Introduction
There is a dynamic balance between osteoblast and
adipocyte differentiation of bone marrow stromal cells (BMSCs).
Under the action of signaling pathways, BMSCs have the potential to
differentiate into osteoblasts or adipocytes (1). Postmenopausal osteoporosis (PMOP) is
a skeletal disorder that results from osteoclastic bone resorption
outpacing osteoblastic bone formation, is induced by estrogen
deficiency, and characterized by decreased bone mass with an
increased risk of fragility fracture (2). Current drugs available to treat PMOP
aim to decrease osteoclastic activity or increase osteoblastic
activity. Although estrogen replacement therapy is commonly used
for PMOP prevention and treatment, it increases the risk of breast
cancer and venous thromboembolism, and therefore, the risks
outweigh the beneficial anti-resorptive effects of estrogen therapy
(3). Denosumab, an androgen
deprivation therapy, has reported side effects including sexual
dysfunction, fatigue and metabolic syndrome (4). Bisphosphonates, the first-line
anti-resorptive drug, have also been used to treat PMOP (5). However, due to the high incidence of
osteonecrosis of the jaw and atypical femoral fractures in patients
administered oral bisphosphonates for PMOP, such drugs cannot be
routinely administered to patients in clinic (6). Therefore, there is a great need to
seek novel therapeutic strategies and/or natural therapies that
stimulate an anabolic effect on osteoporotic bone in PMOP
patients.
The process of rat BMSC (rBMSC) differentiation
involves various signaling pathways including MAPK and Notch
pathway. Recently, plant-derived phytochemicals have received
attention for their ability to induce osteoblast and osteoclast
activity. Phytochemicals with promising therapeutic potential for
osteoporosis include isoflavones, stilbenes and flavonoids
(7). Icariin (ICA) is a major
active flavonoid glycoside extracted from the Chinese herbal
medicinal plant Epimedii, and it acts as a phytoestrogen. A study
has shown that ICA protected against bone loss induced by
ovariectomy or glucocorticoids (8). ICA treatment could increase
osteogenic differentiation and bone formation of BMSCs in
ovariectomized rat model of osteoporosis (9). In addition, ICA has recently been
shown to play a vital role in the proliferation and osteogenic
differentiation of rBMSCs and human BMSCs (10). Song et al (11) showed that ICA induced osteoblast
differentiation through estrogen receptor (ER)-mediated activation
of ERK and JNK signaling. Furthermore, this study showed that
17β-estradiol affects osteogenic and adipogenic differentiation
through ER signaling (12).
Therefore, we hypothesize that ICA may promote osteogenic
differentiation of rBMSCs through an ER-mediated signaling pathway.
ICI182780 (ICI) is a high affinity ER antagonist, which also acts
as an agonist of the membrane-bound G-protein coupled ER
(GPER).
In the experiments presented here, we used ICI as an
ER antagonist. We investigate the effect of ICA on both osteogenic
and adipogenic differentiation in rBMSCs, to further explore
whether ICA regulates differentiation of rBMSCs via ER
signaling.
Materials and methods
Cell culture
rBMSCs were purchased from Cyagen Biological
Sciences (RASMX-01001; Santa Clara, CA, USA), and cultured in α-MEM
(Hyclone, Pittsburg, PA, USA) containing 10% fetal bovine serum
(Tbd, China) in a humidified chamber with 5% CO2 at
37°C. The medium was replaced every three days.
Cell-counting kit-8 (CCK8) assay
rBMSCs were seeded into 96-well plates at a density
of 5×103 cells/well, then treated with different
concentrations of ICA (10−4, 10−5,
10−6, 10−7, 10−8, 10−9
M). After culturing for 24 or 48 h, cell proliferation was measured
using a CCK8 according to the manufacturer's instructions (Dojindo
Laboratories, Kumamoto, Japan). Briefly, the culture medium was
replaced with 100 µl α-MEM containing 10 µl CCK8, and the plates
were incubated for 1 h at 37°C. Absorbance was measured at 450 nm
using a multi-well spectrophotometer (BioTek, Synergy H4).
Alkaline phosphatase (ALP)
activity
rBMSCs were seeded into 6-well plates at a density
of 2×103 cells/well, and treated with different
concentrations of ICA, as described in 2.2. Cells were treated with
ICA for 3 and 7 days, and ALP activity assays were completed using
ALP kits according to the manufacturer's instructions (Nanjing
Jiancheng, Nanjing, China). Absorbance was measured at 520 nm using
a multi-well spectrophotometer (BioTek, Synergy H4).
Alizarin red S staining
To determine calcium deposition, alizarin red
staining was performed on day 7 of ICA treatment to evaluate the
mineralized matrix. Cells were divided into control,
10−6 M ICA and β-estradiol (E2) treatment groups. After
culturing for 7 days, the medium was removed, cells were washed
three times with PBS and fixed in 95% ethanol for 10 min, then
washed twice with PBS, and stained with 0.1% alizarin red at a pH
of 7.2 (Sigma). After incubating for 5–10 min at room temperature,
cells were again washed three times with PBS and examined via light
microscopy.
