Introduction
Growth factor typically acts as signaling molecules
between cells and is an important component required for tissue
engineering. There have been many attempts at developing dental
biomaterial or scaffold supplemented with a single or combination
of active molecules to induce dentine or pulp regeneration. One
growth factor that was widely selected for this purpose is vascular
endothelial growth factor (VEGF).
VEGF-A is one of the four subtypes of VEGF (-A, -B,
-C, and -D) that are expressed in dental pulp with autocrine and
paracrine effects in blood vessels and immune cells (1). It is a potential signaling protein
that can promote angiogenesis and activate dentin regeneration
(2). VEGF has been used in growth
factor delivery-based tissue engineering (3), including pulp tissue regeneration for
regenerative endodontics (4).
Moreover, VEGF has been shown to induce proliferation and
differentiation of human pulp cells into odontoblasts (5). Another interesting property of VEGF
is helping cells survive from stress or hazardous conditions. There
have been some studies reporting that VEGF played a role in
survival of serum-starved endothelial cells (6,7), as
well as survival of these cells in hypoxic conditions (8). A previous study has also revealed
that VEGF expression was upregulated in mouse odontoblast-like
cells (MDPC-23) exposed to 2-hydroxyethyl methacrylate (HEMA)
(9).
HEMA is a major monomer released from incomplete
polymerization of resin-based dental restorative materials. It is
cytotoxic to cells and can damage DNA (10), leading to proliferation impairment
(11) and delayed cell
differentiation (12).
Translationally controlled tumor protein (TCTP) is a
conserved protein found in many eukaryotic cells ranging from plant
to animal kingdoms (13). TCTP has
many functions, including proliferation, maturation, and
antiapoptosis (14). TCTP from
Penaeus merguiensis (Pmer-TCTP) has been shown to
have an antiapoptotic property against HEMA-treated dental pulp
cells (15). A new formula of
resin modified glass ionomer cement supplemented with TCTP can
reduce cell death from residual HEMA release (16). The aim of this study was to compare
cell proliferation, anticytotoxicity, differentiation and
mineralization of VEGF-A with TCTP on HEMA-treated human dental
pulp cells (HDPCs). This will assist the further development of a
restorative material that may have less toxicity and be able to
regenerate pulpal dentine complex.
Materials and methods
Expression and purification of
recombinant TCTP and recombinant VEGF
Expression of Pmer-TCTP gene was performed in
the bacteria Escherichia coli (E. coli) strain BL21.
The protein was purified according to methods previously described
(14). Briefly, E. coli
strain BL21 harboring pGEX-Pmer-TCTP was inoculated and
induced by 1 mM IPTG (isopropyl β-D-thiogalactopyronositol) for
stimulating protein expression. After induction for 3 h, the
bacterial cells were harvested by centrifugation and the soluble
glutathione S-transferase (GST)-TCTP protein was purified by using
Glutathione Sepharose 4 Fast Flow (GE Healthcare Bio-Science,
Piscataway, NJ, USA) and thrombin was used for splitting of
GST-tagged protein. The purified Pmer-TCTP protein with
molecular mass about 19.2 kDa (168 amino acid residues) was
determined by SDS-PAGE and protein concentration was analyzed by a
BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Recombinant human VEGF was purchased from
Gibco® (Gibco; Thermo Fisher Scientific, Inc.). This
protein has about 40 kDa (homodimer) with 165 amino acid
residues/subunit.
Cell culture
HDPCs were cultured from pulp tissue of sound third
molar teeth of adults aged 18–25 years at the Dental Hospital,
Faculty of Dentistry, Prince of Songkla University, with consent
forms approved by the Research Ethics Committee (Number
EC5703-09-P-LR), Faculty of Dentistry, Prince of Songkla
University. Primary culture of pulp cells was performed using an
enzymatic method. Briefly, the pulp tissue was cut into small
pieces before digested in 3 mg/ml of collagenase type I and 4 mg/ml
of dispase for 1 h at 37°C. After centrifugation, cells were
cultured in α-MEM, supplemented with 10% fetal calf serum (FCS),
100 µM L-ascorbic acid 2-phosphate, 100 µM L-glutamate, 100 U/ml
penicillin, and 100 µg/ml streptomycin and incubated at 37°C with
5% CO2. Pulp cell passages between three and five were
used for this study.
