Introduction
Rheumatoid arthritis (RA), a common and systemic
autoimmune disease of unknown etiology, leads to chronic
progressive and aggressive inflammation in the synovial joints,
with subsequent destruction of cartilage and erosion of bone in the
affected joint, which causes severe disability and increased
mortality rates (1,2). In RA, inflammatory cells, including
natural killer cells, T and B lymphocytes and neutrophils,
infiltrate the synovial membrane, contributing to cartilage and
bone degradation (3). Among the
proinflammatory cytokines, tumor necrosis factor-α (TNF-α) and
interleukin 17A (IL-17A) are expressed at high levels in the
rheumatoid joint and are important in the mechanisms underlying the
inflammatory response (4,5). These cytokines, produced by
CD4+ T helper (Th) cells, and CD4+ T cells
are important in the inflammatory process via their cytolytic
activities, and the production of pro- and anti-inflammatory
cytokines, which regulate immune responses. CD4+ T cells
can differentiate into Th1, Th2, Th9, Th17 or regulatory T (Treg)
cells, depending on the cytokine secretion and expression of
specific transcription factors (6). Th17 cells were identified in 2005
based on their ability to produce IL-17A (7,8).
CD4+ lymphopenia in patients is usually caused by
diseases, including RA (9–11). Among the identified T cell subsets,
Th17 and Treg cells have gained increasing scientific interest and
have been extensively investigated in several
autoimmune/inflammatory disorders (12–15).
Pathogenic Th17 cells are key in the development of RA, which
mediate pannus growth, osteoclastogenesis and synovial
neoangiogenesis. By contrast, Treg cells are a T cell subset
functioning to suppress autoreactive lymphocytes. The imbalance
between Th17 cells and Treg cells has been identified as a crucial
event in the pathogenesis of RA (16,17).
According to previous reports, RA affects ~1% of the
adult population in developed countries (18,19).
Treatment for RA has been categorized into the use of
disease-modifying anti-rheumatic drugs (DMARDs) and non-steroidal
anti-inflammatory drugs (NSAIDs). Traditional NSAIDs, including
ibuprofen and diclofenac, inhibit cyclooxygenase (COX) I and COX II
(20). They reduce pain and
swelling in RA, enhance recovery, and promote mobility and physical
activity. However, NSAIDs do not slow the progression of the
disease and may have adverse effects (21). The use of NSAIDs is associated with
cardiovascular risk factors due to their effect in increasing
systolic blood pressure, particularly in high-risk patients with
diabetes, hypertension or heart disease (22,23).
Conventional DMARDs are immunosuppressive agents, of which
methotrexate (MTX) is the most commonly used, and remain the
cornerstone of RA treatment. In previous years, treatment
strategies and the use of DMARDs have changed. DMARDs retard or
halt disease progression, or delay disease onset; ‘tight control’
and ‘treat-to-target’ are the presently used paradigms (24). However, DMARDS do not suppress the
progression of clinical disability (25,26).
Novel therapeutic approaches are required to identify a therapeutic
target for remission or low disease activity, which can reduce the
inflammatory and autoimmune components in RA, promote restoration
of immune tolerance, slow cartilage destruction and reduce the
treatment time for RA (27).
Oxymatrine (OMT), a type of monosomic alkaloid
extracted from the dried roots of the traditional Chinese herb,
Sophora flavescens Ait. (Kushen) or Sophora
alopecuroides (Kudouzi), has a tetracyclic quinolizine
structure, its molecular formula is
C15H24N2O. OMT possesses potent
anti-inflammatory, immunoregulatory, antivirus, anticancer,
antifibrotic and cardiovascular-protective activities (28–32).
Previously, OMT studies have focused predominantly on its
therapeutic effect against other inflammatory diseases, certain
types of tumor and hepatitis (33–35).
There have been few reports on the effect of OMT on autoimmune
diseases, including RA. The aim of the present study was to
evaluate the effect and mechanism of OMT treatment on RA.
Materials and methods
Drugs and chemicals
OMT was purchased from Ningxia Bauhinia Pharmacy
Co., Ltd. (Ningxia, China). OMT 100, 50 and 25 mg/kg (dissolved in
normal saline) was administrated via intraperitoneal injection
(i.p.). Immunization grade bovine type II collagen and complete
Freund's adjuvant were purchased from Chondrex, Inc. (Redmond, WA,
USA). Enzyme-linked immunosorbent assay (ELISA) kits for IL-17 and
TNF-α, and mouse monoclonal antibodies against FOXP3 (ab22510),
RORγt (ab41942) and β-actin (ab8226) were purchased from Abcam
(Cambridge, MA, USA). TRIzol was obtained from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). A reverse transcription kit
was purchased from TransGen Biotech, Inc. (Beijing, China). Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis was performed using GoTaq® qPCR master mix
(Promega Corporation, Madison, WI, USA). BCA and enhanced
chemiluminescence (ECL) kits were from Pierce, Thermo Fishers
Scientific, Inc.
Animals
Male Sprague-Dawley (SD) rats (8 weeks old; 180–220
g) were obtained from the Experimental Animal Center, Ningxia
Medical University (Ningxia, China). They were housed in multilayer
laminar flow racks under a controlled environment (20–25°C and 12 h
light:dark cycle) with free access to food and water. The present
study was performed according to the Guiding Principles for the
Care and Use of Laboratory Animals (36) and all procedures were approved by
the Animal Care and Use Committee of Ningxia Medical
University.
Half lethal dose (LD50)
assay
The LD50 of OMT was measured using a
sequential method with five dose levels according to body weight,
with a single i.p. injection. The mortality rates of the rats were
monitored during the 14 days follow treatment.
