Introduction
Osteosarcoma is a common and highly malignant
osteoblastic tumor that originates from mesenchymal cells.
Osteosarcoma has high metastasis and recurrence rates (1), more than 85% of patients eventually
succumbing to the disease due to lung metastases (2), before the advent of multimodality
treatment methods. Therefore, metastasis is considered to be the
primary cause of mortality in patients with osteosarcoma (2,3). The
prevention of metastases is expected to effectively reduce the rate
of mortality from osteosarcoma. The mechanisms of tumor metastasis
are highly complex, as the pathogenesis of tumors varies due to
differences in the genetic background and the microenvironment of
the tumor. It has been reported that epithelial-mesenchymal
transition (EMT) has a pivotal role in the metastatic process in
many types of tumor cells (4) and
promotes the metastasis of epithelial neoplasms (5). However, as osteosarcoma arises from
cells of a mesenchymal origin, it is unclear whether EMT is
necessary for the metastasis of osteosarcoma. In a previous study
on EMT-related transcription factors, it was found that the
knockout of twist family bHLH transcription factor 2 (Twist2)
promoted EMT and metastasis of osteosarcoma cells; therefore,
Twist2 functions as a tumor suppressor gene (6). Knockout and overexpression
experiments revealed that snail family transcription repressor 2
(SNAI2) regulated the invasion and metastasis of osteosarcoma
cells, and that knockout of SNAI2 resulted in significantly
decreased motility, remodeling of the actin cytoskeleton and loss
of cellular protrusions, which contributed to the negative
regulation of tumor invasion and metastasis (7).
Cyclooxygenase-2 (COX-2), an inducible
cyclooxygenase and a rate-limiting enzyme in prostaglandin
synthesis, is associated with inflammatory diseases, and can
promote angiogenesis and tissue invasion in cancer (8,9). In
osteosarcomas, the rate of tumors with a positive expression of
COX-2 is 67–92%, and the expression of COX-2 in osteosarcoma stem
cells is 141-fold greater than its expression in osteosarcoma cells
(10–12). Previous studies have also found
that the overexpression of COX-2 in osteosarcomas increased the
expression levels of matrix metallopeptidase (MMP)-2 and MMP-9
(13), and promoted cell motility
and invasiveness (14). However,
further work is required to determine whether COX-2 can promote EMT
and migration in osteosarcoma cells.
Previous studies have reported that the
PI3K/AKT/NF-κB signaling pathway is abnormally activated in many
metastatic tumors (15,16). while other studies have found that
mutations in AKT may lead to a higher metastatic risk in patients
with osteosarcoma (17,18). However, to the best of our
knowledge, the relationship between COX-2 and the PI3K/AKT/NF-κB
pathway has not been characterized in the context of osteosarcoma.
Therefore, in the present study, the effects of COX-2 on EMT and
migration in MG-63 osteosarcoma cells were investigated with
respect to PI3K/AKT/NF-κB signaling.
Materials and methods
Cells and reagents
The 293T cell line and the osteosarcoma MG-63 cell
line were purchased from the Cell Bank of the Type Culture
Collection of the Chinese Academy of Sciences.
The lentiviral vector plvx-DsRed (containing DsRed,
a red fluorescent protein) and recombinant lentivirus
plvx-COX2-DsRed (containing DsRed and COX-2) were
constructed by Sangon Biotech Co., Ltd. High glucose DMEM was
purchased from Gibco (Thermo Fisher Scientific, Inc). FBS was
purchased from Beijing Sijiqing Biotechnology Co., Ltd. (https://www.11467.com/beijing/co/739711.htm). Trypsin,
RIPA lysis buffer, super-sensitive enhanced chemiluminescence (ECL)
reagent, SDS-PAGE gel preparation kits, SDS-PAGE sample loading
buffer, SDS-PAGE electrophoresis buffer, Matrigel matrix and
western blot transfer buffer were purchased from Beyotime Institute
of Biotechnology. Human MMP-2 (ml058669), MMP-9 (ml058617) and
vascular endothelial growth factor (VEGF; ml064281) ELISA kits were
purchased from Shanghai Meilian Biotechnology Co., Ltd. E-cadherin
(Abcam, ab194982), vimentin (Abcam, ab193555), MMP-2 (Abcam,
ab92536), MMP-9 (Abcam, ab38898), PI3K (Abcam, ab191606),
phosphorylated (p)-PI3K (Bioss, bs4605), AKT (Abcam, ab179463),
p-AKT (Abcam, ab81283), IKK (Abcam, ab178870), p-IKK (Abcam,
ab38515) and NF-κB (Abcam, ab207297) rabbit monoclonal antibodies
were purchased from Abcam and Bioss. DyLight 488-conjugated goat
anti-rabbit immunoglobulin G (IgG; GTX213110-04) was purchased from
GeneTex Inc., and horseradish peroxidase-conjugated goat
anti-rabbit IgG (7074P2) was purchased from Cell Signaling
Technology, Inc. The COX-2 inhibitor NS398 and the PI3K inhibitor
LY294002 were purchased from MedChemExpress LLC.
Cell culture
MG-63 and 293T cells were cultured in high-glucose
DMEM containing 10% FBS at 37°C in a 5% CO2 incubator.
Subculture was performed when the cells reached a confluence of
80–90%.