Finding effective concentrations of
TCTP and VEGF on HDPCs
The effect of TCTP and VEGF at various
concentrations on HDPCs was examined by the 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. TCTP and VEGF were diluted with cell culture medium at
various concentrations varying from 1 ng/ml to 15 µg/ml (n=5 in
each group). HDPCs at a density of 5×103 cells/well were
cultured in 96-well plates in a humidified atmosphere of 5%
CO2 at 37°C for 24 h and then the culture medium
supplemented with TCTP and VEGF were replaced. After 24 and 72 h,
the medium was removed, 200 µl of fresh medium containing 10 mM
HEPES (pH 7.4) and 50 µl MTT solution (5 mg/ml in PBS) were added
to each well and incubated in the dark for 4 h at 37°C. The medium
and MTT were then removed and 200 µl of DMSO and 25 µl of
Sorensen's glycine buffer (0.1 M glycine plus 0.1 M NaCl
equilibrated to pH 10.5 with 0.1 M NaOH) were added. The optical
density (OD) of formazan production was measured at 570 nm. The OD
values adjusted for a blank (medium only) of the experimental
groups were divided by the control (cell cultured in normal medium
without TCTP and VEGF) and expressed as percentages of the control,
which represented the percentages of viable cells.
Cytotoxicity of HEMA
MTT assay was used to evaluate the cytotoxicity of
HEMA at two concentrations, which were 4 mM and 6 mM. HDPCs were
seeded at 8×103 cells/well in a 96-well plate at a
humidified atmosphere of 5% CO2 at 37°C. After 24 h, the
media was replaced with HEMA mixed in culture medium and left for
24 h. Then the media was refreshed with normal media and incubated
for 48 h before MTT assay was performed as described previously
(n=5 in each group).
The results from HEMA cytotoxicity testing led to
the selection of 4 mM HEMA as a cytotoxic reagent. In addition, the
result from effective concentrations of TCTP and VEGF suggested the
use of TCTP at 1 and 10 ng/ml of VEGF for further
investigation.
The recovery effect of TCTP and VEGF
on HEMA-treated HDPCs
There were five groups of HDPCs according to
different medium conditions: HEMA, HEMA+TCTP, HEMA+VEGF,
HEMA+TCTP+VEGF, and control group (culture medium only). HDPCs were
seeded at 8×103 cells/well on a 96-well plate at a
humidified atmosphere of 5% CO2 at 37°C and fed with
normal medium. After 24 h, the culture medium was replaced with
media supplemented with substances as described above for 24 h.
After that, the fresh media was replaced and incubated for 48 h.
The viable cells were determined by the MTT assay (n=5 in each
group).
Alkaline phosphatase (ALP) activity
assay
ALP activity assay was used to determine
odontoblast-like differentiation. The experiment was divided to six
groups according to substances added to the medium: HEMA,
HEMA+TCTP, HEMA+VEGF, TCTP, VEGF and cells cultured in normal
medium as a control group. HDPCs were seeded at 1×104
cells/well on a 24-well plate and fed with normal culture medium
using 15% FCS at a humidified atmosphere of 5% CO2 at
37°C (n=3 in each group). The medium was changed every 2 days and
the ALP activity of cells was determined after being cultured for
3, 7 and 14 days. The ALP activity was measured by using
p-nitrophenol phosphate as a substrate. Cells were washed
twice with PBS pH 7.4 and lysed in 20 µl/well of lysis buffer (100
mM MgCl2·6H2O, 1 M Tris-HCl pH 7.4, 0.1%
Triton-X 100, pH 10) on ice and centrifuged at 14,000 g at 4°C for
10 min. The supernatant was collected for total protein and ALP
activity determinations. The total protein was determined by using
BCA kit. For ALP activity assay, 5 µl of the supernatant of each
sample was incubated in 50 µl of ALP substrate solution containing
0.2 M 2-amino-2-methyl-1-propanol (AMP), 4 mM MgCl2 and
50 µl of 4 mg/ml p-nitrophenol phosphate at 37°C for 30 min.
Then, the reaction was stopped with 100 µl of 0.1 M NaOH. The
absorbance was measured at 405 nm. ALP activity was calculated as
nanomolar of p-nitrophenol per µg of total protein and
adjusted to the increased percentage compared to the control
(medium only).
Alizarin red S (ARS) staining
assay
Alizarin red S (ARS) staining assay was used to
evaluate calcium deposition. There were six groups according to
different substances added in the inductive medium, which were
HEMA, HEMA+TCTP, HEMA+VEGF, TCTP, VEGF and the last group was cells
cultured in an inductive medium only as a positive control group.