Induction of collagen-induced
arthritis (CIA) and OMT treatment
The Male SD rats [Permit no. SCXK (Ning) 2011–0001]
were randomly divided into six groups (10 rats/group) prior to the
onset of arthritis: Normal control group, positive control group
treated with dexamethasone (DXM; 2 mg/kg, twice a week), CIA model
group, OMT high-dose group (100 mg/kg, once daily), middle-dose
group (50 mg/kg, once daily) and low-dose group (25 mg/kg, once
daily). The 50 male SD rats, excluding those in the normal control
group (10 rats) were administered with a subcutaneous injection of
0.1 ml bovine type II collagen emulsified in complete Freund's
adjuvant (1:1, v/v) into the right hind metatarsal footpad. After 1
week, the rats were administered with a booster subcutaneous
injection of 0.1 ml bovine CII in incomplete Freund's adjuvant
(1:1, v/v) into the left hind metatarsal footpad. The control rats
were treated in the same manner but without the CII antigen.
Between days 1 and 35 following the second immunization, the rats
in the OMT-treated group were administered with OMT at
concentrations of 100, 50 or 25 mg/kg i.p. The rats in the control
group and the CIA model group were administered with 100 mg/kg
saline i.p., and DXM (2 mg/kg) was used as a reference drug,
administered (i.p.) at the same time. The gradual onset of
arthritis usually starts ~10 days following primary
immunization.
Evaluation of CIA
Hindpaw swelling
The volume of the hindpaw swelling was measured with
vernier calipers once every 4 days for 6 weeks. Hindpaw swelling
(mm2)=left hindpaw swelling (mm) × left hind ankle
swelling (mm).
Arthritis score
The SD rats were assessed every 4 days for the
progression of CIA between days 1 and 35 following secondary
immunization. Each paw was examined and graded for severity of
erythema, swelling and scleroma, and the four scores were combined,
resulting in a maximum possible score of 16 per mouse; the maximum
arthritic score per rat was set at 8 (4 points for two hindpaws).
The score was calculated using a five-point scale: 0, no signs of
arthritis; 1, signs involving the ankle/wrist; 2, signs involving
the ankle+tarsal of the hindpaw and/or wrist+carpals of the
forepaw; 3, signs extending to the metatarsals or metacarpals; 4,
severe disease involving the entire hindpaw or forepaw. The
examination was performed by two independent observers who remained
blinded to the treatment groups.
Histological analysis of knee joints
The SD rats were sacrificed via anesthesia and serum
was collected on day 35 following second immunization. The knee
joints were dissected, fixed in 4% paraformaldehyde solution for 24
h, decalcified in 10% ethylene diamine tetraacetate for 30 days,
with the solution renewed once a week, and then embedded in
paraffin. Standard frontal sections of 3 µm were prepared and
stained with hematoxylin and eosin (H&E). The synovial tissue
sections were observed using light microscopy (CX23; Olympus
Corporation, Tokyo, Japan) and evaluated in a blinded-manner.
Measurement of serum levels of IL-17A and
TNF-α
The serum levels of IL-17A and TNF-α were quantified
using ELISA according to the manufacturer's protocol (Abcam). For
measurements of IL-17A and TNF-α, the SD rats were anesthetized on
the final day of the experiment and serum was drawn from the heart.
The reaction product was quantified using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 450 nm. All
samples were analyzed in duplicates using the average optical
density values to calculate concentrations.
Spleen lymphocyte preparation
The spleens were removed immediately following
sacrifice and placed in PBS. The spleens were then mechanically
disrupted through a wire mesh strainer with the end of a 10 ml
plastic syringe plunger. The spleen lymphocytes preparations were
filtered through lens tissue to remove debris and the cells were
collected by centrifugation (at 500 × g for 5 min at 4°C). Finally,
the cells were resuspended, dispersed in trypsin and counted using
a hemocytometer.
RNA extraction and RT-qPCR analysis
Total RNA was extracted from the spleen lymphocytes
using TRIzol. The purity and concentration of RNA was determined
using spectrophotometry at 260 and 280 nm. Complementary DNA was
synthesized using a reverse transcription kit. The RT-qPCR mixture
was denatured at 95°C for 2 min followed by 50 cycles of
amplification including denaturing at 95°C for 15 sec, annealing at
60°C for 15 sec and extension at 72°C for 45 sec. The total
reaction volume was 20 µl with 3 µl cDNA, 10 µl ROX qPCR Master
(2X), 0.5 µl primers individually and 6 µl ddH2O. The
primers used for the RT-qPCR were as follows: RORγt, sense
5′-TCTGGAAGCTGTGGGATAGA-3′ and antisense 5′-GAG-GAG CCT GTG GAG AAA
TAC-3′; FOXP3, sense 5′-GGCCCTTCTCCAGGACAGA-3′ and antisense
5′-GCTGATCATGGCTGGGTTGT-3′; β-actin, sense
5′-CCTCATGCCATCCTGCGTCT-3′ and antisense
5′-GCCACAAGGATTCCATACCCA-3′. Relative gene expression levels were
determined as described previously (37). The results were normalized to the
expression of the housekeeping gene, β-actin. Data shown are
representative of three independent experiments. Data analysis was
performed using the 2−∆∆Cq method (38).
