 35 mm tissue culture dishes in MGM at
a density of 7×103 cells/cm2. The medium was replaced 24 h later
with the basal medium (DMEM containing 10% FBS, 100 mg/ml
streptomycin, 100 U/ml penicillin and 0.29 mg/ml GlutaMAX; all from
Invitrogen; Thermo Fisher Scientific, Inc.) containing different
concentrations (0–50 mg/ml) of recombinant human BMP-2 (Medtronic,
Dublin, Ireland). The cells were dyed with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and then collected 72
h after BMP-2 treatment. Total RNA was extracted using the TRIzol
reagent, and the expression of Gremlin-1 mRNA was analyzed by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). Gremlin-1 expression levels were initially standardized
by β-actin, and then transformed to a ratio over the control 0
mg/ml BMP-2 treatment group. For the time-course study, human MSCs
cultured in 35 mm tissue culture dishes were treated with or
without 0.1 mg/ml BMP-2, and cell samples were collected at 12, 24,
48, 72 and 96 h. Gremlin-1 expression levels were first
standardized by β-actin, and then transformed to the ratio over the
gremlin-1 expression levels at 0 h.
RT-qPCR
Total RNA was quantified with a NanoDrop ND-1000
spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE,
USA), and was reverse transcribed into cDNA with the iScript cDNA
synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription was conducted in a 20 ml reaction volume with
the following protocol: 25°C for 5 min, 42°C for 30 min and 85°C
for 5 min. qPCR was conducted in quadruplicate using the iQ5 system
(Bio-Rad Laboratories, Inc.). The reaction mixture had a volume of
25 ml and contained 10 ng cDNA, 1X iQ SYBR Green supermix (Bio-Rad
Laboratories, Inc.) and 200 nM of each primer.
The primer sequences used in this study were as
follows: β-actin, forward 5′-CATGTACGTTGCTATCCAGGC-3′ and reverse
5′-CTCCTTAATGTCACGCACGAT-3′; Gremlin-1, forward
5′-CGGAGCGCAAATACCTGAAG-3′ and reverse 5′-GGTTGATGATGGTGCGACTGT-3′;
ALP, forward 5′-GTGAACCGCAACTGGTACTC-3′ and reverse
5′-GAGCTGCGTAGCGATGTCC-3′; OC, forward 5′-GAAGCCCAGCGGTGCA-3′ and
reverse, 5′-CACTACCTCGCTGCCCTCC-3′; IBSP, forward
5′-CACTGGAGCCAATGCAGAAGA-3′ and reverse
5′-TGGTGGGGTTGTAGGTTCAAA-3′; OPN, forward
5′-CTCCATTGACTCGAACGACTC-3′ and reverse
5′-CAGGTCTGCGAAACTTCTTAGAT-3′; MSX2, forward
5′-ATGGCTTCTCCGTCCAAAGG-3′ and reverse 5′-CGGCTTCTTGTCGGACATGA-3′;
RUNX2, forward 5′-TGGTTACTGTCATGGCGGGTA-3′ and reverse
5′-TCTCAGATCGTTGAACCTTGCTA-3′. qPCR was conducted with the
following protocols: 1 cycle of 95°C for 3 min, followed by 45
cycles of 95°C for 10 sec, 58°C for 20 sec, and 72°C for 10 sec.
All amplifications were normalized by β-actin. Data were analyzed
using the comparison Cq (2−ΔΔCq) method (27) and expressed as fold change compared
to respective control (28).
Assessment of gremlin-1 small
interfering RNA (siRNA) efficacy
Four synthetic siRNAs (Qiagen, Inc., Valencia, CA,
USA), which were designed to target multiple regions of human
gremlin-1 mRNA (gene accession number: NM_001191322.1), were
obtained and their sequence information is listed in Table I. Human MSCs were transfected with
control siRNA and gremlin-1 siRNAs by Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.) under the optimized condition of
transfection studied previously to validate the knockdown efficacy
of the gremlin-1 siRNAs (28). In
total, 6 groups were designed in triplicate: i) Non-transfected
MSCs (NT control group); ii) MSCs transfected with control siRNA
(control siRNA group); iii) MSCs transfected with gremlin-1 siRNA1
(siRNA1 group); iv) MSCs transfected with gremlin-1 siRNA2 (siRNA2
group); v) MSCs transfected with gremlin-1 siRNA3 (siRNA3 group);
and vi) MSCs transfected with gremlin-1 siRNA4 (siRNA4 group).
Following transfection for 24 h, MSCs were transferred to the basal
medium containing 0.1 mg/ml BMP-2. Total RNA was extracted 72 h
following treatment with BMP-2 using TRIzol reagent according to
the manufacturer's protocols and gremlin-1 mRNA expression was
assessed by RT-qPCR. Gremlin-1 protein expression levels in the
culture supernatant were calculated using a commercial enzyme
linked immunosorbent assay kit for human gremlin-1 (EK1292; Wuhan
Uscn Business Co., Ltd., Wuhan, Hubei, China) 72 h after treatment
with BMP-2. The most effective gremlin-1 siRNA to inhibit gremlin-1
mRNA and protein expression levels was selected for the following
studies. To examine the duration of gremlin-1 inhibition resulting
from a single transfection of gremlin-1 siRNA in the presence of
BMP-2, a time-course study in which gremlin-1 expression was
analyzed following siRNA transfection was performed. Initially,
samples were collected to measure the baseline of gremlin-1
expression (day 0) prior to transfection. For the transfection,
three groups were constructed in triplicate: One group without
transfection (NT control); one group transfected with control siRNA
(control siRNA); and one group transfected with the most effective
gremlin-1 siRNA. The medium was replaced after a 24 h period of
transfection with basal medium containing 0.1 mg/ml BMP-2, and this
medium was replaced twice a week. Cell samples were collected on
days 1, 3, 7, 10 and 14. Total RNA was extracted using TRIzol
reagent according to manufacturer's protocols at the indicated time
points to perform RT-qPCR, in order to assess the mRNA expression
levels of gremlin-1 at these time points.
 | Table I.Sequences of the four synthetic siRNAs
targeting gremlin-1 mRNA. |
Table I.