HDPCs at 1.5×105 cells/well were seeded on a 12-well
plate and fed with 500 µl/well of inductive medium, which was
composed of 1 M β-glycerophosphate, 10 mg/ml ascorbic acid, 100
U/ml penicillin, 100 mg/ml streptomycin and 100 mg/ml
antibiotic-antimycotic, in α-MEM with 20% fetal calf serum (n=3 in
each group) at 37°C in a humidified atmosphere containing 5%
CO2. After the first 24 h, the medium was refreshed and
then the medium was replaced every 2 days. After 7, 14 and 21 days,
the medium was removed and cells were washed with PBS pH 7.4 and
fixed in 10% (v/v) formaldehyde (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at room temperature for 15 min and washed twice
with 1 ml/well of ultrapure water at room temperature for 5 min.
Then Alizarin Red solution was added 1 ml/well and incubated on
shaker for 30 min. After removal of ARS, the plate was washed four
times with ultrapure water, while shaking for 5 min, and then Store
plates at −20°C prior to dye extraction. The monolayers were
incubated by 200 µl cetylpyridinium chloride (CPC) buffer for 2 h
on a shaker. After that, it was centrifuged at 20,000 g for 15 min
and 100 µl of the supernatant was then transferred to a 1.5 ml
tube. It was diluted (10 µl of sample and 90 µl of CPC) to a
96-well plate and the OD values corrected for a blank (CPC only).
The absorbance of the sample was measured in an ELISA reader at 550
nm.
Gene expression
Quantitative real-time reverse transcription
polymerase chain reaction (qRT-PCR) was used to investigate the
effect of each sample on pulp cells differentiation. Dentin
sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP-1), ALP
and bone morphogenetic protein-2 (BMP-2) expressions were examined
and gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was
selected to be an internal control. The primers (5′- and 3′-) were
designed and shown in Table I.
There were six groups in the experiment: HEMA 4 mM alone and with
(1 ng/ml TCTP, 10 ng/ml VEGF) with pulp cells, 1 ng/ml TCTP, 10
ng/ml VEGF with pulp cells and cells cultured in normal medium was
set as the control. The experiments were repeated three times.
HDPCs at 1.5×106 cells were seeded on six-well plate
with 2,000 µl/well of normal medium. After 24 h, the mixed-medium
with selected supplements was replaced and then changed every 2
days. After 1, 3 and 7 days the medium was removed and cells were
washed with PBS pH 7.4. The total RNA of cells was extracted and
purified by RNeasy® Plus Micro kits (Qiagen, Inc.,
Valencia, CA, USA) and converted to cDNA with SuperScript™ III RT
(Invitrogen; Thermo Fisher Scientific). The thermal profile for
RT-PCR, under denaturing conditions was 65°C for 5 min followed by
cDNA synthesis at 50°C for 30 min. The reaction was terminated at
85°C for 5 min. Then the remaining RNA was removed by adding 1 µl
of RNase H at 37°C for 20 min and 3 µl of each cDNA product was
fractionated by 1.2% agarose gel electrophoresis and visualized by
ethidium bromide staining by ultraviolet (UV) transillumination.
SYBR Green PCR master mix (Roche Diagnostics GmbH, Mannheim,
Germany) was used for qPCR analysis. The qPCR mix consisted of 1 µl
of cDNA, 2 µl of forward and reverse primer, 10 µl of SYBR Green
and 5 µl of nuclease-free water, and the negative control was
distilled water instead of cDNA sample. The reaction was performed
manually in triplicates in each sample, at a final volume of 20 µl.
The thermal profile for amplification of the investigated gene was
followed by 40 cycles of denaturation at 95°C for 15 sec, annealing
at 60°C for 30 sec and then elongation at 72°C for 30 sec. After
the end of the last cycle, the final quantification was reported as
an amount after normalization to reference gene GAPDH. Relative
values were analyzed using comparative cycle threshold (CT) method
(∆∆CT method).
 | Table I.qPCR F and R primer sequences. |
Table I.
qPCR F and R primer sequences.