Oil red O staining
rBMSCs were seeded into 6-well plates at a density
of 2×103 cells/well, and divided into control,
10−6 M ICA and adipocyte-induced treatment groups. After
rBMSCs were cultured for 14 days, oil red O staining was used to
evaluate adipocyte differentiation of rBMSCs. Cells were rinsed
twice with PBS, fixed with 4% paraformaldehyde for 15 min, then
rinsed twice more with PBS. treated with 60% isopropanol for 1 min,
and infusion with oil red O (Sigma-Aldrich) that was dissolved in
60% isopropanol for 20 min. This process was followed by three
rinses with PBS. and then photographed.
Western blot analysis
rBMSCs were seeded into 10-cm culture dishes and
divided into control, ICA, ICI and ICI+ICA treatment groups. Total
protein was extracted with RIPA lysis buffer (P0013B, Beyotime,
Haimen, China). Total protein was separated with sodium dodecyl
sulfate polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride membranes. The membranes were washed in
TBST, blocked with 5% skim milk and incubated overnight with
anti-ERα (1:1,000, ab133467, Abcam, USA) and anti-ERβ (1:1,000,
ab32063, Abcam), anti-RUNX2 (1:1,000, 12556S, Cell Signaling
Technology, Danvers, MA, USA), anti-BMP-2 (1:1,000, ab14933,
Abcam), anti-PPARγ (1:1,000, C26H12, Cell Signaling Technology),
anti-CEBP/α (1:1,000, 2295, Cell Signaling Technology). Anti-GAPDH
(1:1,000, D16H11, Cell Signaling Technology) was used as a loading
control. The following day, membranes were incubated with
anti-rabbit IgG secondary antibody (1:4,000, 7074P2, Cell Signaling
Technology). Immunoreactive bands were visualized with an
electrochemiluminescence reagent (5312-1; DOCLAB, Guangzhou, China)
and quantified using Image J (NIH).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
rBMSCs were seeded into 60-mm culture dishes, and
divided into four groups, as aforementioned. Total RNA was isolated
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer's instructions. cDNA was synthesized from mRNA
using reverse transcriptase (AK4001; Takara, Shiga, Japan). After
predenaturation at 95°C for 2 min, 40 RT-qPCR cycles were performed
(95°C for 10 sec; and 60°C for 30 sec), followed by a final
extension at 72°C for 10 min. Target gene expression was calculated
using the formula 2−ΔΔCq (13). Gene expression was normalized to
GAPDH. Gene primer sequences are shown in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence
(5′-3′) |
|---|
| ERα | Forward:
CATCGATAAGAACCGGAGGA |
|
| Reserve:
TCTGACGCTTGTGCTTCAAC |
| ERβ | Forward:
GAAGCTGAACCACCCAATGT |
|
| Reserve:
CCAATCATGTGCACCAGTTC |
| RUNX2 | Forward:
CCACCACTCACTACCACACG |
|
| Reserve:
GGACGCTGACGAAGTACTAT |
| BMP-2 | Forward:
GCCATCGAGGAACTTTCAGA |
|
| Reverse:
TGTTCCCGAAAAATCTGGAG |
| PPARγ | Forward:
GGAATGCGTCATGAAAGGCG |
|
| Reserve:
GCGAACTTCAGTCCAGGTCA |
| C/EBPα | Forward:
TTACAACAGGCCAGGTTTCC |
|
| Reserve:
CTCTGGGATGGATCGATTGT |
Statistical analysis
Data were collected from three separate experiments
and expressed as the mean ± standard deviation. The statistical
differences were analyzed by one-way analysis of variance with
Least Significant Difference and Student-Newman-Keuls post hoc
tests using SPSS 19.0 software (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
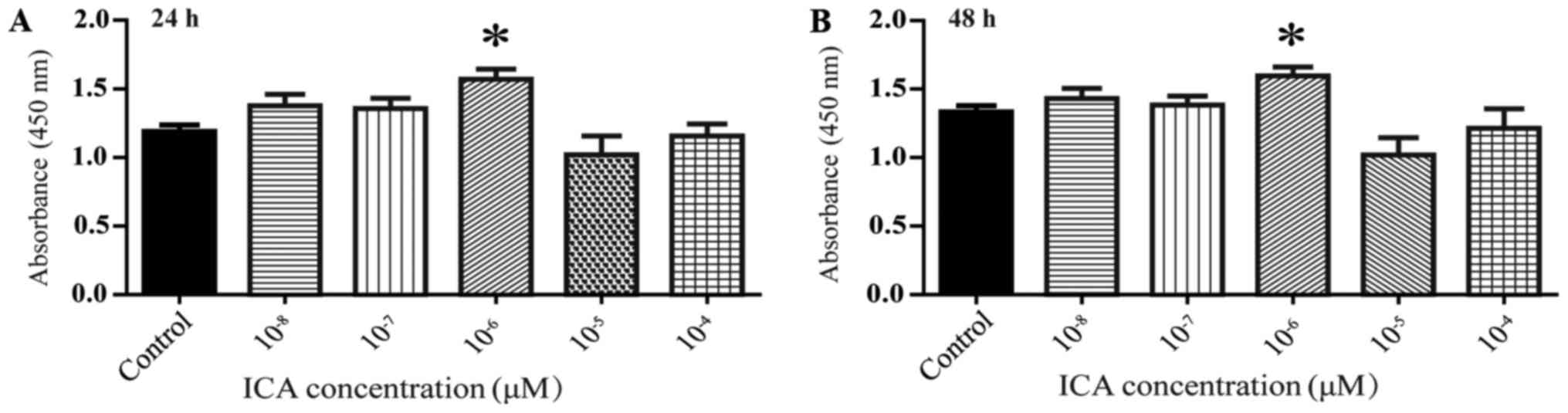
Effects of different ICA
concentrations on rBMSCs activity
CCK8 kits were used to measure cell activity.