Sequences of the four synthetic siRNAs
targeting gremlin-1 mRNA.
| siRNA no. | Target position | Target sequence
(5′-3′) | RNA oligo sequences
(5′-3′) |
|---|
| siRNA1 | 477–499 |
TCGTTGCATATCCATCGATTTGG |
AAAUCGAUGGAUAUGCAACGA |
|
|
|
|
GUUGCAUAUCCAUCGAUUUGG |
| siRNA2 | 559–581 |
GACCTAAAACAACCAGATTCTTA |
AGAAUCUGGUUGUUUUAGGUC |
|
|
|
|
CCUAAAACAACCAGAUUCUUA |
| siRNA3 | 989–1011 |
TCCATCTCTTCTTAAGTTGATAG |
AUCAACUUAAGAAGAGAUGGA |
|
|
|
|
CAUCUCUUCUUAAGUUGAUAG |
| siRNA4 | 1218–1240 |
AAGCTTGAAAGGCCAATACCAGA |
UGGUAUUGGCCUUUCAAGCUU |
|
|
|
|
GCUUGAAAGGCCAAUACCAGA |
Water soluble tetrazolium salt-8
(WST-8) assay
Human MSCs were inoculated in 48-well plates in
quadruplicate with a density of 5.5×103 cells/cm2. Following
culture for 24 h, human MSCs in 300 ml medium were transfected with
control siRNA or the most effective gremlin-1 siRNA. The medium was
altered 24 h later with basal medium containing 0.1 mg/ml BMP-2.
The metabolism of human MSCs was estimated on days 1, 3 and 7. The
WST-8
(2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt; Cedarlane Laboratories Ltd., Burlington, Canada)
reagent is designed to estimate the activity of mitochondrial
dehydrogenase in human MSCs. WST-8 is decreased by mitochondrial
dehydrogenases in viable cells to produce a soluble yellow
formazan, which is directly proportional to the reduced activity of
the cells, and thus is used as a measurement of total cellular
metabolic activity. WST-8 solution (30 µl) was added to 300 µl
medium containing human MSCs (1.0×104), and incubated for a further
3 h. Following this, the absorbance was measured at 450 nm with an
absorbance microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA) and the reference wavelength was 650 nm. The ratios
between the data of individual patients and the non-transfection
group were calculated and then the data of all five patients were
combined.
DNA content analysis
The human MSCs were inoculated in 96-well plates in
quadruplicate at a density of 3×103 cells/cm2. Following culture
for 24 h, human MSCs were transfected with control siRNA or the
most effective gremlin-1 siRNA. The medium was replaced with basal
medium with 0.1 mg/ml BMP-2 24 h later. Cells were dyed using a
CyQUANT Cell Proliferation Assay kit (Invitrogen; Thermo Fisher
Scientific, Inc.) and samples were gathered on days 0, 3 and 6. The
DNA content was measured with an emission wavelength of 530 nm and
an excitation wavelength of 450 nm. The ratios between the data of
individual patients and the non-transfection group were first
calculated and then data of all 5 patients were combined.
Induction of osteogenic
differentiation of human MSCs
To estimate the influence of gremlin-1 inhibition on
osteogenic differentiation in human MSCs, gremlin-1 expression was
knocked down in human MSCs, and human MSCs were induced to perform
osteogenic differentiation in osteogenic medium [DMEM with 10% FBS,
100 mg/ml streptomycin, 100 U/ml penicillin, 0.29 mg/ml L-glutamine
(all from Invitrogen; Thermo Fisher Scientific, Inc.), 5 mM
b-glycerolphosphate, 10 nM dexamethasone, and 50 mg/l ascorbic
acid-2-phosphate (all from Sigma-Aldrich, Merck KGaA)] which
contained 0.1 mg/ml BMP-2 in 12-well plates. Three study groups
were constructed: One group with no transfection (NT control); one
group transfected with control siRNA (control siRNA); and one group
transfected with the most effective gremlin-1 siRNA. All three
groups were treated with osteogenic medium with 0.1 mg/ml BMP-2 24
h post transfection. siRNA transfection was repeated every 7 days,
as advised by the manufacturer (Qiagen, Inc.) to cause prolonged
silencing of gremlin-1. The following experiments were conducted to
assess osteogenic differentiation
RT-qPCR analysis of osteoblastic
genes
On day 14 of osteogenic induction, cell samples were
gathered, total RNA was extracted using TRIzol reagent and reverse
transcription was conducted as described above. Expression levels
of osteoblastic genes including alkaline phosphatase (ALP), msh
homeobox 2 (MSX2), integrin-binding sialoprotein (IBSP),
osteocalcin (OC), runt related transcription factor 2 (RUNX2) and
osteopontin (OPN) were detected by RT-qPCR. Human β-actin, used as
the endogenous reference gene, standardized the osteoblastic gene
expression. Following standardization, the ratio between the data
of individual patients and the non-transfection group were first
calculated and then data of all 5 patients were combined. Gremlin-1
expression levels were also measured to confirm the inhibition of
gremlin-1 on day 14.
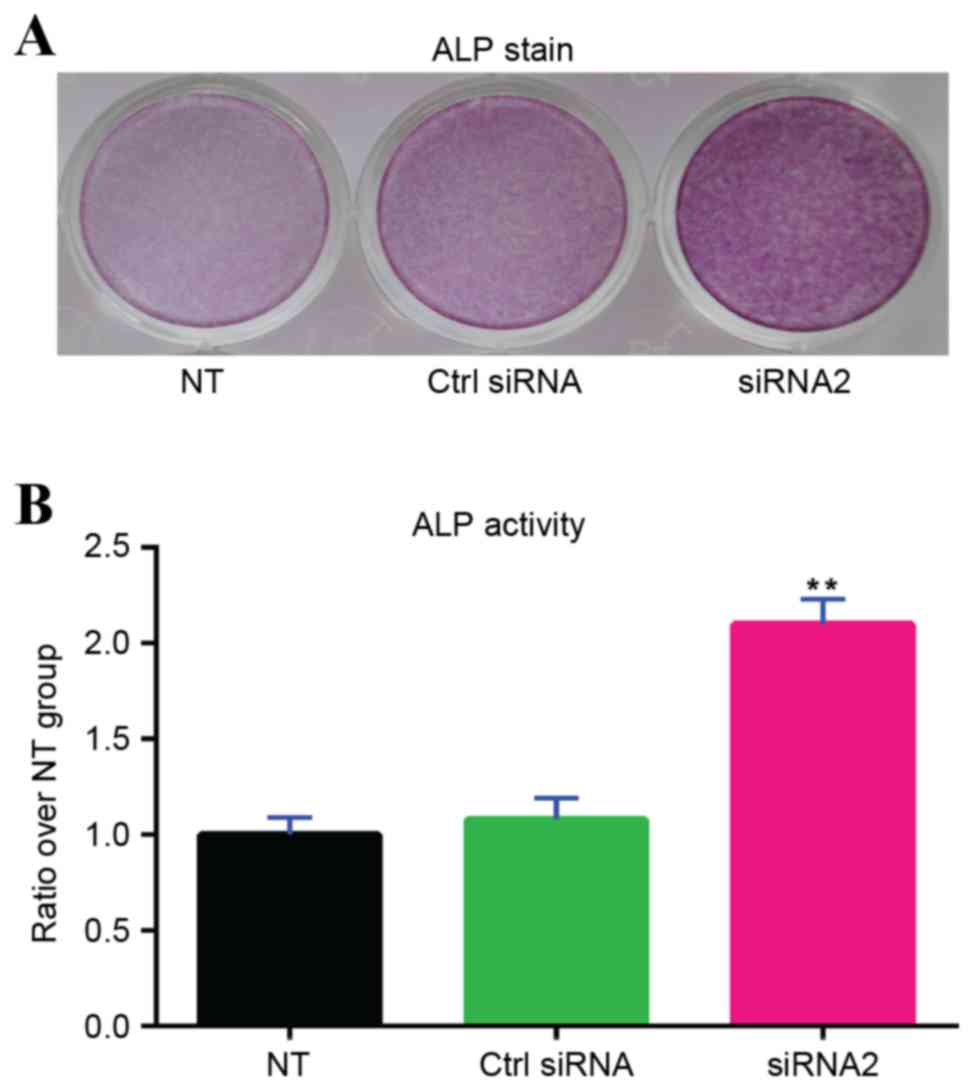
ALP staining
On day 14 of osteogenic induction, ALP staining was
conducted using a Fast Naphthol phosphate kit (Sigma-Aldrich; Merck
KGaA). Cells were washed three times using PBS and were
subsequently fixed for 30 sec by citrate-acetone-formaldehyde
fixative. Samples were then rinsed with deionized water in order to
be stained in the dark for 15 min, and counterstained with neutral
red solution for 2 min (Fig. 5).