| Gene | Primer sequence (5′-
3′) | GenBank accession
no. |
|---|
| DSPP | F:
GGGATGTTGGCGATGCA | NM_014208.3 |
|
| R:
CCAGCTACTTGAGGTCCATCTTC |
|
| DMP1 | F:
GCAGAGTGATGACCCAGAG | NM_004407.3 |
|
| R:
GCTCGCTTCTGTCATCTTCC |
|
| ALP | F:
CCACAAGCCCGTGACAGA | NM_001127501.3 |
|
| R:
GCGGCAGACTTTGGTTTC |
|
| BMP-2 | F:
GCTTCCGCCTGTTTGTGTTTG | NM_007553.2 |
|
| R:
AGAGACATGTGAGGATTAGCAGGT |
|
| GAPDH | F:
GCACCGTCAAGGCTGAGAAC | NM_002046 |
|
| R:
ATGGTGGTGAAGACGCCAGT |
|
Statistical analysis
The results of MTT were analyzed using the one way
analysis of variance (ANOVA) and Tukey's post hoc-test to
investigate the differences among the experimental groups. Unpaired
t-test was used to compare the difference of means between two
groups of TCTP and VEGF. Kruskal-Wallis and Dunnett multiple
comparison were used to analyze the results of ALP assay, ARS assay
and qRT-PCR. P<0.05 was considered as statistically
significant.
Results
Effective concentration of TCTP and
VEGF on HDPCs
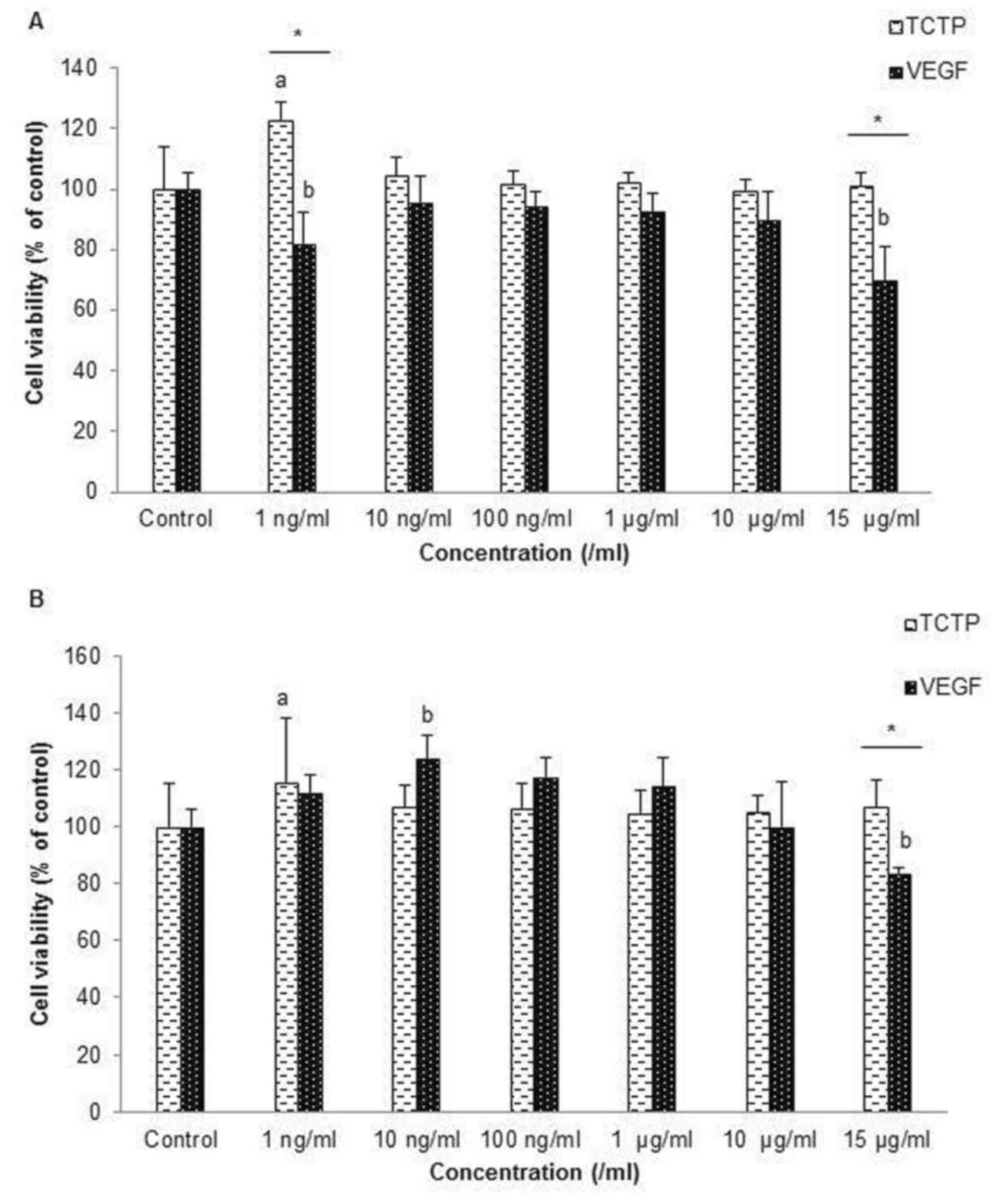
HDPCs were treated with various concentrations from
1 to 15 µg/ml of TCTP and VEGF in order to find the effective
concentrations that are not cytotoxic to cells and can also promote
cell growth. After culturing for 24 h, most of the various
concentrations of TCTP can promote cell growth because the
percentage of viable cells was over 100% compared to the control
(Fig. 1A), especially at 1 ng/ml
of TCTP, which gave the highest percentages of cell viability
(P<0.05). Cells cultured in medium supplemented with VEGF did
not have a higher percentage of viable cells than the control after
culturing for 24 h. However, after 72 h, it was found that at low
concentration, especially at 1 ng/ml of TCTP and 10 ng/ml of VEGF,
had significantly higher cell viability than the control and
compared within groups (P<0.05). High concentration of VEGF at
15 µg/ml significantly reduced cell viability (Fig. 1B). The results led to the
determination to use 1 ng/ml of TCTP and 10 ng/ml of VEGF in
culture medium for further studies.
The recovery effect of TCTP and VEGF
on HEMA-treated HDPCs
HEMA-treated pulp cells at concentration of 4 mM and
6 mM demonstrated its high level of cytotoxicity, because the
percentages of viable cells were all less than 10% (Fig. 2A). The result from Fig. 2B showed that HDPCs cultured in 4 mM