compared to control, treatment of rBMSCs with 10−6 M ICA
resulted in significantly increased cell activity at 24 and 48 h
(Fig. 1; *P<0.05).
ICA promotes osteogenic
differentiation of rBMSCs
ALP is a glycoprotein associated with the formation
of calcified tissue, and it is the most widely recognized marker of
the osteoblast phenotype. ALP activity is partially indicative of
osteogenic differentiation. Treatment of cells with 10−6
M ICA resulted in an increase in ALP activity by day of 3 of
treatment, and this increase persisted through day 7 of treatment.
(Fig. 2; *P<0.05). ALP activity
was also increased by 10−4 M ICA on the seventh day
(Fig. 2; *P<0.05). These
results, together with those detailed in section 3.1, led us to
choose a concentration of 10−6 ICA for the following
experiments.
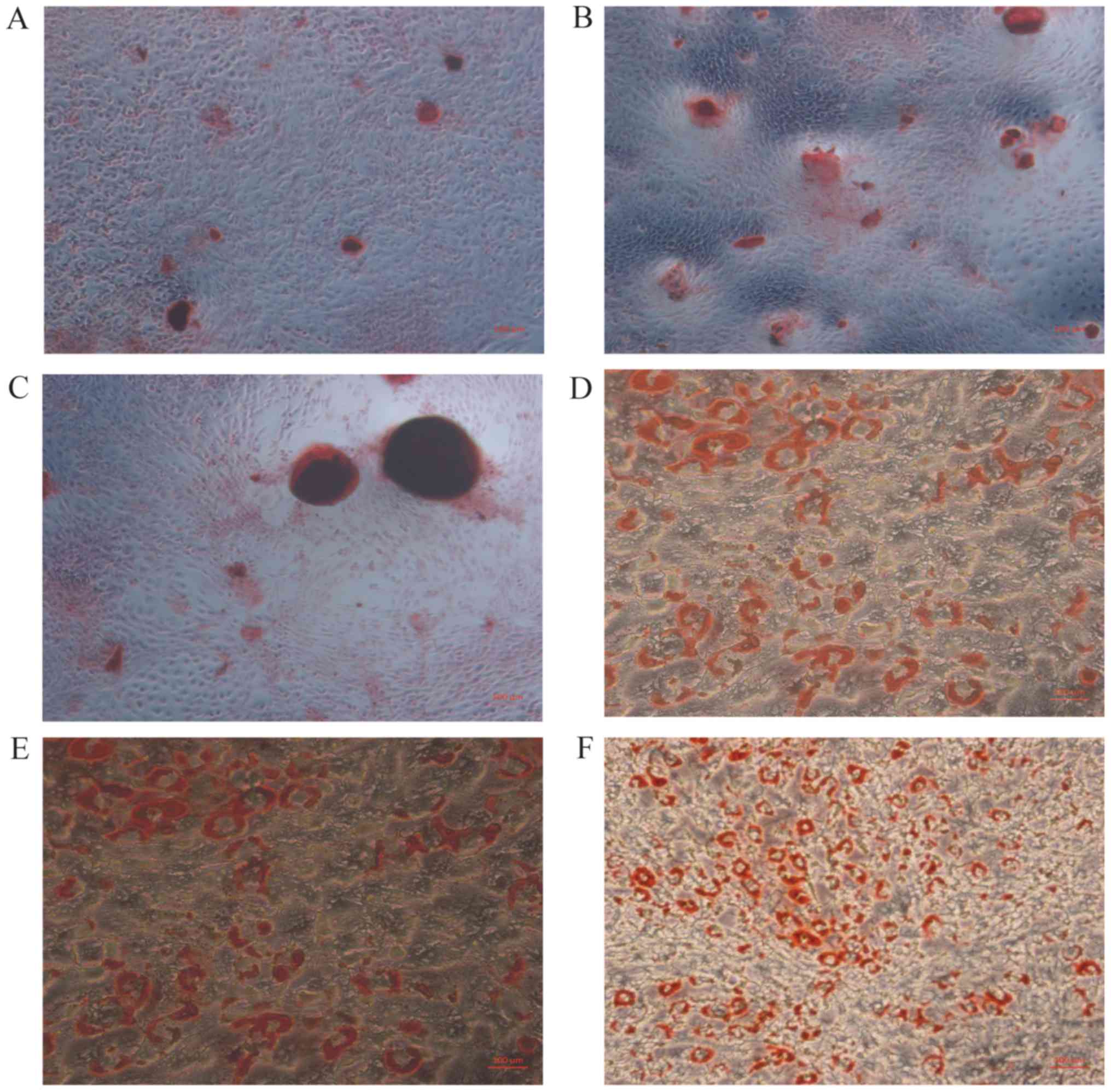
ICA promotes osteogenic
differentiation
The number of mineralized nodules can serve as
measure of osteogenic differentiation. Compare to control, the
number of mineralized nodules was increased in cells treated with
ICA, and was also increased cells treated with E2 (Fig. 3A-C). Fat droplets were identified
by oil red O staining, and there was a reduction in the number and
size of fat droplets in cells treated with ICA. (Fig. 3D-F; *P<0.05).