Samples were then washed three times using tap water to remove the
dissociative dye. The experiment was repeated three times
independently.
ALP activity assay
On day 14 of osteogenic induction, the activity of
ALP was quantitatively measured using a commercial phosphatase
assay kit (BioAssay Systems, Hayward, CA, USA). Lysis buffer
(containing 50 mM Tris-HCl, 0.5% Triton and 5 mM MgCl2;
Sigma-Aldrich; Merck KGaA) was used to lyse the cells in
triplicate. The lysate was then moved to 96-well plates and
incubated with ALP substrate at 37°C for 30 min in the dark. Stop
buffer (0.1 M NaOH) was then added to halt the reaction. The
p-nitrophenol product, which was generated by enzymatic hydrolysis
of p-nitrophenylphosphate substrate was detected at 405 nm using an
absorbance microplate reader (BioTek Instruments, Inc.). The
protein concentration of samples was tested using a detergent
compatible protein assay kit (Bio-Rad Laboratories, Inc.) and FBS
(Bio-Rad Laboratories, Inc.), and a standard curve was created to
transform data. Concentration of ALP was standardized by total
protein amount. The ratio between the data of individual patients
and the non-transfection group were first calculated and then the
data of all 5 patients were combined.
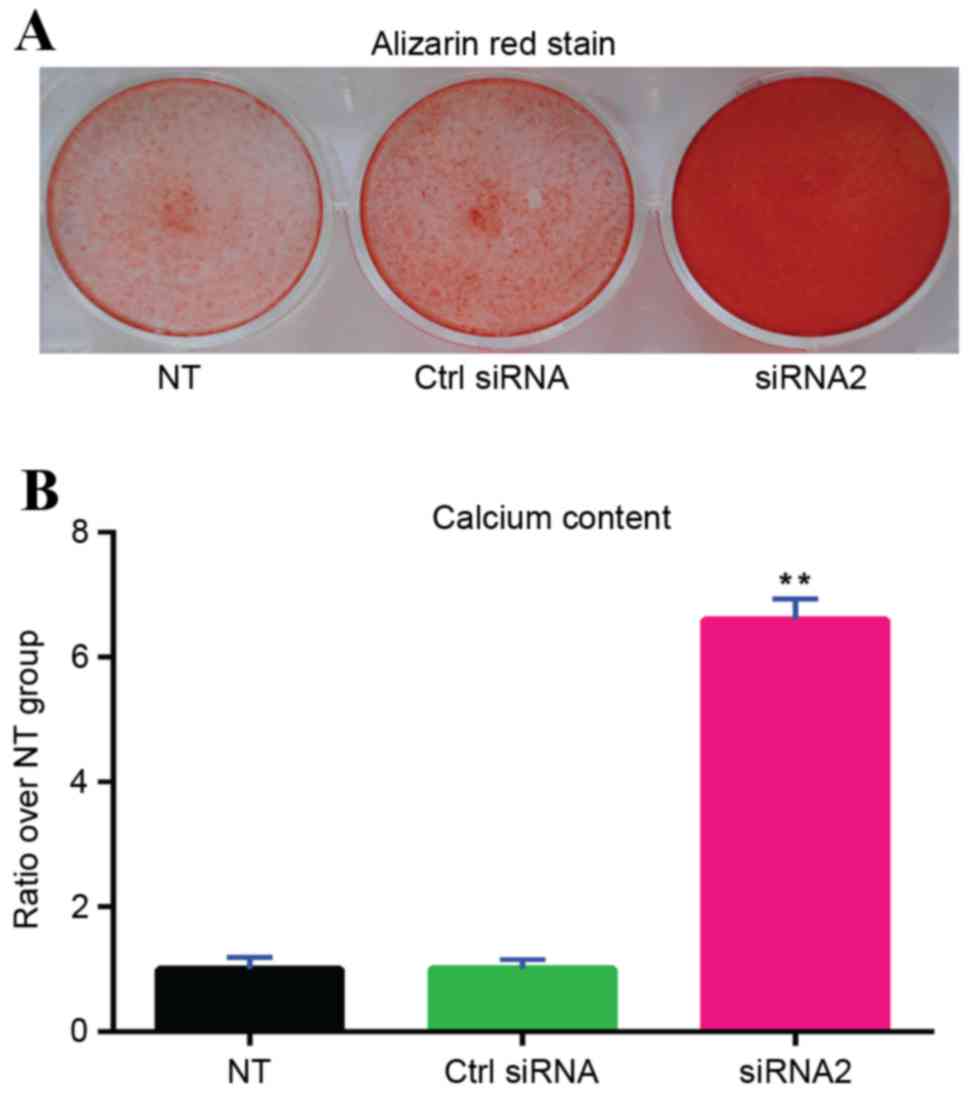
Alizarin Red staining
On day 28 of osteogenic induction, cells and the
extracellular matrix from all groups were fixed in 75% ethanol at
48°C for 1 h, washed with distilled water, and stained with
Alizarin Red S solution (Sigma-Aldrich; Merck KGaA) until an
orange-red color appeared (Fig.
6). Samples were then rinsed three times with deionized water,
and washed once with PBS.
Calcium assay
On day 28 of osteogenic induction, 600 ml 0.5 N
hydrochloric acid solution was added to each well (12-well plates)
to demineralize the cells and the extracellular matrix secreted by
the cells, which were then incubated at 48°C overnight. Following
centrifugation of the samples at 10,000 × g for 10 min, the
supernatant containing calcium extracts was gathered. The calcium
concentration was examined using the QuantiChrom Calcium Assay kit
(BioAssay Systems). The ratio between the data of individual
patients and the non-transfection group were first calculated and
then the data of all 5 patients were combined.