Western blot analysis
Spleen lymphocytes were washed with PBS three times
and lysed with RIPA buffer. The protein concentration was
determined using a BCA kit according to the manufacturer's
protocol. Protein (20 µg) was separated by 10% SDS-PAGE and then
transferred onto a PVDF membrane. The membranes were blocked with
5% dried milk and incubated with primary antibody against FOXP3
(1:1,000) and RORγt (1:2,000) in TBST overnight at 4°C. Following
rinsing in milk-TBST, the blots were incubated with horseradish
peroxidase-conjugated secondary antibody at room temperature for 1
h. The expression levels of FOXP3 and RORγt were detected using the
ECL detection system and X-ray films.
Statistical analyses
Data are presented as the mean ± standard deviation.
Data were analyzed using Student's t-test and one-way analysis of
variance. Statistical significance in the comparison of means of
different groups was calculated using LSD-t analyses with SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference
between data sets.
Results
Toxicity of OMT
To evaluate the toxicity of the i.p. injection of
OMT, the present study determined its LD50 in rats. The
LD50 of OMT was 898.22 mg/kg (95% confidence interval,
832.46–963.98).
OMT attenuates collagen-induced
arthritis in SD rats
There was a significant increase in hindpaw volumes
in all CIA groups, compared with those in the normal control group.
Hindpaw swelling involved tarsal, distal with ankle and
interphalangeal inflammation. The onset of arthritis was induced in
all immunized rats in 24 h. The first manifestation of CIA was
erythema of one or more ankle joints, followed by involvement of
the metatarsal and interphalangeal joints. The development of
erythema reached a peak on day 9, following which there was a mild
decrease in the signs of inflammation between days 10 and 16.
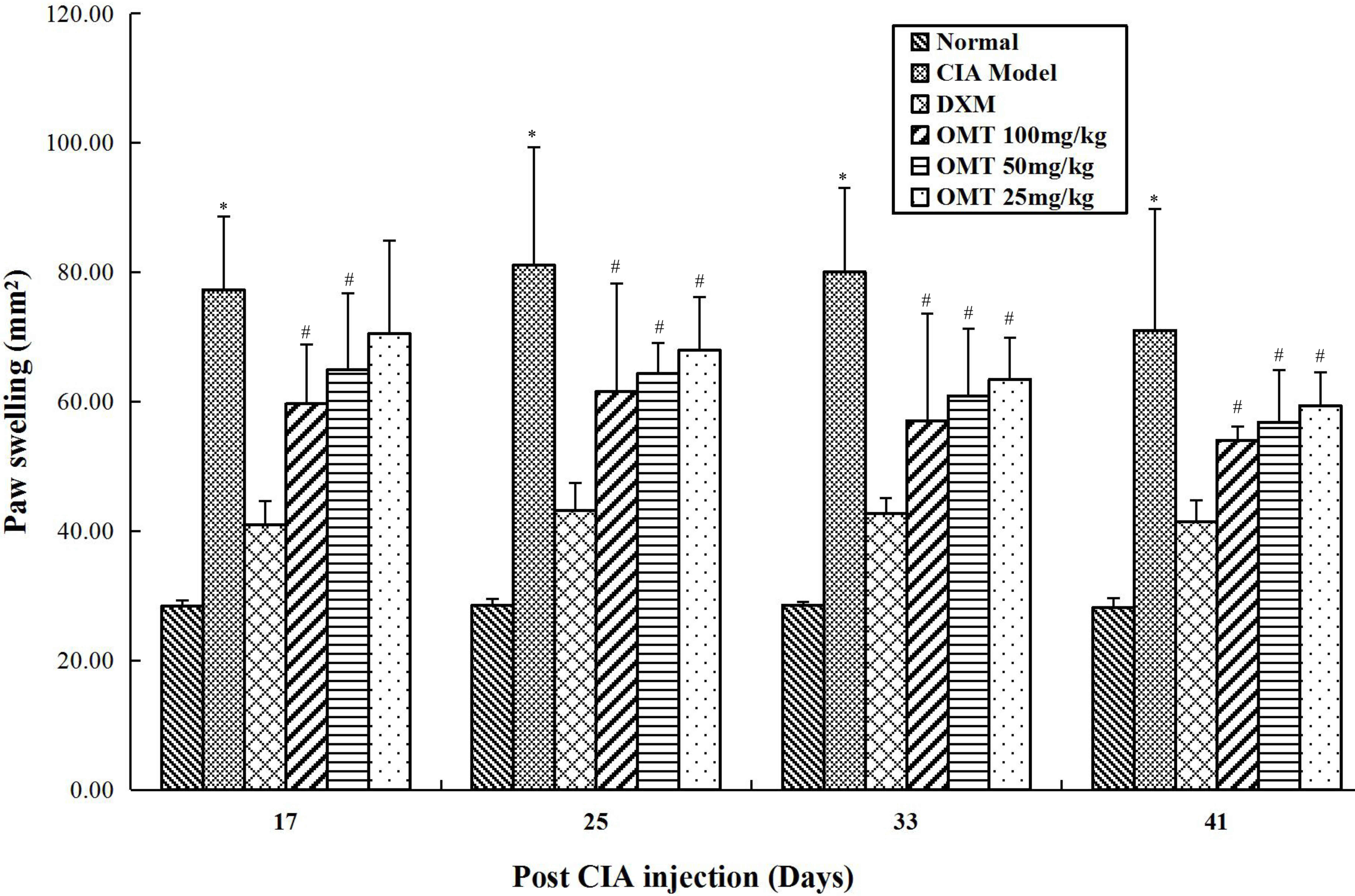
However, on days 17–18, hindpaw swelling was followed by the rapid
reappearance of inflammation. The rats treated with 100 and 50
mg/kg OMT showed significant reduction in paw edema volume,
compared with that in the model group. Treatment with OMT (100, 50
and 25 mg/kg/day, days 8–35) relieved the right hindpaw swelling
and inhibited the progression of polyarthritis between days 18 and
24 following secondary immunization (Fig. 1). The same efficacy of DXM (2
mg/kg, twice/week, ig, days 8–35) were observed.