ICA promotes the expression of
osteogenic differentiation marker proteins and inhibits the
expression of adipose differentiation marker proteins
Runt-related transcription factor 2 (RUNX2) and
collagen type 1 (COL1) are markers of osteogenic differentiation,
whereas peroxisome proliferator-activated receptor gamma (PPARγ)
and CCAAT/enhancer-binding protein alpha (C/EBPα) are important
indicators of adipose differentiation. Compare with control group,
the expression levels of ERα and ERβ proteins were significantly
decreased with ICI treatment (Fig.
4). Treatment of cells with ICA, resulted in a significant
decrease in PPARγ and C/EBPα protein expression, and a significant
increase in ERα, ERβ, RUNX2 protein expression. However, compare to
ICA treatment alone, when combined treatment of ICI+ICA, resulted
in a significant increase in PPARγ protein expression and a
significant decrease in RUNX2 and COL1 protein expression. While
C/EBPα protein expression was not significantly different (Fig. 4; *P>0.05).
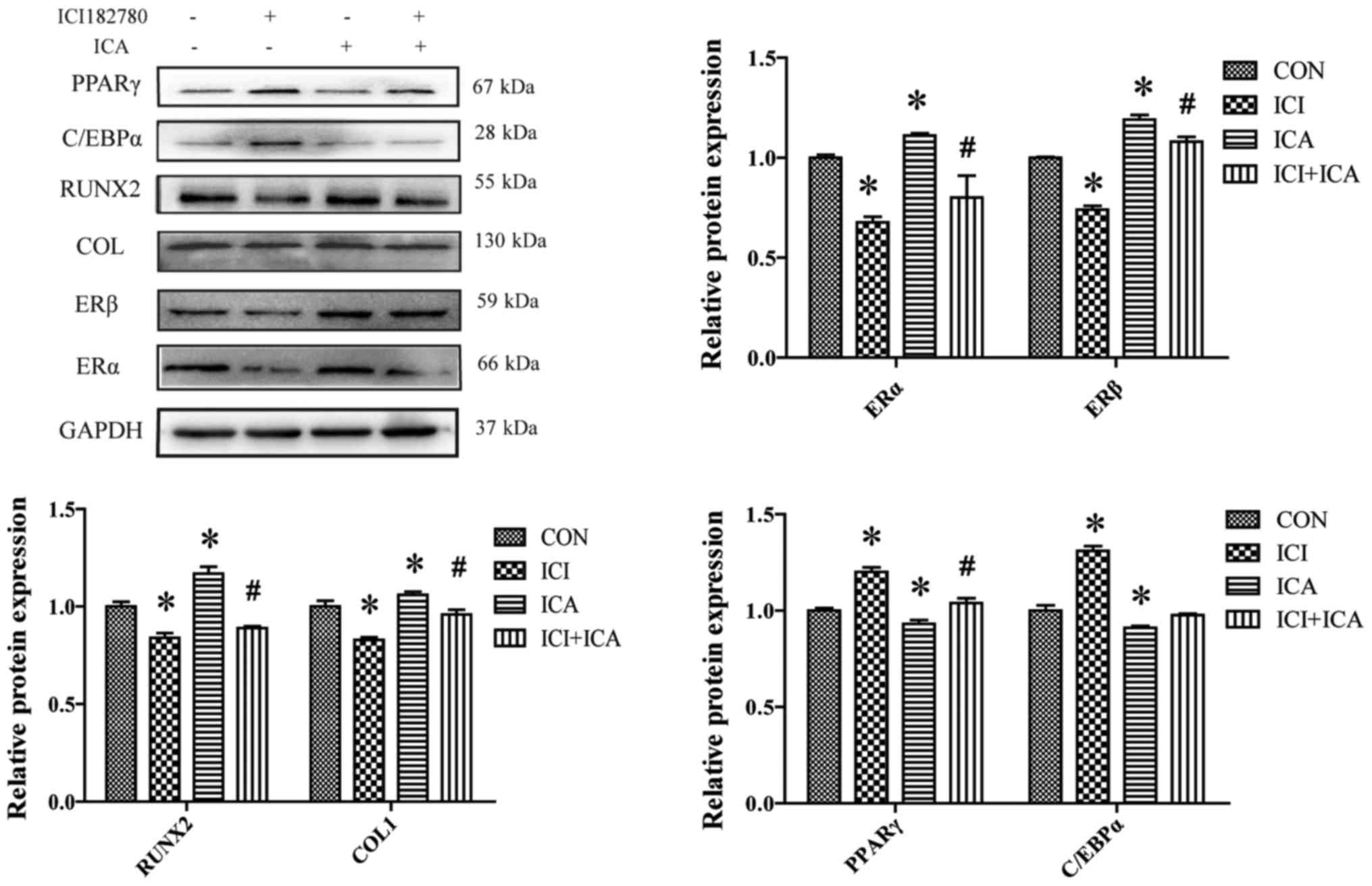 | Figure 4.Western blot analysis of ERα, ERβ,
RUNX2, COL1, C/EBPα and PPARγ protein expression in rBMSCs treated
with ICA and/or ICI. ICA treatment resulted in the downregulation
of C/EBPα and PPARγ expression, and upregulation RUNX2 and COL1
expression. All of the observed effects were blocked by ICI
treatment, except for C/EBPα expression. Data are presented as the
mean ± standard deviation (n=3). *P<0.05 vs. control;
#P<0.05 vs. ICA. ICA, icariin; ICI, ICI182780; ER,
estrogen receptor; RUNX2, runt-related transcription factor 2;
COL1, collagen type 1; PPARγ, peroxisome proliferator-activated
receptor-γ; C/EBPα, CCAAT/enhancer-binding protein α; rBMSCs, rat
bone marrow stromal cells; CON, control. |
ICA promotes the expression of
osteoblast-specific genes and inhibits the expression of
adipose-specific genes
Treatment of cells with ICI resulted in a
significant decrease in ERα and ERβ gene expression,
(Fig. 5); treating cells with ICA
resulted in a significant increase in the gene expression of bone