Statistical analysis
Data are presented as the mean ± standard error and
were analyzed by Student's t-test or one-way analysis of variance
followed by the Bonferroni post hoc test, as appropriate. All tests
were two-tailed, with P<0.05 considered to indicate a
statistically significant difference. All statistical analyses were
conducted with PASW Statistics version 18.0 (SPSS Inc., Chicago,
IL, USA).
Results
BMP-2 induces gremlin-1 mRNA
expression
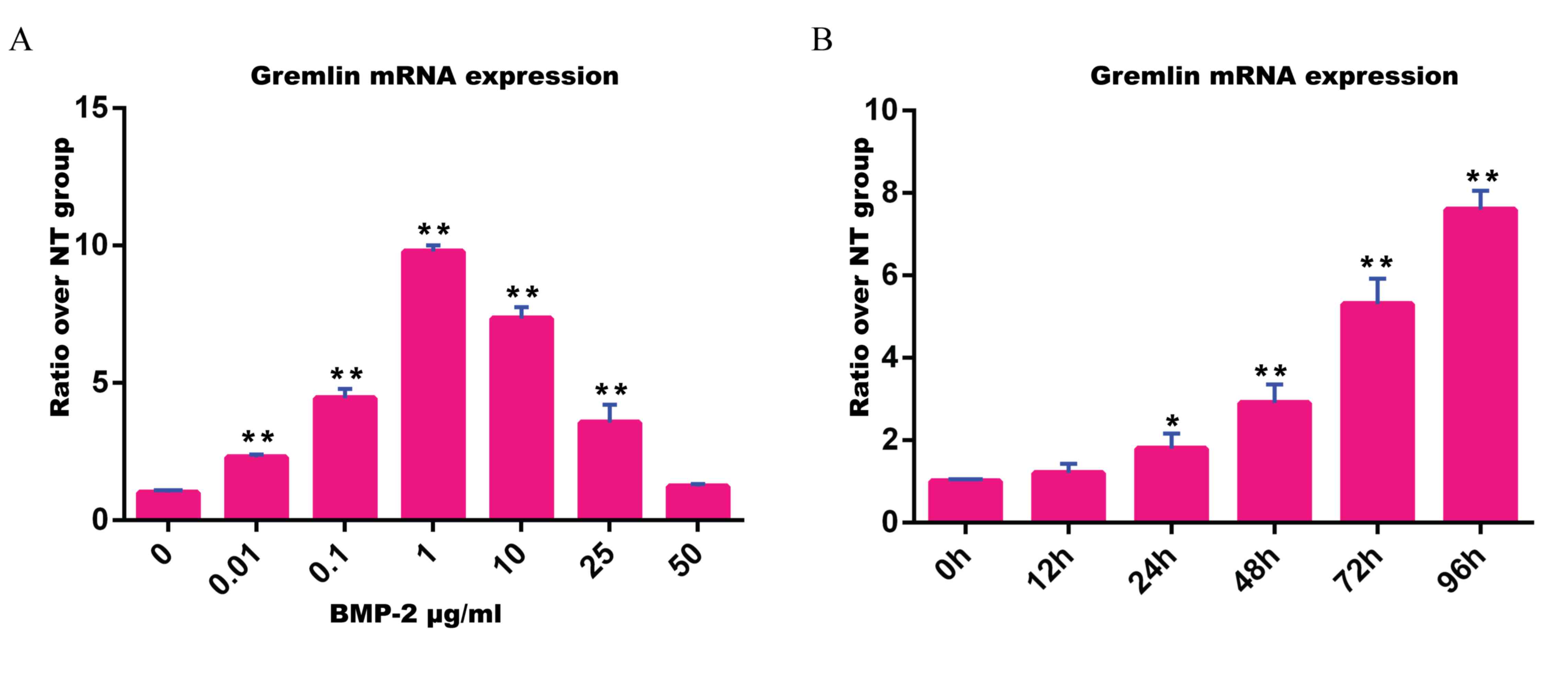
Gremlin-1 mRNA expression was increased by BMP-2,
and the peak expression appeared when induced with 1 mg/ml BMP-2
(Fig. 1A). Increasing
concentrations of BMP-2 resulted in increasing gremlin-1 mRNA
expression levels within the range from 0.01–1 mg/ml BMP-2, whereas
the induction of gremlin-1 expression was reduced as the
concentration of BMP-2 rose from 1 to 50 mg/ml (Fig. 1A). There was no significant
difference in the level of gremlin-1 expression between the group
without BMP-2 treatment and the group treated with 50 mg/ml BMP-2
(P>0.05; Fig. 1A).
Gremlin-1 mRNA expression was also induced by 0.1
mg/ml BMP-2 in a time-dependent manner. Gremlin-1 mRNA expression
levels increased with time and at 24, 48, 72 and 96 h following
BMP-2 treatment the increase was significantly higher than the
group without BMP-2 treatment (P<0.05, P<0.01, P<0.01 and
P<0.01, respectively; Fig.
1B).
Suppression of BMP-2-induced gremlin-1
expression by gremlin-1 siRNA transfection
Following the examination of all transfection
conditions, the optimal condition was 20 nM (final concentration)
of siRNA duplex with 3 µl Lipofectamine RNAiMAX in 1 ml medium for
each well of the 12-well tissue culture plates, which produced the
highest mean fluorescence intensity and the highest percentage of
siRNA-positive cells in the transfected cells. The following siRNA
transfection studies were performed under this optimal condition.