To examine the effects of OMT on arthritic
progression in rats with CIA, the development of arthritis was
evaluated by scoring the clinical disease activity daily following
secondary immunization. The arthritic scores reached a peak on day
25. The OMT-treated groups had significantly decreased arthritic
scores, compared with the model group between days 17 and 41. The
same efficacy of DXM (2 mg/kg) was observed (Fig. 2).
OMT reduces synovial inflammation and
inflammatory articular destruction in SD rats with CIA
To investigate the inhibitory effects of OMT on
arthritic activity in rats with CIA, histopathological assessment
of the knee joints was performed using H&E staining. Compared
with the normal group (Fig. 3A),
the knee joints of the model group rats revealed marked synovial
hyperplasia and inflammatory cell infiltration into the joint
capacity (Fig. 3B). The data of
the DXM group showed a prominent reduction in synovial hyperplasia
and inflammatory cell infiltration, compared with the model group
(Fig. 3C). The CIA rats treated
with 100 or 50 mg/kg OMT had lower levels of inflammatory cells
infiltration, well-preserved joint spaces and minimal synovia
hyperplasia (Fig. 3D-F). These
results suggested that OMT inhibited synovial inflammation and
inflammatory articular destruction at the knee joints in rats with
CIA.
OMT suppresses inflammatory cytokine
production
It is known that numerous cytokines are fundamental
to the processes causing inflammation, articular destruction and
the co-morbidities associated with RA in the joints of patients
with RA. To investigate the mechanisms mediating the decreased
severity of CIA following OMT treatment, the present study
evaluated the effect of OMT on the production of mediators of
inflammation, which decreased the incidence and severity of CIA.
The levels of TNF-α and IL-17A inflammatory cytokines in the serum
drawn from the heart of CIA rats were measured using ELISA kits.
The concentrations of total TNF-α and IL-17A in the serum of the