morphogenic protein-2 (BMP-2) and runx2 compared to
control (Fig. 5). These genes
expression changes are in accordance with the changes of protein
expression detected with Western-blot, suggesting that ICA
stimulates osteogenic differentiation of rBMSCs by upregulating
runx2 and bmp-2 expression. In addition, pparγ
and c/ebpα mRNA expression were decreased following
treatment with ICA, suggesting that ICA acts to inhibit adipogenic
differentiation. However, compared with ICA treatment, pparγ
and c/ebpα were significantly increased, whereas
bmp-2 and runx2 expression were significantly
decreased with combined treatment of ICI+ICA. (Fig. 5; *P<0.05).
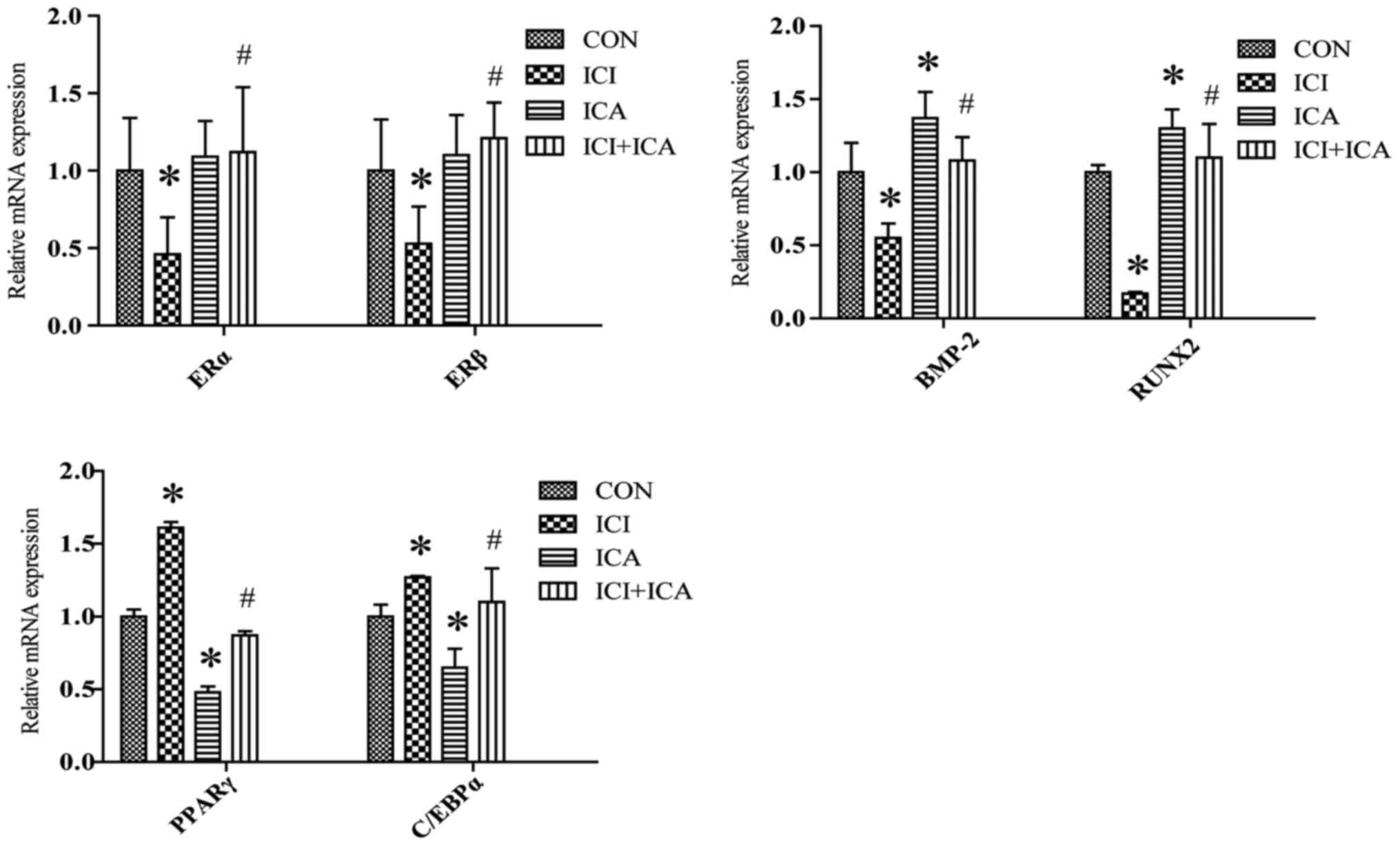 | Figure 5.Reverse transcription-quantitative
polymerase chain reaction analysis of ERα, ERβ,
RUNX2, BMP-2, C/EBPα and PPARγ gene relative
expression in rat bone marrow stromal cells treated with ICA or
ICI. Data are presented as the mean ± standard deviation (n=3).
*P<0.05 vs. control; #P<0.05 vs. ICA. ICA,
icariin; ICI, ICI182780; ER, estrogen receptor; RUNX2, runt-related
transcription factor 2; BMP-2, bone morphogenetic Protein 2; PPARγ,
peroxisome proliferator-activated receptor-γ; C/EBPα,
CCAAT/enhancer-binding protein α. |
Discussion
ER regulates physiological functions in almost all
tissues in both males and females. Recent studies have shown that
ER signaling plays an important role in many bone metabolism
diseases (14). The classic ERs
include ERα and ERβ. ERα is predominantly expressed in cortical
bone, whereas ERβ shows higher levels of expression in cancellous
bone (15). ERα signaling
activation regulates matrix mineralization in vitro, and its
deficiency may lead to osteoporosis (16), ERβ signaling activation induces
osteogenic differentiation of MC3T3-E1 cells and upregulates the
expression of the osteogenesis-related factors including bone GLa
protein and osteopontin. Previous study has indicated that ER
signaling could regulate osteogenic and adipogenic differentiation
(12). Therefore, in this study,