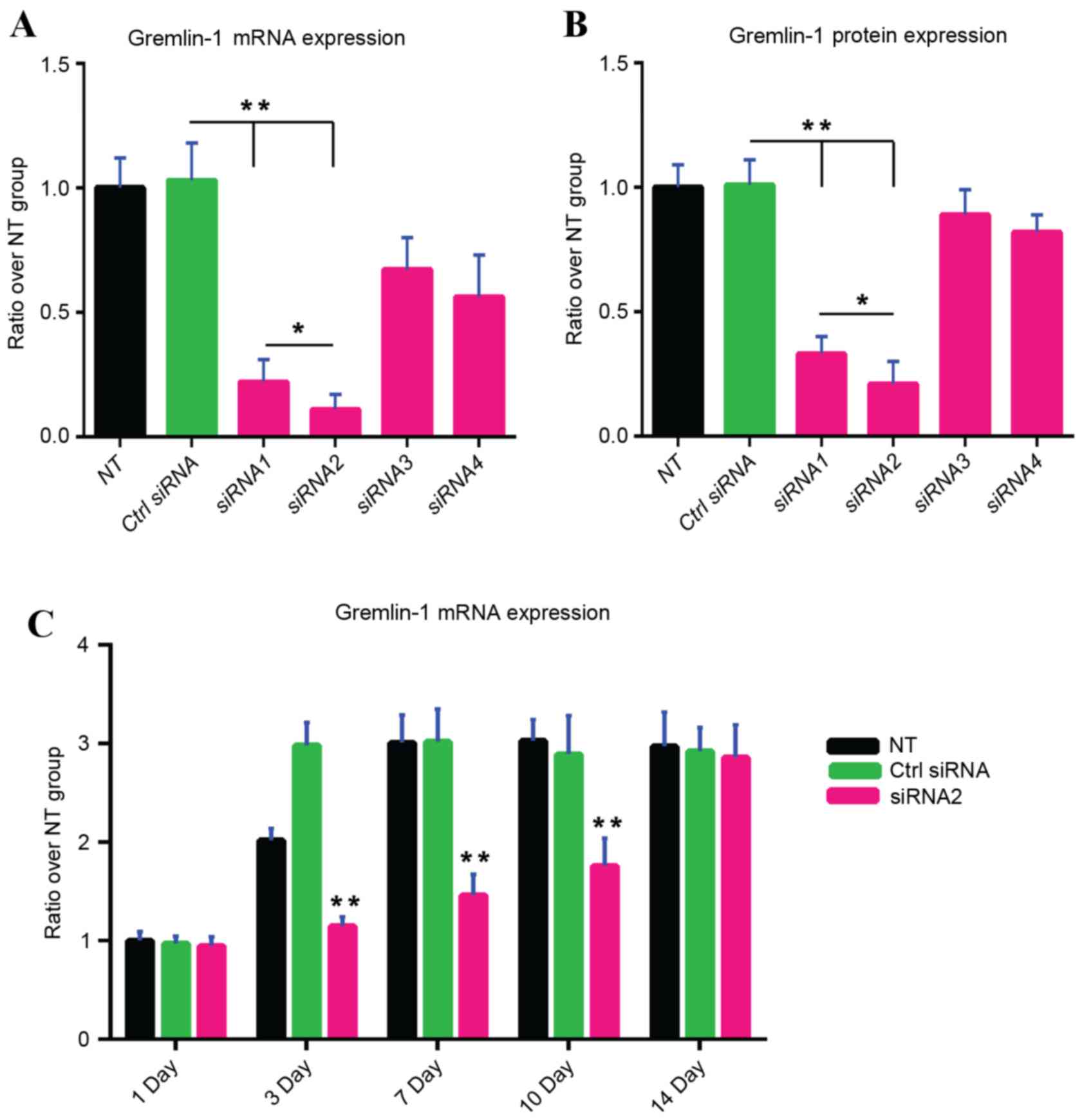
Compared with the non-transfected group, gremlin-1 mRNA (Fig. 2A) and protein (Fig. 2B) levels were not changed by
transfection with control siRNA. By contrast, gremlin-1 mRNA
expression levels in human MSCs and gremlin-1 protein levels in the
culture supernatant were significantly reduced by the transfection
of gremlin-1 siRNA1 (P<0.01 and P<0.01, respectively;
Fig. 2A and B, respectively) and
siRNA2 (P<0.01 and P<0.01, respectively; Fig. 2A and B, respectively). Gremlin-1
mRNA and protein expression were not altered by transfection of
gremlin-1 siRNA3 and siRNA4 (Fig. 2A
and B). As gremlin-1 mRNA and protein expression levels were
significantly more inhibited with gremlin-1 siRNA1 compared with
gremlin-1 siRNA1 (P<0.05 and P<0.05, respectively; Fig. 2A and B, respectively), further
experiments were conducted with gremlin-1 siRNA2 only. A single
gremlin-1 siRNA2 transfection significantly inhibited BMP-2 induced
gremlin-1 expression on days 3, 7 and 10 compared with the
untransfected group and the control siRNA group, but the inhibitive
effect was not evident on day 14 (Fig.
2C).
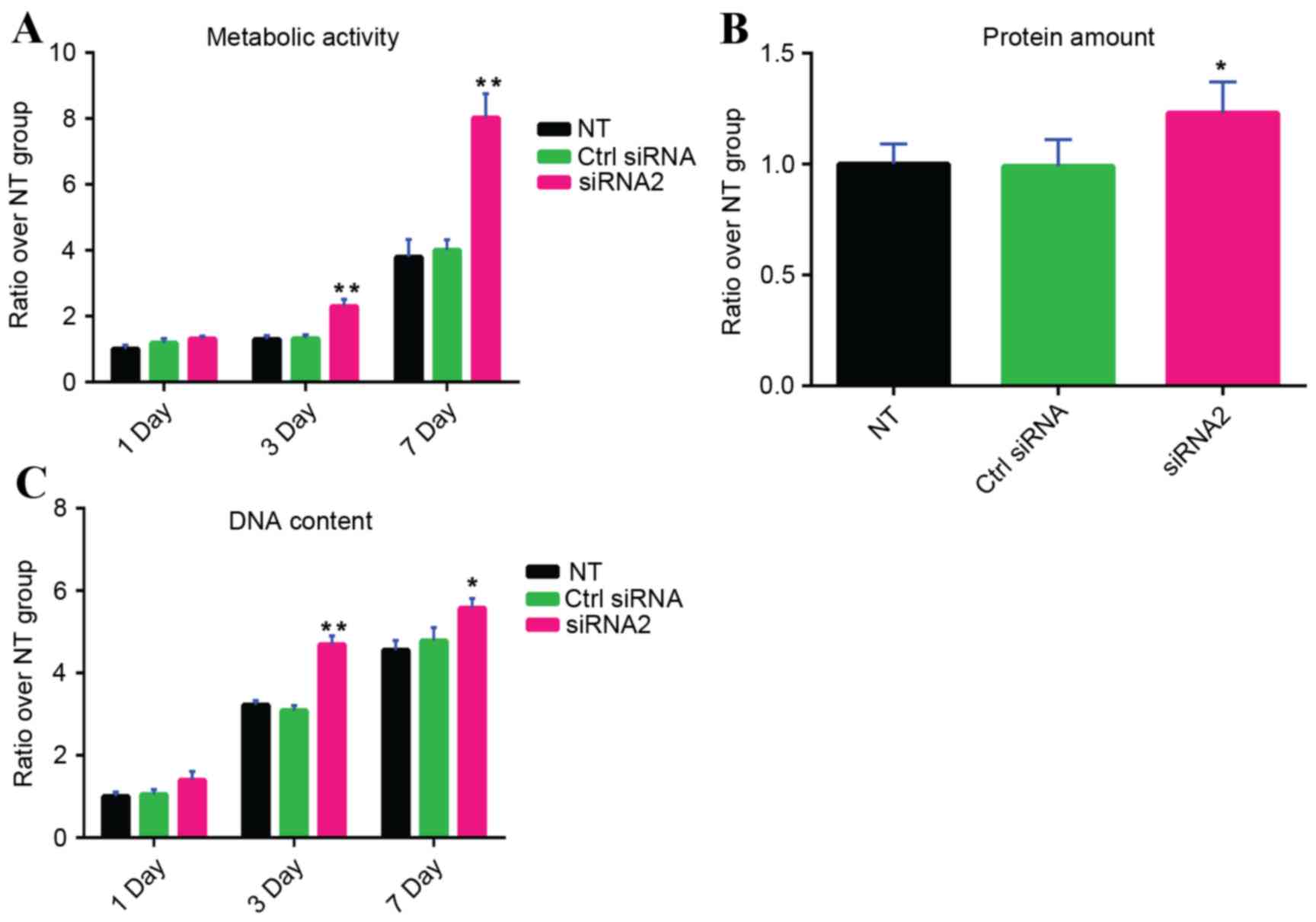
Increased viability of human MSCs by
gremlin-1 suppression
Following siRNA transfection, the WST-8 assay
indicated that metabolism of MSCs was significantly increased by
transfection with gremlin-1 siRNA2 on days 3 and 7 compared with
the untransfected group and the control siRNA group (P<0.01;
Fig. 3A). The total protein
content in the group transfected with gremlin-1 siRNA2-was also
significantly higher compared with the untransfected group and the
control siRNA group on day 14 (P<0.05; Fig. 3B). In addition, following siRNA
transfection, the total DNA content was significantly increased by
gremlin-1 siRNA2 on days 3 and 7 compared with the untransfected
group and the control siRNA group (day 3, P<0.01 and P<0.01,
respectively; day 7, P<0.05 and P<0.05, respectively;
Fig. 3C).