of HEMA with either 1 ng/ml TCTP or 10 ng/ml VEGF and both of
combinations TCTP and VEGF had percentages of cell viability that
were not statistically different from the control group, while cell
viability in HEMA-treated cells was significantly lower than other
groups (P<0.05) (Fig. 2B). The
morphological features of HDPCs were shown in Fig. 2C. The results suggested that TCTP
and VEGF had an ability to recover the viability of HEMA-treated
pulp cells. It was noted that HEMA-treated cells supplemented with
TCTP+ VEGF did not increase the percentages of cell viability, when
compared to either TCTP or VEGF.
ALP activity
The result of ALP activity was revealed in Fig. 3. HEMA significantly reduced ALP
activity compared to the control and TCTP or VEGF groups in all
three periods (day 3 P<0.01), (day 7 and day 14 P<0.001).
HEMA+TCTP and HEMA+VEGF groups had slightly higher ALP activity
than HEMA within each time period.
Mineralization effect of TCTP and VEGF
on HEMA-treated HDPCs
The Alizarin red S (ARS) assay was used to determine
calcium deposition and the results have been shown in Fig. 4. After culture for 7 days, the
levels of calcium deposition in TCTP and VEGF groups were higher
than the others (P<0.001). However, calcium in group HEMA+VEGF
was significantly higher than HEMA+TCTP and HEMA alone (P<0.01).
In addition, after culture for 14 days, cells in groups HEMA+TCTP,
HEMA+VEGF, TCTP, and VEGF had increased calcium deposition compared
to the control. In particular, the HEMA+TCTP group had the highest
calcium deposition (P<0.001). At this period, the level of
calcium deposition in cells treated with TCTP and VEGF was reduced
from 7 days. After culture for 21 days, HDPCs cultured in an
inductive medium with added TCTP had increased calcium deposition
and became the highest compared to other groups.
It was noted that HDPCs cultured in inductive medium
only (control) had increased calcium deposition by time and reached
its highest after 21 days. Cells treated with HEMA+TCTP had reached
its highest calcium deposition after 14 days and then reduced, but
were still the same level as control after 21 days. Furthermore,
the groups of HEMA+TCTP and HEMA+VEGF had higher calcium deposition
than the HEMA group at all three time periods.