Experimental grouping and lentiviral
infection
The plvx-DsRed (1 µl) or plvx-COX2-DsRed (1 µl)
plasmid stock solution (1.67 µg/ml) was transfected (Polybrene,
Thermo Fisher Scientific, Inc.) to 293T cells in log phase for
virus propagation, in order to obtain recombinant plvx-DsRed and
plvx-COX2-DsRed lentiviruses with a titer of 5×1010
particle forming units/µl. The virus was collected 72 h after
transfection.
MG-63 cells were subcultured at a confluence of
50–60% and the corresponding lentiviruses with an optimal
multiplicity of infection (MOI; 25) were individually added; the
expression of DsRed in the cells was observed and recorded after 24
h. The experimental groups were as follows: i) plvx-DsRed group,
MG-63 cells infected with the empty lentiviral vector plvx-DsRed;
ii) plvx-COX2-DsRed group, MG-63 cells infected with the
recombinant lentivirus plvx-COX2-DsRed; iii) NS398 group, MG-63
cells infected with plvx-COX2-DsRed and then treated with the COX-2
inhibitor NS398 (3.8 µM) for 24 h; and iv) LY294002 group, MG-63
cells infected with plvx-COX2-DsRed and then treated with the PI3K
inhibitor LY294002 (5 µM) for 24 h.
Transwell chamber migration and
invasion assays
For the migration assay, cells in the plvx-DsRed,
plvx-COX2-DsRed, NS398 and LY294002 groups were collected and
digested with pancreatin. Single-cell suspensions of
1.5×105 cells/ml were prepared using serum-free medium.
For each group, 400 µl/well of the suspension was loaded into the
upper chamber of the Transwell insert and 600 µl of culture medium
containing 20% FBS was added to the lower chamber; six parallel
controls were established for each group. After culturing at 37°C
in a 5% CO2 incubator for 24 h, the Transwell inserts
were removed from the culture plate and fixed in anhydrous methanol
at −20°C for 5 min. Cells in the upper chamber, which had not
migrated, were gently removed using a cotton swab. The migrated
cells were stained with 0.25% crystal violet for 5 min and
subsequently washed with PBS to remove the excess crystal violet.
The stained cells were observed and images captured using a
microscope (magnification, ×200, routine light microscopy) and cell
counting was performed in 6 randomly selected fields to determine
the number of migrated cells.
The invasion assay was performed in the same manner
as the migration assay, except that Matrigel-coated Transwell
chambers were used.
Determination of changes in
E-cadherin, vimentin and NF-κB protein expression levels using
immunofluorescence assays
Cells from each group were collected and digested
with pancreatin. Single-cell suspensions of 1×104
cells/ml were prepared using high-glucose DMEM containing 10% FBS.
For each group, a 6-well plate was inoculated with 1 ml/well of the
suspension and cultured at 37°C in a 5% CO2 incubator
for 24 h. Subsequently, the cells were washed with PBS, fixed with
4% paraformaldehyde at room temperature for 15 min, washed again
with PBS, permeabilized in 0.25% Triton for 15 min and blocked with
blocking buffer (5% BSA in 0.25% Triton) for 30 min at 37°C. After
the removal of excess blocking buffer, primary antibodies in
blocking buffer were added at the following dilutions: E-cadherin
(1:500), vimentin (1:500) and NF-κB (1:200). The cells were
incubated at 4°C overnight, washed with PBS and the DyLight
488-conjugated secondary antibody (1:2,000) was added. After
incubation at room temperature for 1 h, the cells were washed with
PBS. Cells were imaged using a fluorescent microscope. The optical
density (OD) values of the cells were analyzed as the integrated
optical density (IOD)/total area, and IOD values were calculated
using Image-Pro Plus (version 6.0, Media Cybernetics, Inc.). The
concentration of DAPI used 15 µg/ml, for 5 min at 37°C.
Determination of changes in the levels
of MMP-2, MMP-9 and VEGF in the supernatant of MG-63 cells using
ELISA
The culture supernatant was obtained from the cells
of each group following growth for 48 h in the media and
centrifugation (1,200 × g for 5 min at 37°C). Changes in the levels
of MMP-2, MMP-9 and VEGF were measured using ELISA kits, according
to the manufacturer's instructions.
Determination of changes in the levels
of PI3K, p-PI3K, AKT, p-AKT, IKK and p-IKK protein expression using
western blotting
Cells from each group were collected, lysed on ice
with RIPA buffer, collected in a centrifuge tube and further lysed
for 30 min. After centrifugation at 13,000 × g for 10 min at 4°C,
the supernatant was collected and protein concentration measured
with a BCA assay. After the addition of 4X sample loading buffer,
the cells were boiled for 5 min to allow sufficient denaturation
and stored at −20°C before use. SDS-PAGE was performed using a 10%
separating gel and 5% stacking gel. The loading volumes were
determined based on protein concentration (20 µg per lane). After
electrophoresis, the samples were transferred onto PVDF membranes
and blocked with 5% skimmed milk/TBST (0.1% Tween-20) at 37°C for
90 min. Subsequently, the cells were incubated overnight with the
following primary antibodies: PI3K (1:1,000), p-PI3K (1:2,000), AKT
(1:750), p-AKT (1:1,000), IKK (1:1,000) or p-IKK (1:1,500).