Increased osteogenic differentiation
of human MSCs by gremlin-1 suppression
siRNA transfection was performed every 7 days during
the osteogenic differentiation of human MSCs, as the inhibitive
effect of a single gremlin-1 siRNA2 transfection lasted for a
maximum of 7 days (Fig. 2C).
Gremlin-1 siRNA2 significantly increased the mRNA expression levels
of all osteoplastic genes examined, including ALP (P<0.01;
Fig. 4A), IBSP (P<0.01;
Fig. 4B), MSX2 (P<0.01;
Fig. 4C), OC (P<0.05; Fig. 4D), OPN (P<0.01; Fig. 4E) and RUNX2 (P<0.01; Fig. 4F) compared with the untransfected
group and the control siRNA group. The qualitative naphthol
phosphate staining test indicated that following 14 days of
osteogenic induction, transfection of gremlin-1 siRNA2 increased
the ALP activity of the cells (Fig.
5A). Similar results were obtained in the quantitative ALP
assay, which revealed that transfection of gremlin-1 siRNA2
significantly increased ALP activity of the MSCs compared with the
untransfected group and the control siRNA group (P<0.01;
Fig. 5B).
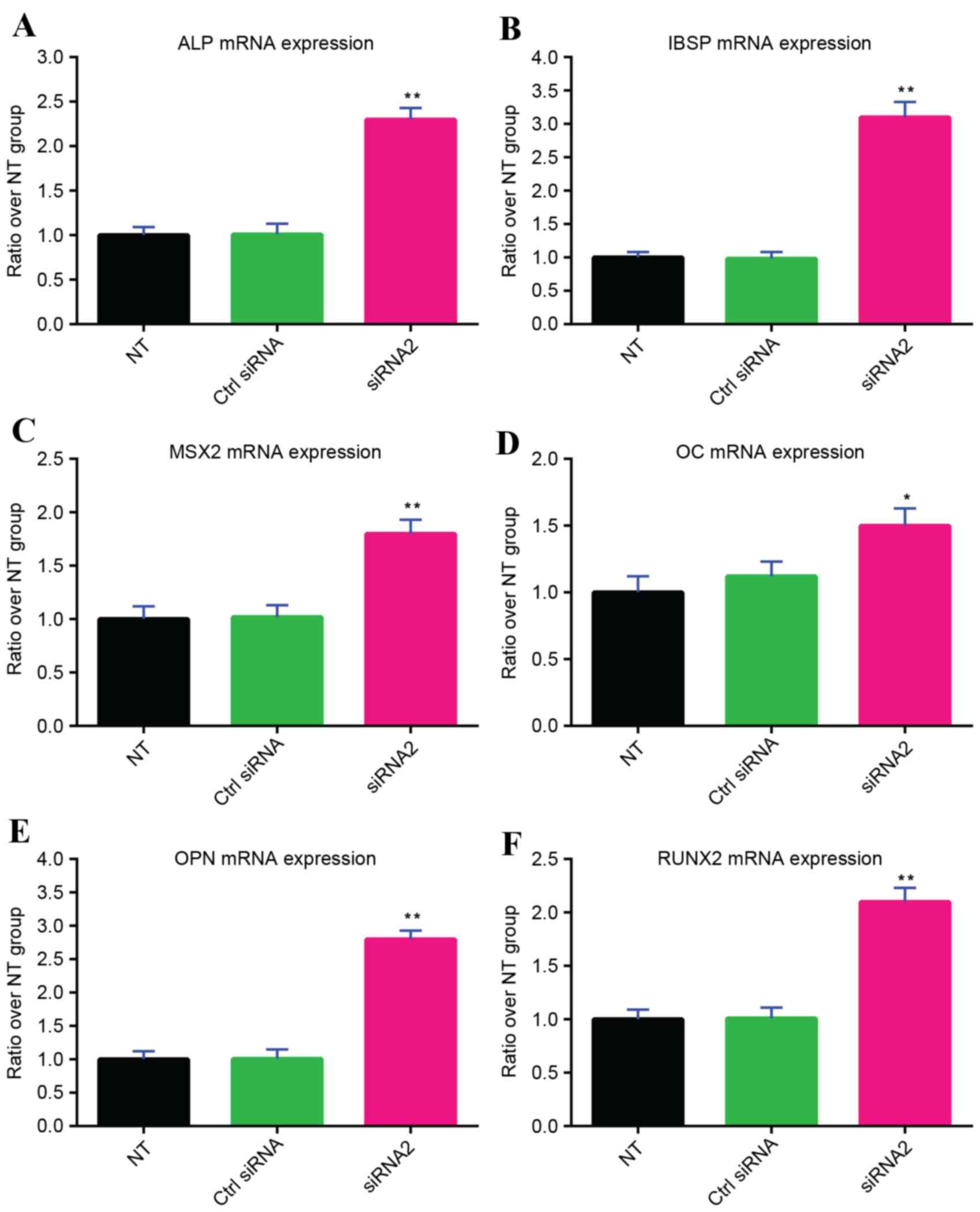 | Figure 4.Expression levels of the osteoblastic
genes (A) ALP, (B) IBSP, (C) MSX2, (D) OC, (E) OPN and (F) RUNX2 on
day 14 of osteogenic induction by osteogenic medium containing 0.1
mg/ml BMP-2. *P<0.05 and **P<0.01 vs. NT and Ctrl siRNA
groups. ALP, alkaline phosphatase; IBSP, integrin-binding
sialoprotein; MSX2, msh homeobox 2; OC, osteocalcin; OPN,
osteopontin; RUNX2, runt related transcription factor 2; siRNA,
small interfering RNA; NT, not transfected; Ctrl, control. |
Qualitative Alizarin Red staining indicated that
compared with the untransfected group and the control siRNA group,
more calcium deposits were observed in the group transfected with
gremlin-1 siRNA2 (Fig. 6A). The
results of the quantitative calcium assay demonstrated that
gremlin-1 siRNA2 transfection significantly increased calcium
deposits in MSCs compared with the untransfected group and the
control siRNA group (P<0.01; Fig.