we used ICI as an ER antagonist to determine whether ICA promotes
osteogenic differentiation and inhibits adipogenic differentiation
in an ER-dependent fashion. We demonstrated that ICI significantly
downregulated ERα and ERβ protein and gene expression, suggesting
that ICI successfully blocks ER-mediated signaling.
Bone metabolism is a balance between
osteoblast-driven bone formation and osteoclast-mediated bone
resorption. Osteogenic differentiation and adipogenic
differentiation of rBMSCs are mutual inhibitory processes (11). Previous studies have demonstrated
that ICA promotes osteogenic differentiation of MC3T3-E1 cells
(17) and rBMSCs (18). Treatment with 10−6 M ICA
significantly increased rBMSCs activity, demonstrating that ICA
promotes rBMSCs proliferation. Alizarin red S staining was used to
visualize mineral deposition, and adipocyte accumulation was
assessed with oil red O staining. The number of mineralized nodules
was significantly increased with ICA treatment; while the number of
fat droplets was significantly decreased, suggesting that ICA
induces osteogenic differentiation but inhibits adipogenic
differentiation. ALP is an early marker of osteoblast
differentiation, and a peak in ALP activity is indicative of
osteogenic differentiation (19).
Treatment of 10−6 M ICA significantly increased ALP
activity by the third day of treatment, and ALP activity remained
elevated on the seventh day of treatment. These findings correspond
to those from a previous study, which showed that ICA stimulated
osteogenic differentiation (20)
and inhibited adipocyte differentiation of rBMSCs (21).
RUNX2 acts as a scaffold for nucleic acids and
regulatory factor involved in skeletal gene expression, and shows
significantly increased expression in osteogenic differentiation
(22); RUNX2 along with COL1,
another important molecular marker of bone formation and bone
remodeling, represent the foundation for matrix mineralization and
have been shown to have increased expression or synthesis during
osteogenic differentiation (23).