Membranes were washed with PBS, incubated for 1.5 h at room
temperature with the secondary antibody (1:6,000), washed with PBS
again, and then visualized using ECL reagent. The OD values of the
samples were analyzed using Image-Pro Plus.
Statistical analysis
The experimental data (n=3) were analyzed using SPSS
21.0 (IBM Corp.). Quantitative values were expressed as the mean ±
standard error of the mean. Comparisons between multiple groups
were performed using one-way ANOVA, and pairwise comparisons were
performed using the least significance difference test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Generation of recombinant
lentivirus-infected MG-63 cells overexpressing COX-2
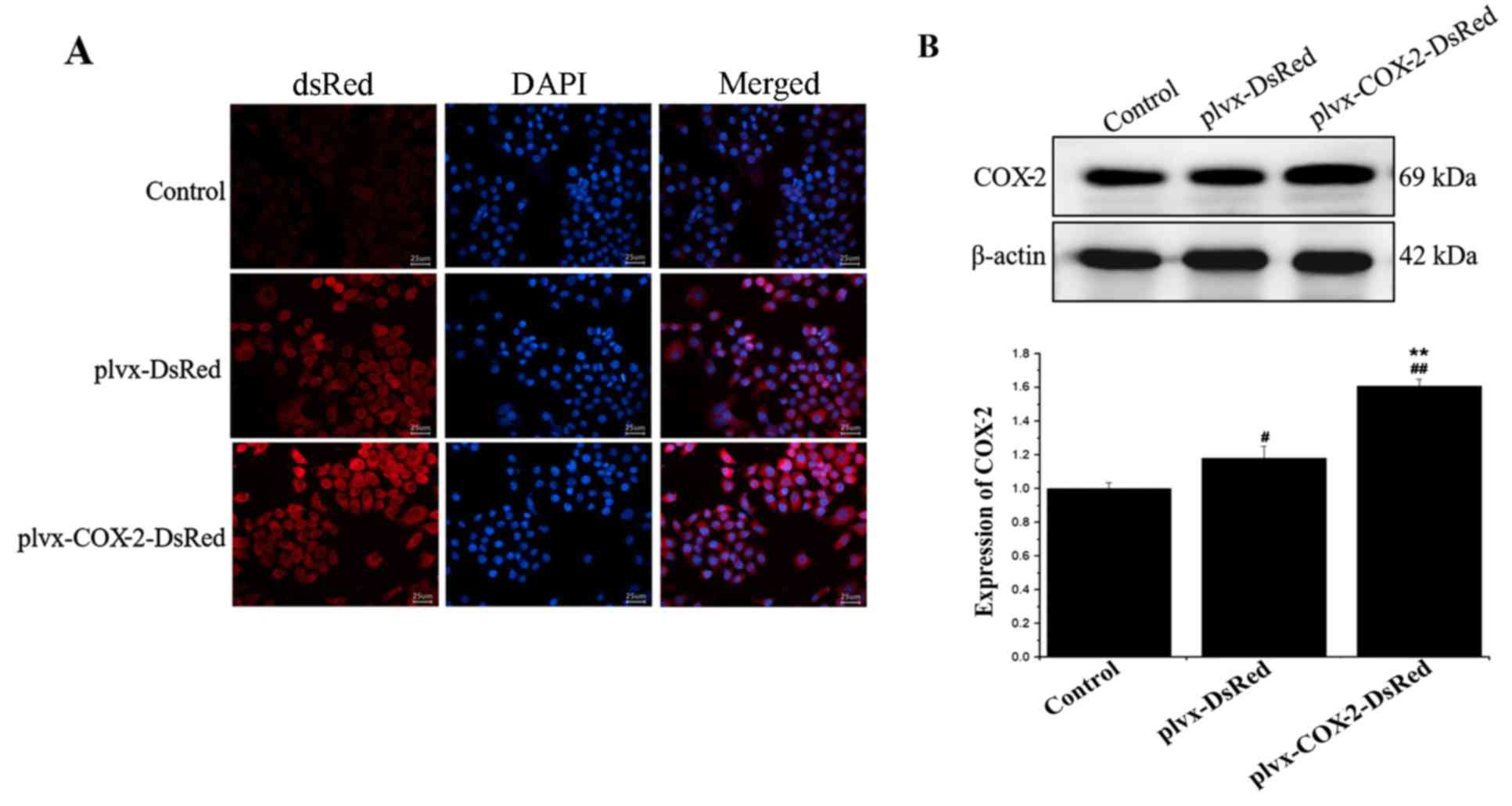
As shown in Fig.
1A, strong red fluorescence was observed in the plvx-DsRed and
plvx-COX2-DsRed infected groups. Western blotting results further
demonstrated a significant increase in COX-2 protein expression
levels in cells of the plvx-COX2-DsRed group compared with that in
the plvx-DsRed group (Fig. 1B).
These results indicated that the lentiviral infections and COX-2
overexpression were successful.