CIA model group were significantly higher compared with the normal
group (P<0.05), whereas OMT (100, 50 and 25 mg/kg) treatment of
CIA rats markedly reduced the production of TNF-α and IL-17A
inflammatory cytokines in the serum of the CIA rats (Fig. 4A and B). In addition, dose of 100
mg/kg OMT significantly inhibited the production of TNF-α and
IL-17A, compared with a dose of 25 mg/kg (P<0.05). The
inhibitory effect on TNF-α and IL-17A levels in the serum of the
CIA rats was higher in the OMT-treated groups, compared with those
in the DXM-treated group (P<0.05). These data suggested that the
administration of OMT may deactivate the inflammatory response of
infiltrating and proliferating synovial cells in a dose-dependent
manner.
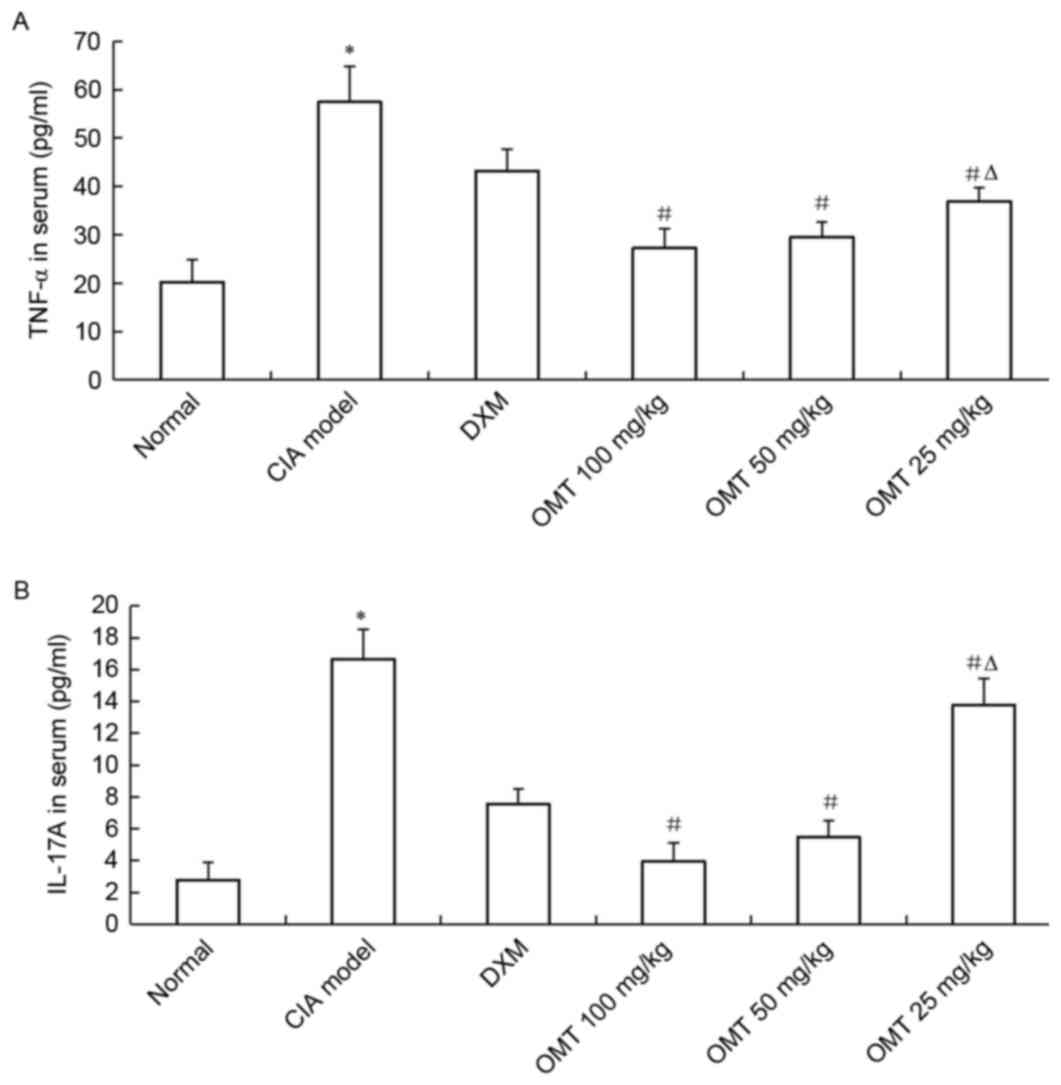 | Figure 4.Effects of OMT on serum
concentrations of TNF-α and IL-17A in CIA rats, measured using
enzyme-linked immunosorbant assays. (A) TNF-α; (B) IL-17A. Levels
of TNF-α and IL-17A were markedly reduced by OMT concentrations of
100, 50 and 25 mg/kg, in a dose-dependent manner. Results are
representative of three independent experiments. *P<0.05,
compared with the normal group; #P<0.05, compared
with the CIA model group; ∆P<0.05, compared with the
100 mg/kg group. OMT, oxymatrine; CIA, collagen-induced arthritis;
DXM, dexamethasone; TNF-α, tumor necrosis factor-α; IL-17A,
interleukin-17A. |
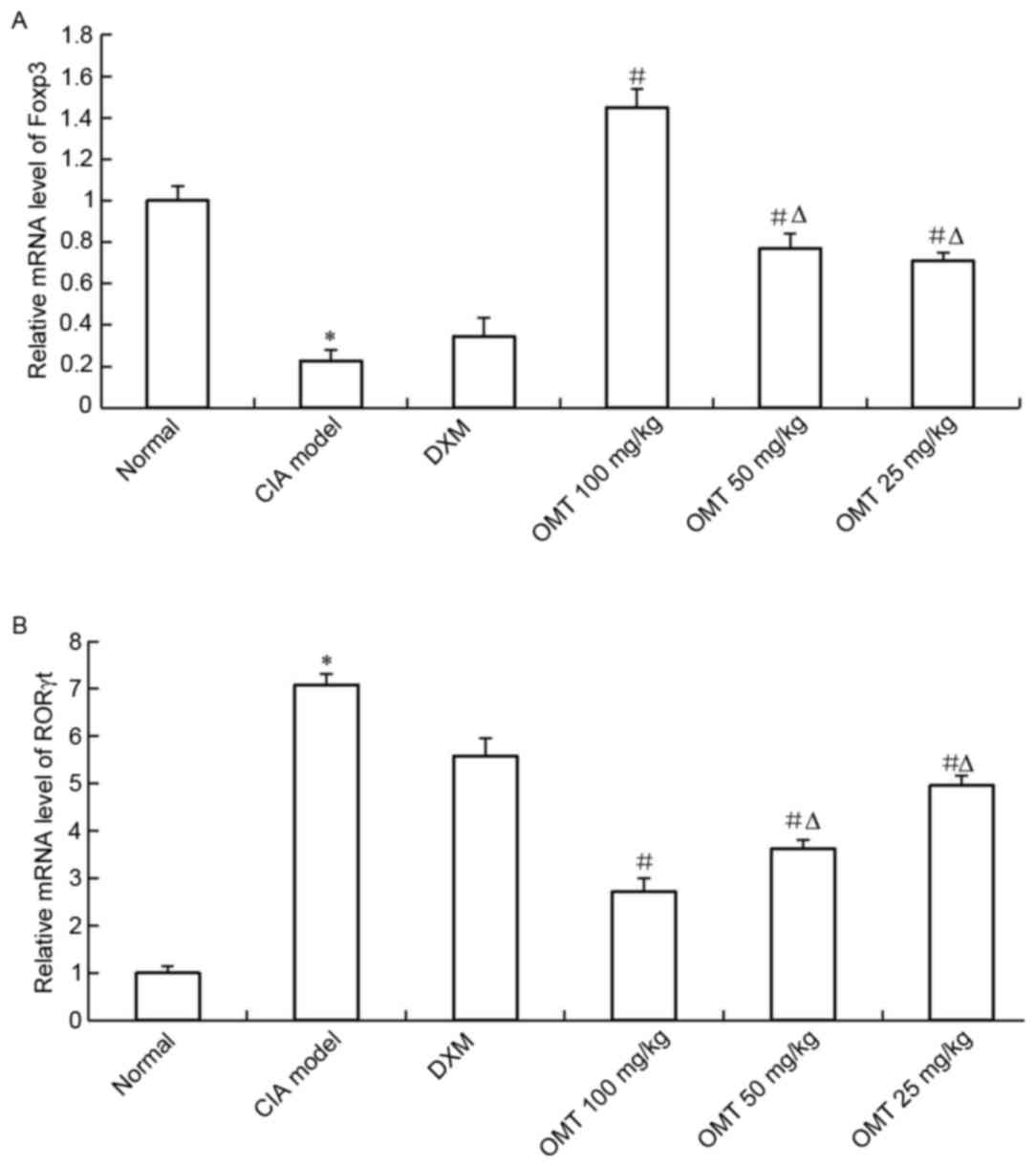
OMT increases the expression of FOXP3
and decreases the expression of RORγt in rat splenocytes
Total RNA was isolated from the spleen lymphocyte
cells of either the OMT-treated CIA rats or CIA model rats, and the
mRNA expression of Treg and Th17 cell-associated markers were
examined using RT-qPCR analysis. The results showed that the mRNA
level of FOXP3, a Treg cell-related molecule, was significantly
upregulated in the spleen lymphocyte cells of the OMT-treated rats
(Fig. 5A). By contrast, the mRNA
level of the Th17 cell-associated molecule, RORγt, was decreased in
the OMT-treated CIA rats (Fig.