Differentiation-related gene
expression of HDPCs by qRT-PCR
The result of qRT-PCR was shown in Fig. 5. The response of DSPP gene was
reported as an average of fold expression (Fig. 5A). At day 1, the expression of DSPP
gene in cells treated with HEMA was lower than control, while
upregulation was found in cells treated with TCTP and VEGF. At day
3, cells treated with HEMA+TCTP (P<0.001) and HEMA+VEGF
(P<0.01) had significantly higher upregulation than other
groups. However, downregulation was found in all groups on day
7.
The high level of BMP-2 gene expression was observed
in cells treated with HEMA+TCTP on day 1 (P<0.01) and on day 3
(P<0.001) and HEMA+VEGF on day 1 and day 3 (P<0.001)
(Fig. 5B). In addition, the
expression of BMP-2 gene upregulated significantly highest in group
HEMA+VEGF on day 1 and day 3 (P<0.001). BMP-2 gene was down
regulated in all groups on day 7.
On day 1, the expression of ALP gene, in control
group, HEMA+TCTP and TCTP was higher than other groups (Fig. 5C). Moreover, the upregulation of
ALP expression in TCTP and VEGF groups increased immediately on day
3, and ALP expression in VEGF groups was significantly higher
(P<0.01) compared to others which corresponded with the result
of ALP activity in Fig. 3. ALP
gene expression down regulated in all groups on day 7.
DMP-1 expression was reduced by time in all groups
(Fig. 5D). DMP-1 expression in the
control group, HEMA+VEGF and VEGF, were higher than other groups on
day 1. The expression of DMP-1 gene upregulated significantly
highest in VEGF group on day 3 (P<0.05). HDPCs treated with
HEMA+VEGF (P<0.05), TCTP (P<0.05), and VEGF (P<0.01) were
significantly higher compared to others on day 7.
Discussion
This study evaluated some biological properties of
VEGF-A or VEGF, especially the anticytotoxicity, differentiation
and mineralization in pulp cells compared to TCTP. Previous studies
have reported that VEGF (3,5) and
Pmer-TCTP (15) can promote
pulp cell proliferation and differentiation, which were confirmed
by the results of this study. The VEGF used in this study is in a
homodimer form, which has 165 amino acid residues per monomer.
Pmer-TCTP is composed of 168 amino acid residues and the
molecular mass is not much different from this VEGF monomer. The
effective concentration of VEGF that can promote cell growth after
72 h and also used to promote survival of HEMA-treated HDPCs was 10
ng, which was the working concentration that induced stem cells to
differentiate (3). The
concentration that promoted growth of HDPCs of TCTP was lower (1
ng/ml) than VEGF (10 ng/ml), which may suggest that TCTP has a
higher potential than VEGF in promoting HDPCs growth.
Both TCTP and VEGF can survive HEMA-treated pulp
cells. But they did not have a synergistic effect, as pulp cells
treated with HEMA+TCTP+VEGF did not increase the percentages of
viable cells compared to HDPCs exposed to either HEMA+TCTP or
HEMA+VEGF. This result may suggest that the mechanism whereby both
VEGF and TCTP survive HEMA-treated pulp cells might relate to each
other or share the same carrier or pathway. However, further
investigation is required.
The mechanism of the added VEGF that can promote
HDPCs proliferation and survive cells from HEMA exposure may
involve signal transduction through receptor tyrosine kinases,
vascular endothelial growth factor receptor 2 (VEGF-R2), and lead
to cell proliferation by either activation of the mitogen-activated
protein kinases or MAPK cascade via Raf stimulation or PLC
(phospholipase C)-γ. Activation of PI3K (phosphatidylinositol
3-kinase) leads to activation of PKB (protein kinase B) and the
cell survival process (7,17). VEGF can also survive cells from
hypoxic conditions via the PI3K/Akt/FoxO1 pathway (8). VEGF induces expression of the
anti-apoptotic proteins Bcl-2 in vascular endothelial cells
(18) and in leukemic cells
(19).
TCTP and its anti-apoptotic effect against
HEMA-treated pulp cells has previously been reported (15). The mechanism whereby TCTP can
survive HEMA-treated pulp cells remains unknown. It was shown that
TCTP can inhibit apoptosis by maintaining mitochondria stability
and inhibiting Bax function (20),
as well as forming heterocomplexes with Bcl-xL (21). However, TCTP also inhibits
apoptosis by stabilizing anti-apoptotic Bcl-2 family proteins, MCL1
and by inhibiting p53-dependent apoptosis by down regulating this
protein (14).