COX-2 overexpression promotes the
migration and invasion of MG-63 cells
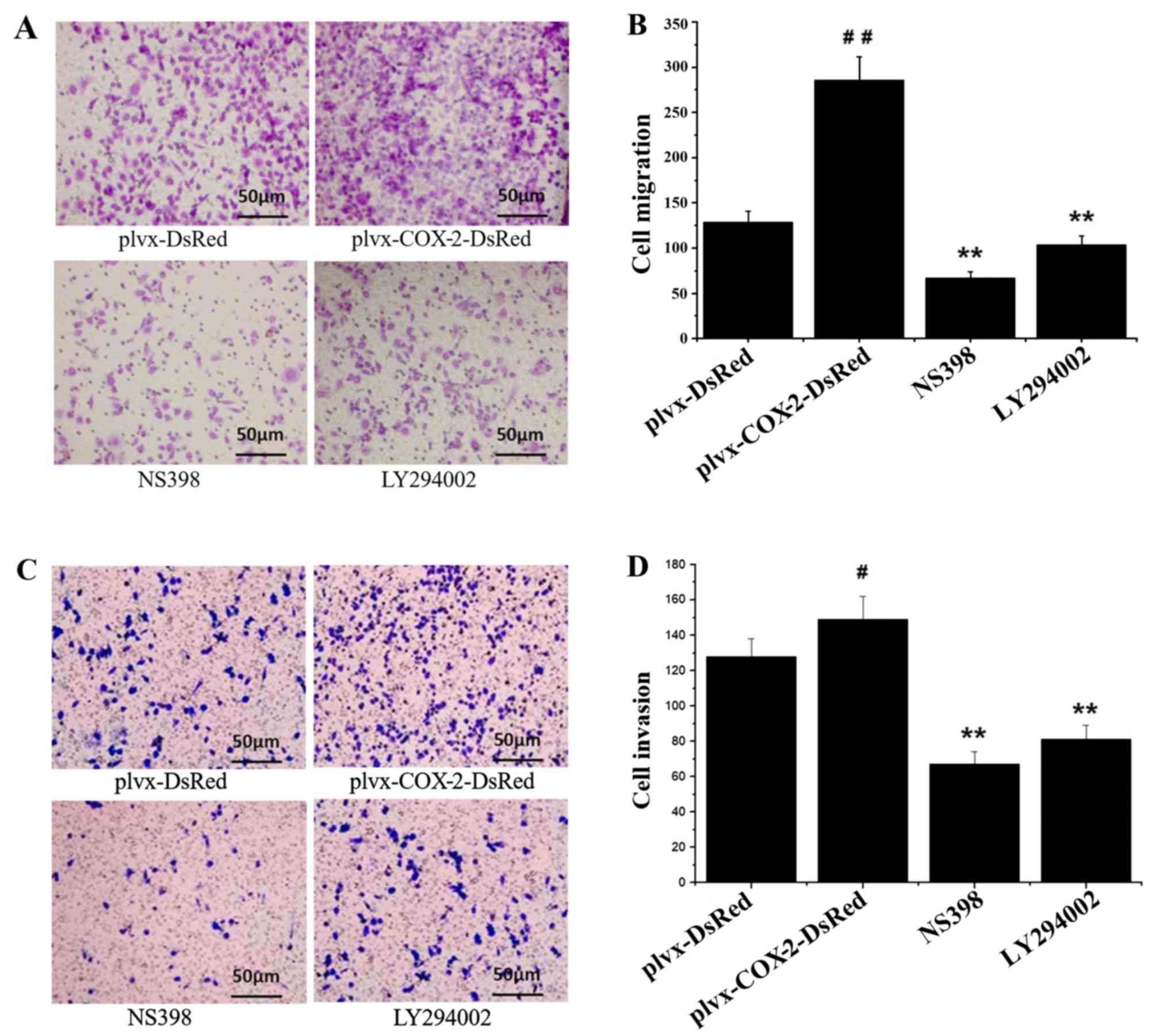
Results of the Transwell chamber assay indicated
that the number of migrated and invaded MG-63 cells was
significantly higher in the plvx-COX2-DsRed group compared with the
plvx-DsRed group (Fig. 2). The
number of migrated and invaded cells were significantly decreased
in the NS398- and LY294002-treated groups compared with the cells
infected with plvx-COX2-DsRed alone (Fig. 2). The number of migrated and
invaded cells in the NS398 and LY294002 groups were not
significantly different from each other (Fig. 2). These results indicated that
COX-2 overexpression promoted the migration and invasion of MG-63
cells.
COX-2 overexpression inhibits the
expression of E-cadherin and promotes the expression of vimentin in
MG-63 cells
As shown in Fig. 3A and
B, the results of the immunofluorescence assay indicated that
E-cadherin expression was significantly lower in MG-63 cells
infected with plvx-COX2-DsRed compared with cells infected with
plvx-DsRed control. Expression of E-cadherin was significantly
higher in cells treated with NS398 or LY294002 compared with cells
infected with plvx-COX2-DsRed alone (Fig. 3A and B). The expression of vimentin
was significantly higher in MG-63 cells infected with
plvx-COX2-DsRed than in cells infected with plvx-DsRed (Fig. 3C and D). Vimentin expression was
significantly lower in cells treated with NS398 or LY294002 than in
cells infected with plvx-COX2-DsRed alone (Fig. 3C and D).