5B). The results of the western blot analysis also demonstrated
that OMT treatment significantly suppressed the protein level of
RORγt and significantly increased that of FOXP3 in the rats' spleen
lymphocytes (Fig. 6A-C). Thus, the
administration of OMT in SD rats may ameliorate CIA by regulating
the imbalance between Treg cells and Th17 cells.
Discussion
RA is an autoimmune disease, which can cause chronic
joint inflammation and disability (39). The common treatment of such
diseases is typically with immunosuppressants, which inhibit the
immune response. However, the long-term use of immunosuppressive
medications can have harmful side effects (40,41).
Therefore, novel drugs with high efficacy and low toxicity are
urgently required. OMT is an alkaloid obtained from the traditional
Chinese medicine, Sophora flavescens Ait, which has been
reported to benefit patients suffering from inflammatory diseases,
cancer and chronic hepatitis B (42). However, mechanistic evidence of the
effect of OMT on the immune-inflammatory response in RA remains
limited. In the present study, the potential therapeutic function
of OMT in the CIA animal model was investigated. Paw swelling and
arthritic scores are indices for measuring the anti-arthritic
activity of various drugs, and were used in the present study to
determine the activity of OMT at concentrations of 100, 50 and 25
mg/kg/d/i.p. The results showed that rats in the OMT-administered
groups had a significant reduction in paw volume and marked
decrease in arthritic scores, compared with those in the CIA model
group. In addition, histopathologic assessment of the joints of the
OMT-treated rats revealed that OMT reduced synovial inflammation
and inflammatory articular destruction, and ameliorated symptoms in
the affected knee joint.
The most meaningful observation of the present study
was that OMT inhibited the level of IL-17A induced by the
inflammatory response, and upregulated Treg cells. It has been
reported that the balance between Th17 and Treg cells has a
significant role in the induction and progression of RA (43). Th17 cells, a novel CD4+
Th cell subtype, produce cytokine profiles, including IL-17, IL-21,
IL-22, IL-6 and TNF-α. The IL-17 cytokine family includes six
members: IL-17A, B, C, D, E (IL-25) and F (44,45).
It is well-documented that IL-17A is vital in the
additive/synergistic effects induced with TNF-α and IL-1, two key
pro-inflammatory cytokines in destructive arthritis, whereas Treg
cells expressing FOXP3 possess anti-inflammatory activity (5–47).
The significant suppression of pro-inflammatory cytokines,
including IL-17A and TNF-α, was examined in the spleen of
OMT-treated CIA rats. As the reduction in the expression of IL-17
and Th17 cells, and the upregulation of Treg cells are significant
properties in inhibiting inflammation, the results of the present
study revealed that OMT possessed an anti-inflammatory function in
autoimmune arthritis. RORγt, lineage-specific transcription factors
are essential for Th17 cell differentiation and the expression of
IL-17. Thus, the inhibition of Th17 differentiation is
therapeutically valuable, with suppression of differentiation and
reduced production of Th17-associated pro-inflammatory cytokines.
OMT markedly increased the expression of FOXP3 and suppressed the
expression of RORγt in the rat splenocytes.
In previous years, substantial evidence has
supported that an imbalance between Treg and Th17 cells is crucial
in the immunopathogenesis of RA (48,49).
The differentiation of Th17 cells was induced by the activation of
signal transducer and activator of transcription (STAT)3, however,
the activation of STAT5 promoted the expression of FOXP3 in Treg
cells (50). It has been reported
that the transcription of IL-17 is regulated by the competitive
binding of phosphorylated (p)STAT3 and pSTAT5 (51). Thus, pSTAT5 is a critical
transcriptional factor for FOXP3 in regulating the differentiation
of Treg cells. In the present study, OMT treatment enhanced the
differentiation of Treg cells and inhibited Th17 cells. Information
on the control of Th17 and Treg cells by OMT is limited. The
results of the present study showed the possibility of OMT
regulating the Treg/Th17 balance, which appears to be caused by the
downregulation of T cell transcriptional regulators, including
RORγt, and the upregulation of FOXP3. Therefore, further
investigations are required to clarify the effects of OMT on
modulating Janus kinase-STAT signaling.
In conclusion, the present study investigated OMT as
an immunomodulatory agent, involved in attenuating the inflammatory
response at multiple levels, and suppressing synovial inflammation
and cartilage destruction in CIA rats. These results assist in
further elucidating the pathogenesis of immune-mediated
inflammatory diseases, including RA. In addition, OMT may be a
potent strategy for the treatment or prevention of RA.
Acknowledgements
The authors would like to thank all members of the
Immunology Laboratory (Ningxia Medical University, Yinchuan, China)
for their assistance. The present study was supported by the
National Natural Science Foundation of China (grant no. 81560701)
and the Ningxia Natural Science Foundation Program (grant nos.
NZ14087 and NZ14139).