6B).
Discussion
The results of the present study suggested that
gremlin-1 inhibition improved viability and osteogenic
differentiation of human MSCs by BMP-2-inducement. These results
add to the understanding of the involvement of gremlin-1 in human
MSC osteogenesis, and imply that gremlin-1 may inhibit bone
formation in humans. In human MSC cultures, gremlin-1 expression
was induced by BMP-2 in experiments of dose- and time-dependence.
To the best of our knowledge, the present study is the first to
observe the biophysical nature of time-dependent gremlin-1
induction by BMP-2, and the variable effect depending on the dose.
The induction of gremlin-1 was enhanced by BMP-2 within the
concentrations from 0.01–1 mg/ml, although the induction was
decreased at concentrations of BMP-2 from 1–50 mg/ml (Fig. 1). However, the reason why BMP-2
concentrations >1 mg/ml reduces gremlin-1 induction remains to
be elucidated. DNA content, cellular metabolism and protein content
of human MSCs were increased by gremlin-1 inhibition, and this
influence was unlikely to result from the transfection system as
the transfection of control siRNA did not change these features of
human MSCs in culture (Fig. 3A-C).
These data suggest that gremlin-1 is involved in proliferation of
stem cells. Although the data indicated that gremlin-1 inhibition
increased the viability of human MSCs, the expression levels of
osteoblastic genes and the increased ALP induction on day 14 may
have been the secondary effect of gremlin-1 inhibition on cell
viability, as expression levels of osteoblastic genes in the
present study were standardized by β-actin from the matching group,
and the concentration of ALP was standardized by the total protein
amount. An increase in osteogenic activity was still apparent
following such standardization, so the differences cannot be
explained by stagnated cell growth alone. The expression levels of
osteoblastic genes and increased ALP production by gremlin-1
inhibition indicated increased osteogenic differentiation on a per
cell basis. Nevertheless, the number of calcium deposits was not
standardized, and therefore the enhanced calcium deposits caused by
gremlin-1 inhibition may be partly due to increased cell growth.
The inhibition of gremlin-1 expression by a single transfection of
gremlin-1 siRNA2 in the present study was maintained for a maximum
of 7 days (Fig. 2C). Based on this
observation, gremlin-1 siRNA2 was used to transfect cells every 7
days during the period of osteogenic induction, so as to knock down
the expression of gremlin-1 constantly. Repeated transfections of
gremlin-1 siRNA2 continuously inhibited gremlin-1 expression.
Instead of studying the influence of exogenous
gremlin-1 addition, the present study illustrated the influence of
endogenous gremlin-1 on BMP-2-induced osteogenesis in human MSCs
via the knockdown of endogenous gremlin-1 expression. The different
observations between studies using human MSCs and rodent cells or
animal models indicate that a species-specific difference in
BMP-2-induced osteogenesis-associated functions of gremlin-1 in
MSCs may exist. Dexamethasone and BMP-2 resulted in different
osteoinductive effects on MSCs of humans, rats, and mice (29). Rat MSCs expressed mRNA for activin
receptor-like kinase-6, a type 1 receptor for BMPs, but MSCs from
humans did not express this particular receptor (30). In human MSCs, MSX2 was upregulated
up to 10-fold by BMP-2, but in rat MSCs expression of MSX2 was not
altered by BMP-2 (30). These
observations and the data from the present study suggest that a
species-specific difference may exist when gremlin-1 functions in
BMP-2-induced osteogenesis of MSCs.
The present study indicated that in the presence of
BMP-2, gremlin-1 is harmful to osteogenic differentiation in human
MSCs. One of the possible mechanisms may be that BMP-2 is not
inactivated by gremlin-1 that is already bound to BMP-2 in human
MSC cultures. Gremlin-1 is known to bind to proteoglycans on the
surface of cell. In addition, regarding the possible intracellular
signaling, based on the following observations gremlin-1 may partly
regulate osteogenic differentiation by modulating the intracellular
canonical BMP/Smad signaling, then MSX2, RUNX2, or other
unidentified transcription factors, followed by the subsequent gene
expression of OC, IBSP, OPN and ALP. A previous report (31) has demonstrated that gremlin-1
actively inhibits BMP-2 and the production of its type 2 receptor.
The data from the present study suggested that gremlin-1 inhibition
increased the expression of MSX2 and RUNX2, which are important
transcription factors in the process of osteogenic differentiation.
In addition, the expression levels of the osteoblastic markers
IBSP, OC, ALP and OPN were all increased by gremlin-1 inhibition
(Fig. 4). Further studies are
required regarding the intracellular signaling pathways through
which gremlin-1 regulates osteogenic differentiation in human
MSCs.
In conclusion, expression of gremlin-1 in human MSCs
was upregulated by BMP-2 in a time-dependent manner, with the
effect of dose being variable. Gremlin-1 inhibition increased
BMP-2-induced osteogenic differentiation and viability in human MSC
culture, which indicates that gremlin-1 has an inhibitory effect on
human MSC osteogenesis. The underlying mechanisms that resulted in
these observations remain unknown, and further studies are needed
to clarify the intracellular signaling pathways through which
gremlin-1 regulates osteogenic differentiation in human MSCs. In
addition, similar studies with a larger sample size on human MSCs
are important to discover whether there is any significant
difference between different sexes in response to gremlin-1
inhibition.
Acknowledgements
The present study was supported by a grant from the
Anhui Natural Science Foundation (grant no. 1408085MH182).
References
|
1
|
Granero-Moltó F, Weis JA, Miga MI, Landis
B, Myers TJ, O'Rear L, Longobardi L, Jansen ED, Mortlock DP and
Spagnoli A: Regenerative effects of transplanted mesenchymal stem
cells in fracture healing. Stem Cells. 27:1887–1898. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marie PJ and Fromigué O: Osteogenic
differentiation of human marrow-derived mesenchymal stem cells.