COX-2 overexpression increases the
secreted levels of MMP-2, MMP-9 and VEGF in MG-63 cells
ELISA results indicated that the levels of MMP-2,
MMP-9 and VEGF were significantly higher in the culture supernatant
of cells infected with plvx-COX2-DsRed than of cells infected with
plvx-DsRed (Fig. 4A). The
expression levels of MMP-2, MMP-9 and VEGF in MG-63 cells were also
significantly higher in cells infected with plvx-COX2-DsRed than in
cells infected with plvx-DsRed (Fig.
4B). By contrast, the levels of MMP-2, MMP-9 and VEGF in the
culture supernatant were significantly lower in MG-63 cells treated
with NS398 or LY294002 compared with cells infected with
plvx-COX2-DsRed alone (Fig. 4A and
B).
COX-2 overexpression promotes the
expression of NF-κB
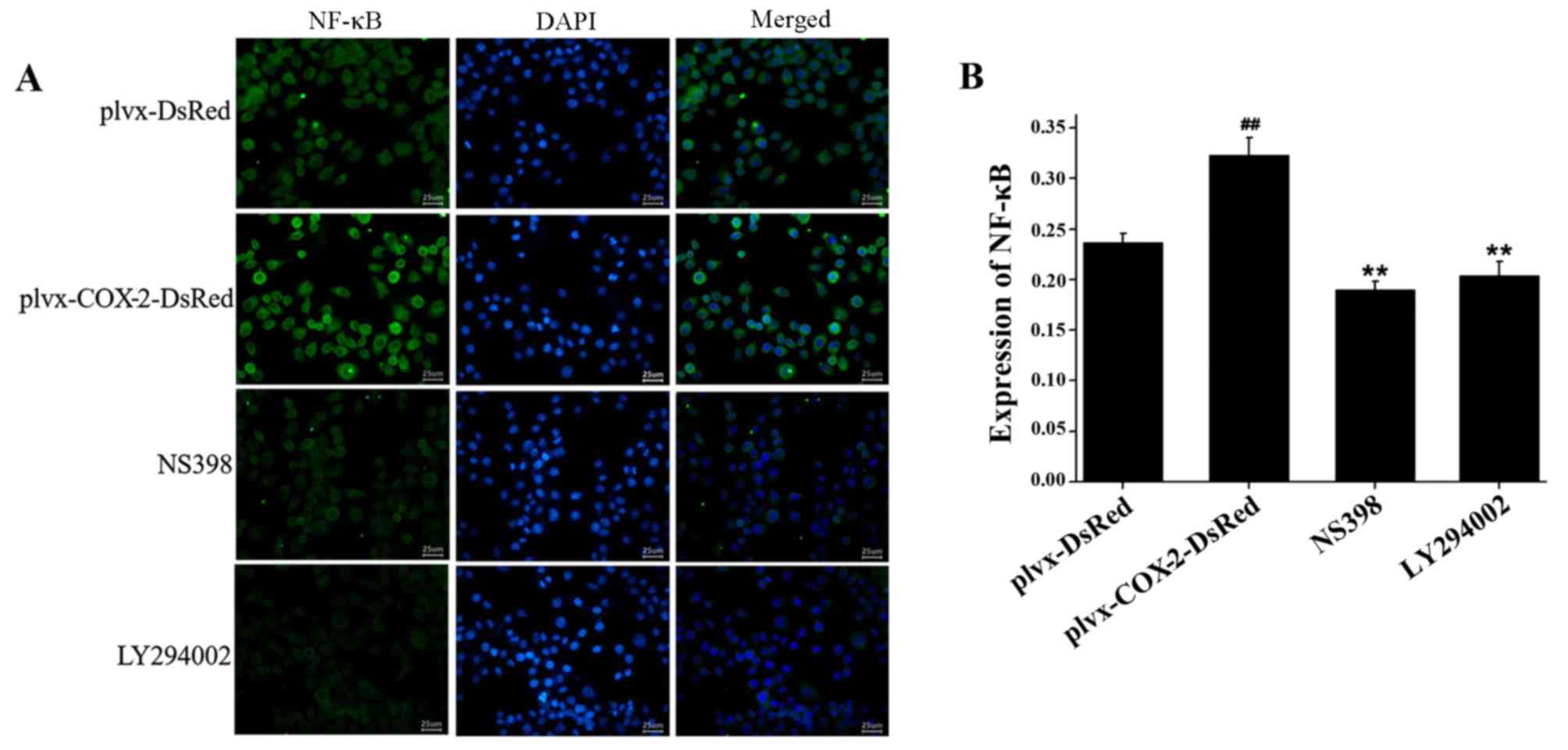
Immunofluorescence assay results indicated that the
total protein expression levels of NF-κB were significantly higher
in MG-63 cells infected with plvx-COX2-DsRed than in cells infected
with plvx-DsRed (Fig. 5). The
total protein expression levels of NF-κB were significantly lower
in cells treated with NS398 or LY294002 than in cells infected with
plvx-COX2-DsRed alone (Fig.
5).
COX-2 overexpression promotes the
phosphorylation of PI3K, AKT and IKK proteins
Western blotting results indicated that the
expression levels of p-PI3K, p-AKT and p-IKK were significantly
higher in MG-63 cells infected with plvx-COX2-DsRed than in cells
infected with plvx-DsRed (Fig. 6).
The expression levels of p-PI3K, p-AKT and p-IKK were significantly
lower in cells treated with NS398 or LY294002 than in cells
infected with plvx-COX2-DsRed alone (Fig. 6). However, no significant
differences were observed in the total protein levels of PI3K, AKT,
and IKK among the groups.