The results of ALP activity assay and cell
mineralization by the ARS assay confirmed the results of other
studies, which showed that TCTP (16) and VEGF (2) can enhance differentiation and
mineralization of pulp cells. This was also consistent with qRT-PCR
results that both TCTP and VEGF had up regulated ALP expression on
day 3 compared to the control. TCTP may promote cell mineralization
longer than VEGF, as shown in the results of calcium deposition
after 21 days. DMP-1 is a phosphoprotein that activates both bone
and dentin mineralization by inducing the deposition of mineral
particles along the collagen fibrils (22). HDPCs had lower expression of DMP-1
in longer culture time in all groups, but it was noticed that TCTP
and VEGF groups still had higher expression than the control after
culture for 3 and 7 days, respectively.
This study revealed that HEMA at low concentration
(4 mM) was cytotoxic to cells and caused down regulation of DSPP,
BMP-2, ALP and DMP-1 expression. TCTP and VEGF up regulated DSPP
expression on day 1 and BMP-2 expression on day 1 and day 3, which
supports that both proteins can promote pulp cells differentiation,
especially in TCTP/VEGF supplemented in HEMA-treated pulp cells. A
recent study revealed that VEGF may have synergist effect to BMPs,
including on cell survival and mineralization (23). Further investigation may be
required concerning the potential connection of the two mechanisms
protecting cell death and differentiation, since both VEGF and TCTP
had anticytotoxicity and promoted cell differentiation.
In conclusion both TCTP and VEGF promoted pulp cells
proliferation and survived HEMA-treated cells, but TCTP was
required a lower concentration for these functions. They did not
have a synergistic effect; however both of them can enhance cell
differentiation and mineralization.
Acknowledgements
The authors would like to thank Professor Peter
Leggat (James Cook University, Australia) for his advice and proof
reading of the manuscript.
Funding
This study was supported by the Higher Education
Research Promotion and National Research University Project of
Thailand (grant no. DEN580513S), Office of the Higher Education
Commission and a Postgraduate Grant, Prince of Songkla
University.
Availability of data and material
The datasets generated and analyzed during the
current study are not currently publicly available due to work in
progress on a suitable repository in Thailand, however they are
available from the corresponding author on reasonable request.
Authors' contributions
The current study was conceived by UK. CW performed
the experiments, analyzed and interpreted the data in conjunction
with WC and UK. CW and UK were the major contributors in writing
the draft manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Human Research Ethics
Committee, Faculty of Dentistry, Prince of Songkla University
(approval number EC5703-09-P-LR) and by the Dental Hospital,
Faculty of Dentistry, Prince of Songkla University. This included
the use of approved consent forms for the collection of
specimens.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Virtej A, Løes S, Iden O, Bletsa A and
Berggreen E: Vascular endothelial growth factors signalling in
normal human dental pulp: A study of gene and protein expression.
Eur J Oral Sci. 121:92–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Liu X, Yu W, Zhang Y, Shi C, Ni
S, Liu Q, Li X, Sun Y, Zheng C and Sun H: Effects of human vascular
endothelial growth factor on reparative dentin formation. Mol Med
Rep. 13:705–712. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yadlapati M, Biguetti C, Cavalla F, Nieves
F, Bessey C, Bohluli P, Garlet GP, Letra A, Fakhouri WD and Silva
RM: Characterization of a vascular endothelial growth factor-loaded
bioresorbable delivery system for pulp regeneration. J Endod.
43:77–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Ma C, Xie X, Sun H and Liu X: Pulp
regeneration in a full-length human tooth root using a hierarchical
nanofibrous microsphere system. Acta Biomater. 35:57–67. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsushita K, Motani R, Sakuta T,
Yamaguchi N, Koga T, Matsuo K, Nagaoka S, Abeyama K, Maruyama I and
Torii M: The role of vascular endothelial growth factor in human
dental pulp cells: Induction of chemotaxis, proliferation and
differentiation and activation of the AP-1-dependent signaling
pathway. J Dental Res. 79:1596–1603. 2000. View Article : Google Scholar
|
|
6
|
Gerber HP, McMurtrey A, Kowalski J, Yan M,
Keyt BA, Dixit V and Ferrara N: Vascular endothelial growth factor
regulates endothelial cell survival through the
phosphatidylinositol 3′-kinase/Akt signal transduction pathway.