BMP-2 plays a pivotal role in growth and differentiation. Huang
et al (24) demonstrated
that BMP-2 overexpression significantly stimulated osteocalcin and
ALP gene expression. ICA treatment significantly upregulated the
protein expression of RUNX2 and COL1 and the gene expression of
runx2 and bmp-2. Together, these data suggest that
ICA can promotes osteogenic differentiation. C/EBPα and PPARγ are
the two molecules mostly likely to influence the regulation of
adipocytes (25). In this study,
the protein expression of PPARγ was significantly downregulated, as
well as the genes expression of pparγ and c/ebpα,
suggesting that treatment with ICA can inhibit the adipogenic
differentiation of rBMSCs.
Our previous study has shown that ICA can improve
osteoporosis via the Notch signaling pathway (21), as well as the MAPK signaling
pathway has also been shown to be involved in ICA-mediated
osteogenic differentiation (26).
In the present study, we use ICI as an ER signaling pathway
antagonist, to investigate the correlation between ICA and the ER
signaling pathway. The results showed that ICA treatment
significantly upregulate the expression of ERα and ERβ, suggesting
that ICA may function by regulating ER signaling. Compared with ICA
treatment alone, combined treatment with ICI+ICA resulted in a
significant decrease in RUNX2 and COL1 protein expression, and a
significant increase in PPARγ, but there is no change in C/EBPα
protein expression. Furthermore, genes expression of runx2 and
bmp-2 were also significantly decreased, while pparγ and c/ebpα
were significantly with combined treatment increased. Together,
these data suggest that the effects of ICA are blocked by ICI. Tao
et al (27) have shown that
prenylated flavonols can act as ER modulators. Taken together,
these data demonstrate that ICA stimulates osteogenic
differentiation and inhibits adipogenic differentiation via
activation of ER signaling.
In conclusion, ICA promotes proliferation of rBMSCs,
stimulates the osteogenic differentiation and mineralization of
rBMSCs by regulating RUNX2, COL1 and BMP-2 expression, and inhibits
adipogenic differentiation of rBMSCs by decreasing the expression
of PPARγ and C/EBPα. These effects can be blocked by ICI, which
suggests that ICA stimulates osteogenic differentiation and
inhibits adipocyte differentiation via activation of ER
signaling.
Acknowledgements
The authors would like to thank the Cancer Research
Institution of Jinan University (Shandong, China) for their
contribution to the study.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81473509 and
81673837), the National Natural Fund Youth Science Fund Project
(no. 81503384) and the Fundamental Research Funds for the Central
Universities.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RZ and LY conceived and designed the present study.
XyL and BP performed western blotting, reverse
transcription-quantitative polymerase chain reaction and Alizarin S
staining, and wrote the manuscript. YP completed CCK8 counting and
data analysis. PW provided the cell line and performed cell
culture. XtL and KS analyzed the western blotting data. LO and ZW
performed the ALP activity experiments. XgL and HW conducted
complete Oil red staining. HH, SM, YT, XP and XZ designed the
structure of the article, performed the literature review, and
critically revised the manuscript for important intellectual
content.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Berendsen AD and Olsen BR:
Osteoblast-adipocyte lineage plasticity in tissue development,
maintenance and pathology. Cell Mol Life Sci. 71:493–497. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Devlin MJ and Rosen CJ: The bone-fat
interface: Basic and clinical implications of marrow adiposity.
Lancet Diabetes Endocrinol. 3:141–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eriksen EF, Díez-Pérez A and Boonen S:
Update on long-term treatment with bisphosphonates for
postmenopausal osteoporosis: A systematic review. Bone. 58:126–135.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nguyen PL, Alibhai SM, Basaria S, D'Amico
AV, Kantoff PW, Keating NL, Penson DF, Rosario DJ, Tombal B and
Smith MR: Adverse effects of androgen deprivation therapy and
strategies to mitigate them. Eur Urol. 67:825–836. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Francis MD and Valent DJ: Historical
perspectives on the clinical development of bisphosphonates in the
treatment of bone diseases. J Musculoskelet Neuronal Interact.
7:2–8. 2007.PubMed/NCBI
|
|
6
|
Gao B, Huang Q, Lin YS, Wei BY, Guo YS,
Sun Z, Wang L, Fan J, Zhang HY, Han YH, et al: Dose-dependent
effect of estrogen suppresses the osteo-adipogenic
transdifferentiation of osteoblasts via canonical Wnt signaling
pathway. PLoS One. 9:e991372014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang YL, Jiang JJ, Shen H, Chai Y, Wei X
and Xie YM: Total flavonoids from Rhizoma Drynariae
(Gusuibu) for treating osteoporotic fractures: Implication in
clinical practice. Drug Design Dev Ther. 11:1881–1890. 2017.