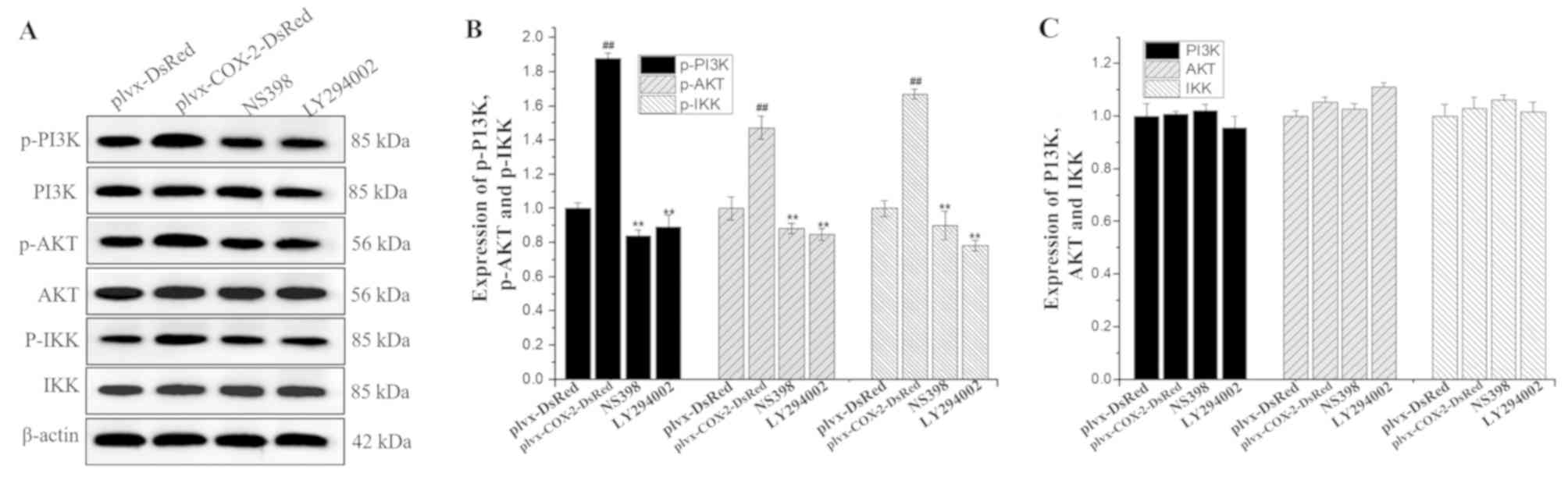 | Figure 6.Phosphorylation levels of PI3K, AKT
and IKK are regulated by COX-2. (A) The expression levels of PI3K,
p-PI3K, AKT, p-AKT, IKK and p-IKK proteins in each group were
determined using western blotting. (B) Quantification of the
expression levels of p-PI3K, p-AKT and p-IKK. (C) Quantification of
the expression levels of total PI3K, AKT and IKK proteins.
##P<0.01 vs. plvx-DsRed; **P<0.01 vs.
plvx-COX2-DsRed. COX-2, cyclooxygenase-2; IKK, inhibitor of NF-κΒ;
p-, phosphorylated; plvx-DsRed, empty vector; plvx-COX2-DsRed,
vector overexpressing COX-2. |
Discussion
The results of the present study demonstrated that
COX-2 overexpression decreased the expression of E-cadherin and
increased the levels of vimentin, MMP-2, MMP-9 and VEGF through the
activation of the PI3K/AKT/NF-κB signaling pathway, enhancing the
invasive ability of MG-63 osteosarcoma cells.
Metastatic invasion and migration are important
hallmarks of malignant tumors, and the acquisition of these
characteristics leads to a significant increase in the mortality
rate of cancer (19,20). Currently, the mechanisms by which
tumor metastasis occurs remain poorly understood; however, many
studies have indicated that tumor metastasis is not an isolated
event, but the influence of a combination of internal and external
factors leads to changes in the signaling networks within tumor
cells following an accumulation-mutation model, When the
accumulation of such changes reaches a critical threshold, tumor
cells acquire metastatic characteristics resulting from the changes
in these gene-signaling networks (21,22).
Osteosarcoma is a highly malignant tumor that is
extremely prone to metastasis during the early stages and has a
high incidence of hematogenous metastasis, which often occurs
during the early stages, and has high recurrence and metastasis
rates (3). The lungs are the most
common site of osteosarcoma metastasis, and lung metastases are
associated with poor clinical efficacy and prognosis (3). As there are relatively few studies
investigating the mechanism of osteosarcoma metastasis, the present
study was conducted to explore the mechanism by which the
metastasis of osteosarcoma occurs using the human osteosarcoma cell
line MG-63. In osteosarcoma tissue and SaOS-2 cells, the positive
expression rate of COX-2 can be as high as 67–92% (10,11).
Furthermore, in osteosarcoma, the expression of COX-2 in stem cells
spheres is 141-fold greater than in daughter adherent cells, thus
endowing these tumors with the characteristics of drug resistance,
metastasis and recurrence (12).
COX-2 is also the predominant rate-limiting enzyme in prostaglandin
synthesis; its overexpression in tissues of advanced osteosarcoma
results in the production of large amounts of prostaglandin 2,
which aggravates inflammation and promotes metastasis.