Requirement for Flk-1/KDR activation. J Biol Chem. 273:30336–30343.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Latham AM, Odell AF, Mughal NA, Issitt T,
Ulyatt C, Walker JH, Homer-Vanniasinkam S and Ponnambalam S: A
biphasic endothelial stress-survival mechanism regulates the
cellular response to vascular endothelial growth factor A. Exp Cell
Res. 318:2297–2311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong Q, Zhou Y, Ye W, Cai T, Zhang X and
Deng DY: Hypoxia-inducible factor 1-alpha-AA-modified bone marrow
stem cells protect PC12 cells from hypoxia-induced apoptosis,
partially through VEGF/PI3K/Akt/FoxO1 pathway. Stem Cells Dev.
21:2703–2717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mantellini MG, Botero T, Yaman P, Dennison
JB, Hanks CT and Nor JE: Adhesive resin and the hydrophilic monomer
HEMA induce VEGF expression on dental pulp cells and macrophages.
Dent Mater. 22:434–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ginzkey C, Zinnitsch S, Steussloff G,
Koehler C, Hackenberg S, Hagen R, Kleinsasser NH and Froelich K:
Assessment of HEMA and TEGDMA induced DNA damage by multiple
genotoxicological endpoints in human lymphocytes. Dent Mater.
31:865–876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Nisio C, D'Aurora M, di Giacomo V,
Stuppia L, Cataldi A and Gatta V: Transcriptome modifications in
human gingival fibroblasts exposed to 2-hydroxyethyl methacrylate.
Gene. 582:38–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwon JH, Park HC, Zhu T and Yang HC:
Inhibition of odontogenic differentiation of human dental pulp
cells by dental resin monomers. Biomater Res. 19:82015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amson R, Pece S, Marine JC, Di Fiore PP
and Telerman A: TPT1/TCTP-regulated pathways in phenotypic
reprogramming. Trends Cell Biol. 23:37–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagano-Ito M and Ichikawa S: Biological
effects of Mammalian translationally controlled tumor protein
(TCTP) on cell death, proliferation and tumorigenesis. Biochem Res
Int. 2012:2049602012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wanachottrakul N, Chotigeat W and
Kedjarune-Leggat U: Translationally controlled tumor protein
against apoptosis from 2-hydroxy-ethyl methacrylate in human dental
pulp cells. J Mater Sci Mater Med. 22:1479–1487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wanachottrakul N, Chotigeat W and
Kedjarune-Leggat U: Effect of novel chitosan-fluoroaluminosilicate
resin modified glass ionomer cement supplemented with
translationally controlled tumor protein on pulp cells. J Mater Sci
Mater Med. 25:1077–1085. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grando Mattuella L, Westphalen Bento L, de
Figueiredo JA, Nor JE, de Araujo FB and Fossati AC: Vascular
endothelial growth factor and its relationship with the dental
pulp. J Endod. 33:524–530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerber HP, Dixit V and Ferrara N: Vascular
endothelial growth factor induces expression of the antiapoptotic
proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem.
273:13313–13316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dias S, Shmelkov SV, Lam G and Rafii S:
VEGF(165) promotes survival of leukemic cells by Hsp90-mediated
induction of Bcl-2 expression and apoptosis inhibition. Blood.
99:2532–2540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Susini L, Besse S, Duflaut D, Lespagnol A,
Beekman C, Fiucci G, Atkinson AR, Busso D, Poussin P, Marine JC, et
al: TCTP protects from apoptotic cell death by antagonizing bax
function. Cell Death Different. 15:1211–1220. 2008. View Article : Google Scholar
|
|
21
|
Thebault S, Agez M, Chi X, Stojko J, Cura
V, Telerman SB, Maillet L, Gautier F, Billas-Massobrio I, Birck C,
et al: TCTP contains a BH3-like domain, which instead of
inhibiting, activates Bcl-xL. Sci Rep. 6:197252016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki S, Haruyama N, Nishimura F and
Kulkarni AB: Dentin sialophosphoprotein and dentin matrix
protein-1: Two highly phosphorylated proteins in mineralized
tissues. Arch Oral Biol. 57:1165–1175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Wang H, Qiu G, Su X and Wu Z:
Synergistic effects of vascular endothelial growth factor on bone
morphogenetic proteins induced bone formation in vivo: Influencing
factors and future research directions. Biomed Res Int.
2016:28695722016. View Article : Google Scholar : PubMed/NCBI
|