View Article : Google Scholar
|
|
8
|
Hu J, Mao Z, He S, Zhan Y, Ning R, Liu W,
Yan B and Yang J: Icariin protects against glucocorticoid induced
osteoporosis, increases the expression of the bone enhancer DEC1
and modulates the PI3K/Akt/GSK β/β-catenin integrated signaling
pathway. Biochem Pharmacol. 136:109–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y, Cao L, Xia L, Wu Q, Wang J, Wang X,
Xu L, Zhou Y, Xu Y and Jiang X: Evaluation of osteogenesis and
angiogenesis of icariin in local controlled release and systemic
delivery for calvarial defect in ovariectomized rats. Sci Rep.
7:50772017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu S, Yang L, Hong H and Zhang R:
Wnt/β-catenin signaling is involved in the Icariin induced
proliferation of bone marrow mesenchymal stem cells. J Tradit Chin
Med. 36:360–368. 2016.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song L, Zhao J, Zhang X, Li H and Zhou Y:
Icariin induces osteoblast proliferation, differentiation and
mineralization through estrogen receptor-mediated ERK and JNK
signal activation. Eur J Pharmacol. 714:15–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niada S, Giannasi C, Ferreira LM, Milani
A, Arrigoni E and Brini AT: 17β-estradiol differently affects
osteogenic differentiation of mesenchymal stem/stromal cells from
adipose tissue and bone marrow. Differentiation. 92:291–297. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martin A, Yu J, Xiong J, Khalid AB,
Katzenellenbogen B, Kim SH, Katzenellenbogen JA, Malaivijitnond S,
Gabet Y, Krum SA and Frenkel B: Estrogens and androgens inhibit
association of RANKL with the pre-osteoblast membrane through
post-translational mechanisms. J Cell Physiol. 232:3798–3807. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bord S, Horner A, Beavan S and Juliet C:
Estrogen receptors alpha and beta are differentially expressed in
developing human bone. J Clin Endocrinol Metab. 86:2309–2314. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Song QS, Wang JY, Leng HJ, Chen ZQ,
Liu ZJ, Dang GT and Song CL: Simvastatin induces estrogen
receptor-alpha expression in bone, restores bone loss, and
decreases ERα expression and uterine wet weight in ovariectomized
rats. J Bone Miner Metab. 29:396–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
An X, Ma K, Zhang Z, Zhao T, Zhang X, Tang
B and Li Z: miR-17, miR-21, and miR-143 enhance adipogenic
differentiation from porcine bone marrow-derived mesenchymal stem
cells. DNA Cell Biol. 35:410–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei Q, Zhang J, Hong G, Chen Z, Deng W, He
W and Chen MH: Icariin promotes osteogenic differentiation of rat
bone marrow stromal cells by activating the ERα-Wnt/β-catenin
signaling pathway. Biomed Pharmacother. 84:931–939. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stucki U, Schmid J, Hämmerle CF and Lang
NP: Temporal and local appearance of alkaline phosphatase activity
in early stages of guided bone regeneration. A descriptive
histochemical study in humans. Clin Oral Implants Res. 12:121–127.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei QS, He MC, Chen MH, Chen ZQ, Yang F,
Wang HB, Zhang J and He W: Icariin stimulates osteogenic
differentiation of rat bone marrow stromal stem cells by increasing
TAZ expression. Biomed Pharmacother. 91:581–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu H, Xiong Y, Zhu X, Gao H, Yin S, Wang
J, Chen G, Wang C, Xiang L, Wang P, et al: Icariin improves
osteoporosis, inhibits the expression of PPARγ, C/EBPα, FABP4 mRNA,
N1ICD and jagged1 proteins, and increases Notch2 mRNA in
ovariectomized rats. Exp Ther Med. 13:1360–1368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Jiang Z, Zhang J, Xie Z, Wang Y
and Yang G: Enhanced osteogenic differentiation of rat bone marrow
mesenchymal stem cells on titanium substrates by inhibiting Notch3.
Arch Oral Biol. 80:34–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim J, Lee HW, Rhee DK, Paton JC and Pyo
S: Pneumolysin-induced autophagy contributes to inhibition of
osteoblast differentiation through downregulation of Sp1 in human
osteosarcoma cells. Biochim Biophys Acta. 1861:2663–2673. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang W, Rudkin GH, Carlsen B, Ishida K,
Ghasri P, Anvar B, Yamaguchi DT and Miller TA: Overexpression of
BMP-2 modulates morphology, growth, and gene expression in
osteoblastic cells. Exp Cell Res. 274:226–234. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Rong Y, Bao L, Nie B, Ren G, Zheng
C, Amin R, Arnold RD, Jeganathan RB and Huggins KW: Suppression of
adipocyte differentiation and lipid accumulation by stearidonic
acid (SDA) in 3T3-L1 cells. Lipids Health Dis. 16:1812017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Y, Xia L, Zhou Y, Xu Y and Jiang X:
Icariin induces osteogenic differentiation of bone mesenchymal stem
cells in a MAPK-dependent manner. Cell Prolif. 48:375–384. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tao ZR, Liu J, Jiang YM, Gong L and Yang
B: Synthesis of prenylated flavonols and their potents as estrogen
receptor modulator. Sci Rep. 7:124452017. View Article : Google Scholar : PubMed/NCBI
|