In the present study, COX-2 overexpression increased
the migratory and invasive ability of MG-63 cells, while the
inhibition of COX-2 activity decreased MG-63 cell migration and
invasion. Furthermore, it was found that COX-2 promoted the
production of MMP-2, MMP-9, VEGF and vimentin, and inhibited
E-cadherin production, in MG-63 cells. The use of the COX-2
inhibitor NS398 reversed the aforementioned changes in protein
expression caused by the overexpression of COX-2. MMP-2 and MMP-9
can promote cell metastasis through the degradation of the
extracellular matrix (23,24). The increased expression of vimentin
and decreased expression of E-cadherin also promotes metastasis
(25,26). These changes indicated that the
cells were undergoing EMT, leading to the dedifferentiation of
epithelial cells into mesenchymal cells, and changes to cell
morphology and polarity, providing cells with the conditions for
the acquisition of metastatic characteristics. Therefore, it can be
deduced that COX-2 affects metastasis by influencing the EMT
process in osteosarcoma MG-63 cells.
Multiple signaling pathways are involved in the
acquisition of tumor characteristics by cells, including the ERK
pathway, the Wnt/β-catenin pathway, the PI3K/AKT pathway, the NF-κB
pathway and the transforming growth factor (TGF)-β1/Smad pathway.
These pathways can be activated through continuous stimulation by a
large number of external signals or by mutation-induced changes in
pathway components, thus promoting tumor incidence and growth.
Previous studies have found that activation of the PI3K/AKT pathway
in osteosarcoma promotes cell proliferation and metastasis,
provides cells with drug resistance, participates in angiogenesis
and regulates changes in the cell cycle (17,27).
Other previous studies have revealed that the PI3K/AKT pathway is
abnormally activated during lung metastasis of osteosarcoma cells.
He et al (18) analyzed the
relationship between AKT single nucleotide polymorphisms and
osteosarcoma, and demonstrated that Chinese patients with
osteosarcoma who possessed the genotype AA of AKT rs6973569 had a
higher risk of metastasis. Furthermore, a study by Guo et al
(28) showed that TGF-β1 could
induce the metastasis of Saos-2 cells through the activation of the
PI3K/AKT signaling pathway. Hou et al (29) found that the knockdown of TGF-α
inhibited the activation of the PI3K/AKT/NF-κB signaling pathway,
which downregulated the expression of intercellular adhesion
molecule-1 and resulted in decreased distant metastases of
osteosarcoma cells. Another previous study showed that the
inhibition of the AKT pathway decreased MMP-2 secretion, thereby
inhibiting the development of pulmonary metastasis in nude mice
implanted with LM8 cells (30). In
addition, a previous study reported that the blockade of the
Ras/PI3K/AKT signaling pathway in a xenograft mouse model of
osteosarcoma decreased the expression and activity of MMP-1, MMP-2
and MMP-9, leading to a decreased level of LM8 cell metastasis
(31).
In view of the aforementioned studies, it can be
deduced that activation or inhibition of the PI3K/AKT signaling
pathway in osteosarcoma can affect tumor cell metastasis. Further
experiments were conducted to verify whether this pathway is a
signal pathway dependent on COX2 to promote MG63 cell metastasis.
The findings of the present study indicated that when
COX-2-overexpressing MG-63 cells were treated with the PI3K
inhibitor LY294002, the invasive ability of the cells decreased
significantly. In addition, the expression of MMP-2, MMP-9, VEGF
and vimentin decreased significantly, while the expression of
E-cadherin significantly increased, indicating that inhibition of
PI3K activity reversed the increased invasive ability of MG-63
cells caused by COX-2 overexpression. Furthermore, quantification
of the protein expression levels of PI3K, p-PI3K, AKT, p-AKT, IKK
and p-IKK revealed that COX-2 overexpression in MG-63 cells was
accompanied by an increase in the phosphorylation levels of PI3K,
AKT and IKK. Conversely, the inhibition of COX-2 or PI3K activity
resulted in a reversal of the increased phosphorylation of PI3K,
AKT and IKK, while the total protein levels did not change.
Activated IKK can activate NF-κB through the degradation of IκB
(inhibitor of NF-κB), allowing the import of NF-κB into nuclei
(32). A previous study found that
the nuclear import of NF-κB can initiate MMP expression and promote
EMT (33). In the present study,
it was found that the protein expression of NF-κB was upregulated
by COX-2 overexpression and reduced by the inhibition of COX-2 and
PI3K.
In conclusion, the present study reported for the
first time, to the best of our knowledge, that COX-2 overexpression
promoted EMT and invasion in osteosarcoma MG-63 cells by activating
the PI3K/AKT/NF-κB signaling pathway. This provides a theoretical
basis for the development of drugs targeting COX-2. However, owing
to the complexity of intracellular signaling networks and the
limitations of in vitro experiments, further studies are
required to verify whether COX-2 can be used as a therapeutic
target for the development of drugs to treat osteosarcoma.
Acknowledgements
The authors would like to thank Dr Lu (College of
Chemical Engineering, Lanzhou University) for providing excellent
technical assistance and helpful discussion.
Funding
The present study was supported by a grant from The
Natural Science Foundation of Gansu province (grant no.