Regen Med. 1:539–548. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koehler KC, Alge DL, Anseth KS and Bowman
CN: A Diels-Alder modulated approach to control and sustain the
release of dexamethasone and induce osteogenic differentiation of
human mesenchymal stem cells. Biomaterials. 34:4150–4158. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yokota J, Chosa N, Sawada S, Okubo N,
Takahashi N, Hasegawa T, Kondo H and Ishisaki A: PDGF-induced
PI3K-mediated signaling enhances the TGF-β-induced osteogenic
differentiation of human mesenchymal stem cells in a
TGF-β-activated MEK-dependent manner. Int J Mol Med. 33:534–542.
2014.PubMed/NCBI
|
|
5
|
Tsuji K, Bandyopadhyay A, Harfe BD, Cox K,
Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ and Rosen V: BMP2
activity, although dispensable for bone formation, is required for
the initiation of fracture healing. Nat Genet. 38:1424–1429. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin L, Fu X, Zhang X, Chen LX, Zhang JY,
Yu CL, Ma KT and Zhou CY: Rat adipose-derived stromal cells
expressing BMP4 induce ectopic bone formation in vitro and in vivo.
Acta Pharmacol Sin. 27:1608–1615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kotajima S, Kishimoto KN, Watanuki M,
Hatori M and Kokubun S: Gene expression analysis of ectopic bone
formation induced by electroporatic gene transfer of BMP4. Ups J
Med Sci. 111:231–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kugimiya F, Kawaguchi H, Kamekura S,
Chikuda H, Ohba S, Yano F, Ogata N, Katagiri T, Harada Y, Azuma Y,
et al: Involvement of endogenous bone morphogenetic protein (BMP) 2
and BMP6 in bone formation. J Biol Chem. 280:35704–35712. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuji K, Cox K, Gamer L, Graf D,
Economides A and Rosen V: Conditional deletion of BMP7 from the
limb skeleton does not affect bone formation or fracture repair. J
Orthop Res. 28:384–389. 2010.PubMed/NCBI
|
|
10
|
Levet S, Ciais D, Merdzhanova G, Mallet C,
Zimmers TA, Lee SJ, Navarro FP, Texier I, Feige JJ, Bailly S and
Vittet D: Bone morphogenetic protein 9 (BMP9) controls lymphatic
vessel maturation and valve formation. Blood. 122:598–607. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo
X, Chen J, Bi Y, He BC, Park JK, et al: A comprehensive analysis of
the dual roles of BMPs in regulating adipogenic and osteogenic
differentiation of mesenchymal progenitor cells. Stem Cells Dev.
18:545–559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Foletta VC, Lim MA, Soosairajah J, Kelly
AP, Stanley EG, Shannon M, He W, Das S, Massague J and Bernard O:
Direct signaling by the BMP type II receptor via the cytoskeletal
regulator LIMK1. J Cell Biol. 162:1089–1098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lavery K, Swain P, Falb D and
Alaoui-Ismaili MH: BMP-2/4 and BMP-6/7 differentially utilize cell
surface receptors to induce osteoblastic differentiation of human
bone marrow-derived mesenchymal stem cells. J Biol Chem.
283:20948–20958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ali IH and Brazil DP: Bone morphogenetic
proteins and their antagonists: Current and emerging clinical uses.
Br J Pharmacol. 171:3620–3632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Passa O, Tsalavos S, Belyaev NN, Petryk A,
Potocnik AJ and Graf D: Compartmentalization of bone morphogenetic
proteins and their antagonists in lymphoid progenitors and
supporting microenvironments and functional implications.
Immunology. 134:349–359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yanagita M: Antagonists of bone
morphogenetic proteins in kidney disease. Curr Opin Investig Drugs.
11:315–322. 2010.PubMed/NCBI
|
|
18
|
Dean DB, Watson JT, Moed BR and Zhang Z:
Role of bone morphogenetic proteins and their antagonists in
healing of bone fracture. Front Biosci (Landmark Ed). 14:2878–2888.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsu DR, Economides AN, Wang X, Eimon PM
and Harland RM: The Xenopus dorsalizing factor Gremlin identifies a
novel family of secreted proteins that antagonize BMP activities.
Mol Cell. 1:673–683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Topol LZ, Bardot B, Zhang Q, Resau J,
Huillard E, Marx M, Calothy G and Blair DG: Biosynthesis,
post-translation modification, and functional characterization of
Drm/Gremlin. J Biol Chem. 275:8785–8793. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karagiannis GS, Musrap N, Saraon P, Treacy
A, Schaeffer DF, Kirsch R, Riddell RH and Diamandis EP: Bone
morphogenetic protein antagonist gremlin-1 regulates colon cancer
progression. Biol Chem. 396:163–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khokha MK, Hsu D, Brunet LJ, Dionne MS and
Harland RM: Gremlin is the BMP antagonist required for maintenance
of Shh and Fgf signals during limb patterning. Nat Genet.
34:303–307. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lappin DW, McMahon R, Murphy M and Brady
HR: Gremlin: An example of the re-emergence of developmental
programmes in diabetic nephropathy. Nephrol Dial Transplant. 17
Suppl 9:S65–S67. 2002. View Article : Google Scholar
|
|
24
|
Bardot B, Lecoin L, Fliniaux I, Huillard
E, Marx M and Viallet JP: Drm/Gremlin, a BMP antagonist, defines
the interbud region during feather development. Int J Dev Biol.
48:149–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michos O, Panman L, Vintersten K, Beier K,
Zeller R and Zuniga A: Gremlin-mediated BMP antagonism induces the
epithelial-mesenchymal feedback signaling controlling metanephric
kidney and limb organogenesis. Development. 131:3401–3410. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gazzerro E, Smerdel-Ramoya A, Zanotti S,
Stadmeyer L, Durant D, Economides AN and Canalis E: Conditional
deletion of gremlin causes a transient increase in bone formation
and bone mass. J Biol Chem. 282:31549–31557. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zuo B, Zhu J, Li J, Wang C, Zhao X, Cai G,
Li Z, Peng J, Wang P, Shen C, et al: microRNA-103a functions as a
mechanosensitive microRNA to inhibit bone formation through
targeting Runx2. J Bone Miner Res. 30:330–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diefenderfer DL, Osyczka AM, Reilly GC and
Leboy PS: BMP responsiveness in human mesenchymal stem cells.
Connect Tissue Res. 44 Suppl 1:S305–S311. 2003. View Article : Google Scholar
|
|
30
|
Osyczka AM, Diefenderfer DL, Bhargave G
and Leboy PS: Different effects of BMP-2 on marrow stromal cells
from human and rat bone. Cells Tissues Organs. 176:109–119. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wellbrock J, Harbaum L, Stamm H, Hennigs
JK, Schulz B, Klose H, Bokemeyer C, Fiedler W and Lüneburg N:
Intrinsic BMP antagonist gremlin-1 as a novel circulating marker in
pulmonary arterial hypertension. Lung. 193:567–570. 2015.
View Article : Google Scholar : PubMed/NCBI
|