References
|
1
|
Helmick CG, Felson DT, Lawrence RC,
Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD,
Merkel PA, et al: Estimates of the prevalence of arthritis and
other rheumatic conditions in the United States. Part I. Arthritis
Rheum. 58:15–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Innala L, Sjöberg C, Möller B, Ljung L,
Smedby T, Södergren A, Magnusson S, Rantapää-Dahlqvist S and
Wållberg-Jonsson S: Co-morbidity in patients with early rheumatoid
arthritis-inflammation matters. Arthritis Res Ther. 18:332016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shegarfi H, Naddafi F and Mirshafiey A:
Natural killer cells and their role in rheumatoid arthritis: Friend
or foe? ScientificWorld Journal. 2012.4919742012.PubMed/NCBI
|
|
4
|
MacNaul KL, Hutchinson NI, Parsons JN,
Bayne EK and Tocci MJ: Analysis of IL-1 and TNF-alpha gene
expression in human rheumatoid synoviocytes and normal monocytes by
in situ hybridization. J Immunol. 45:4154–4166. 1990.
|
|
5
|
Azizi G, Jadidi-Niaragh F and Mirshafiey
A: Th17 Cells in Immunopathogenesis and treatment of rheumatoid
arthritis. Int J Rheum Dis. 16:243–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu J, Yamane H and Paul WE:
Differentiation of effector CD4 T cell populations (*). Annu Rev
Immunol. 28:445–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin
17-producing CD4+ effector T cells develop via a lineage distinct
from the T helper type 1 and 2 lineages. Nat Immunol. 6:1123–1132.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aarvak T, Chabaud M, Källberg E, Miossec P
and Natvig JB: Change in the Th1/Th2 phenotype of memory T-cell
clones from rheumatoid arthritis synovium. Scand J Immunol. 50:1–9.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Talaat RM, Mohamed SF, Bassyouni IH and
Raouf AA: Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus
erythematosus (SLE) patients: Correlation with disease activity.
Cytokine. 72:146–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heo YJ, Joo YB, Oh HJ, Park MK, Heo YM,
Cho ML, Kwok SK, Ju JH, Park KS, Cho SG, et al: IL-10 suppresses
Th17 cells and promotes regulatory T cells in the CD4+ T cell
population of rheumatoid arthritis patients. Immunol Lett.
127:150–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Louten J, Boniface K and de Waal Malefyt
R: Development and function of TH17 cells in health and disease. J
Allergy Clin Immunol. 123:1004–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dejaco C, Duftner C, Klauser A and
Schirmer M: Altered T-cell subtypes in spondyloarthritis,
rheumatoid arthritis and polymyalgia rheumatica. Rheumatol Int.
30:297–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Komatsu N and Takayanagi H: Regulatory T
cells in Arthritis. Prog Mol Biol Transl Sci. 136:207–215. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gizinski AM and Fox DA: T cell subsets and
their role in the pathogenesis of rheumatic disease. Curr Opin
Rheumatol. 26:204–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alunno A, Manetti M, Caterbi S,
Ibba-Manneschi L, Bistoni O, Bartoloni E, Valentini V, Terenzi R
and Gerli R: Altered Immunoregulation in Rheumatoid Arthritis: The
Role of Regulatory T Cells and Proinflammatory Th17 Cells and
Therapeutic Implications. Mediators Inflamm. 2015:7517932015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nam JL, Winthrop KL, Van Vollenhoven RF,
Pavelka K, Valesini G, Hensor EM, Worthy G, Landewé R, Smolen JS,
Emery P and Buch MH: Current evidence for the management of
rheumatoid arthritis with biological disease-modifying
antirheumatic drugs: A systematic literature review informing the
EULAR recommendations for the management of RA. Ann Rheum Dis.
69:976–986. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romas E, Gillespie MT and Martin TJ:
Involvement of receptor activator of NFkappaB ligand and tumor
necrosis factor-alpha in bone destruction in rheumatoid arthritis.
Bone. 30:340–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan FK: The David Y. Graham lecture: Use
of nonsteroidal antiinflammatory drugs in a COX-2 restricted
environment. Am J Gastroenterol. 103:221–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mosleh W and Farkouh ME: Balancing
cardiovascular and gastrointestinal risks in patients with
osteoarthritis receiving nonsteroidal anti-inflammatory drugs. A
summary of guidelines from an international expert group. Pol Arch
Med Wewn. 126:68–75. 2016.PubMed/NCBI
|
|
22
|
Pope JE, Anderson JJ and Felson DT: A
meta-analysis of the effects of nonsteroidal anti-inflammatory
drugs on blood pressure. Arch Intern Med. 153:477–484. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McGettigan P and Henry D: Cardiovascular
risk with non-steroidal anti-inflammatory drugs: Systematic review
of population-based controlled observational studies. PLoS Med.
8:e10010982011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harrold LR, Reed GW, Harrington JT, Barr
CJ, Saunders KC, Gibofsky A, Greenberg JD, John A, Devenport J and
Kremer JM: The rheumatoid arthritis treat-to-target trial: A
cluster randomized trial within the Corrona rheumatology network.
BMC Musculoskelet Disord. 15:3892014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smolen JS and Steiner G: Therapeutic
strategies for rheumatoid arthritis. Nat Rev Drug Discov.
2:473–488. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olsen NJ and Stein CM: New drugs for
rheumatoid arthritis. N Engl J Med. 350:2167–2179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JD, Huh JE, Baek YH, Cho KC, Choi DY
and Park DS: The Efficacy and Mechanism Action of RvCSd, a New
herbal agent, on immune suppression and cartilage protection in a
mouse model of rheumatoid arthritis. J Pharmacol Sci. 109:211–221.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guzman JR, Koo JS, Goldsmith JR, Mühlbauer
M, Narula A and Jobin C: Oxymatrine prevents NF-κB nuclear
translocation and ameliorates acute intestinal inflammation. Sci
Rep. 3:16292013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong XQ, Yu WH, Hu YY, Zhang ZY and Huang
M: Oxymatrine reduces neuronal cell apoptosis by inhibiting
Toll-like receptor 4/nuclear factor kappa-B-dependent inflammatory
responses in traumatic rat brain injury. Inflamm Res. 60:533–539.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang G, Liu X, Wang M, Chen H, Chen Z and
Qiu T: Oxymatrine ameliorates renal ischemia-reperfusion injury
from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras.
30:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao N and Wang X: In vitro
immunomodulatory activity of oxymatrine on Toll-like receptor 9
signal pathway in chronic hepatitis B. Am J Chin Med. 42:1399–1410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Jiang K and Zhao F: Oxymatrine
suppresses proliferation and facilitates apoptosis of human ovarian
cancer cells through upregulating microRNA-29b and downregulating
matrix metalloproteinase-2 expression. Mol Med Rep. 12:5369–5374.
2015.PubMed/NCBI
|
|
33
|
Wang W, Pei X, Xu M, Sun S, Zhang C, Mu K
and Liu Z: The Protective Effect of Sodium Ferulate and Oxymatrine
Combination on Paraquat-induced Lung Injury. Iran J Pharm Res.
14:573–583. 2015.PubMed/NCBI
|
|
34
|
Wu C, Huang W, Guo Y, Xia P, Sun X, Pan X
and Hu W: Oxymatrine inhibits the proliferation of prostate cancer
cells in vitro and in vivo. Mol Med Rep. 11:4129–4134.
2015.PubMed/NCBI
|
|
35
|
Parvez MK, Arbab AH, Al-Dosari MS and
Al-Rehaily AJ: Antiviral natural products against chronic hepatitis
B: Recent Developments. Curr Pharm Des. 22:286–293. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mason TJ and Matthews M: Aquatic
environment, housing and management in the eighth edition of the
guide for the care and use of laboratory animals: Additional
considerations and recommendations. J Am Assoc Lab Anim Sci.
51:329–332. 2012.PubMed/NCBI
|
|
37
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Klarenbeek NB, Kerstens PJ, Huizinga TW,
Dijkmans BA and Allaart CF: Recent advances in the management of
rheumatoid arthritis. Br Med J. 341:c69422010. View Article : Google Scholar
|
|
40
|
Katsuragi T, Iwashige A and Tsukada J:
Immunodeficiency-associated Burkitt lymphoma developed in a patient
receiving a long-term methotrexate therapy for rheumatoid
arthritis. Rinsho Ketsueki. 57:9–14. 2016.(In Japanese). PubMed/NCBI
|
|
41
|
Scott FI, Mamtani R, Brensinger CM, Haynes
K, Chiesa-Fuxench ZC, Zhang J, Chen L, Xie F, Yun H, Osterman MT,
et al: Risk of nonmelanoma skin cancer associated with the use of
immunosuppressant and biologic agents in patients with a history of
autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol.
152:164–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang X, Liu C, Wang J, Fan Y, Wang Z and
Wang Y: Oxymatrine inhibits the migration of human colorectal
carcinoma RKO cells via inhibition of PAI-1 and the TGF-β1/Smad
signaling pathway. Oncol Rep. 37:747–753. 2017.PubMed/NCBI
|
|
43
|
Estrada-Capetillo L, Hernández-Castro B,
Monsiváis-Urenda A, Alvarez-Quiroga C, Layseca-Espinosa E,
Abud-Mendoza C, Baranda L, Urzainqui A, Sánchez-Madrid F and
González-Amaro R: Induction of Th17 lymphocytes and Treg cells by
monocyte-derived dendritic cells in patients with rheumatoid
arthritis and systemic lupus erythematosus. Clin Dev Immunol.
2013:5843032013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bettelli E, Korn T and Kuchroo VK: Th17:
The third member of the effector T cell trlogy. Curr Opin Immunol.
19:652–657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weaver CT, Harrington LE, Mangan PR,
Gavrieli M and Murphy KM: Th17: An effector CD4 T cell lineage with
regulatory T Cell ties. Immunity. 24:677–688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Komatsu N and Takayanagi H: Regulatory T
cells in Arthritis. Prog Mol Biol Transl Sci. 136:207–215. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
An Haack I, Derkow K, Riehn M, Rentinck
MN, Kühl AA, Lehnardt S and Schott E: The Role of Regulatory CD4 T
Cells in Maintaining Tolerance in a Mouse Model of autoimmune
hepatitis. PLoS One. 10:e01437152015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lina C, Conghua W, Nan L and Ping Z:
Combined treatment of etanercept and MTX reverses Th1/Th2,
Th17/Treg imbalance in patients with rheumatoid arthritis. J Clin
Immunol. 31:596–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Venkatesha SH, Dudics S, Weingartner E, So
EC, Pedra J and Moudgil KD: Altered Th17/Treg balance and
dysregulated IL-1β response influence susceptibility/resistance to
experimental autoimmune arthritis. Int J Immunopathol Pharmacol.
28:318–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fischer A: Human immunodeficiency:
Connecting STAT3, Th17 and human mucosal immunity. Immunol Cell
Biol. 86:549–551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang XP, Ghoreschi K, Steward-Tharp SM,
Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L,
Vahedi G, et al: Opposing regulation of the locus encoding IL-17
through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol.
12:247–254. 2011. View Article : Google Scholar : PubMed/NCBI
|