1606RJZA126).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and NC conceptualized and designed the study. HZ,
PQ and TZ made substantial contributions to conception and design,
acquisition of data, analysis and interpretation of data and
figures. XZ and TZ wrote the manuscript. XZ, HZ, PQ and TZ agreed
to be accountable for all aspects of the work in ensuring that
questions related to the accuracy and integrity of any part of the
work are appropriately investigated and resolved. NC revised the
manuscript critically and advised revisions. All authors have read
and approved the manuscript, and take public responsibility for
appropriate portions of the content.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He X, Gao Z, Xu H, Zhang Z and Fu P: A
meta-analysis of randomized control trials of surgical methods with
osteosarcoma outcomes. J Orthop Surg Res. 12:52017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geller DS and Gorlick R: Osteosarcoma: A
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.PubMed/NCBI
|
|
4
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morel AP, Hinkal GW, Thomas C, Fauvet F,
Courtois-Cox S, Wierinckx A, Devouassoux-Shisheboran M, Treilleux
I, Tissier A, Gras B, et al: EMT inducers catalyze malignant
transformation of mammary epithelial cells and drive tumorigenesis
towards claudin-low tumors in transgenic mice. PLoS Genet.
8:e10027232012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amatangelo MD, Goodyear S and Varma D:
c-Myc expression and MEK1-induced Erk2 nuclear localization are
required for TGF-beta induced epithelial-mesenchymal transition and
invasion in prostate cancer. Carcinogenesis. 33:1965–1975. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharili AS, Allen S, Smith K, Price J and
McGonnell IM: Snail2 promotes osteosarcoma cell motility through
remodelling of the actin cytoskeleton and regulates tumor
development. Cancer Lett. 333:170–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsujii M, Kawano S and Dubois RN:
Cyclooxygenase-2 expression in human colon cancer cells increases
metastatic potential. Proc Natl Acad Sci USA. 94:3336–3340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsujii M, Kawano S, Tsuji S, Sawaoka H,
Hori M and DuBois RN: Cyclooxygenase regulates angiogenesis induced
by colon cancer cells. Cell. 93:705–716. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masi L, Recenti R, Silvestri S, Pinzani P,
Pepi M, Paglierani M, Brandi ML and Franchi A: Expression of
cyclooxygenase-2 in osteosarcoma of bone. Appl Immunohistochem Mol
Morphol. 15:70–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodriguez NI, Hoots WK, Koshkina NV,
Morales-Arias JA, Arndt CA, Inwards CY, Hawkins DS, Munsell MF and
Kleinerman ES: COX-2 expression correlates with survival in
patients with osteosarcoma lung metastases. J Pediatr Hematol
Oncol. 30:507–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pang LY, Gatenby EL, Kamida A, Whitelaw
BA, Hupp TR and Argyle DJ: Global gene expression analysis of
canine osteosarcoma stem cells reveals a novel role for COX-2 in
tumour initiation. PLoS One. 9:e831442014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee EJ, Choi EM, Kim SR, Park JH, Kim H,
Ha KS, Kim YM, Kim SS, Choe M, Kim JI and Han JA: Cyclooxygenase-2
promotes cell proliferation, migration and invasion in U2OS human
osteosarcoma cells. Exp Mol Med. 39:469–476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Urakawa H, Nishida Y, Naruse T, Nakashima
H and Ishiguro N: Cyclooxygenase-2 overexpression predicts poor
survival in patients with high-grade extremity osteosarcoma: A
pilot study. Clin Orthop Relat Res. 467:2932–2938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ji C, Guo H, Zhang P, Kuang W, Fan Y and
Wu L: AnnexinA5 promote glioma cell invasion and migration via the
PI3K/Akt/NF-κB signaling pathway. J Neurooncol. 138:469–478. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu LB, Jiang J, Zhu XP, Wang TF, Chen XY,
Luo QF, Shu Y, Liu ZL and Huang SH: Knockdown of Aurora-B inhibits
osteosarcoma cell invasion and migration via modulating
PI3K/Akt/NF-κB signaling pathway. Int J Clin Exp Pathol.
7:3984–3991. 2014.PubMed/NCBI
|
|
17
|
Dong Y, Liang G, Yuan B, Yang C, Gao R and
Zhou X: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol.
36:1477–1486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He ML, Wu Y, Zhao JM, Wang Z and Chen YB:
PIK3CA and AKT gene polymorphisms in susceptibility to osteosarcoma
in a Chinese population. Asian Pac J Cancer Prev. 14:5117–5122.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Orgaz JL, Ladhani O, Hoek KS,
Fernández-Barral A, Mihic D, Aguilera O, Seftor EA, Bernad A,
Rodríguez-Peralto JL, Hendrix MJ, et al: ‘Loss of pigment
epithelium-derived factor enables migration, invasion and
metastatic spread of human melanoma’. Oncogene. 28:4147–4161. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luan W, Yao Q, Xin N, Bu X, Xia Y, Wang J,
Ruan H, Ma S and Xu B: miR-204-5p acts as a tumor suppressor by
targeting matrix metalloproteinases-9 and B-cell lymphoma-2 in
malignant melanoma. Onco Targets Ther. 10:1237–1246. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vincent CT and Fuxe J: EMT, inflammation
and metastasis. Semin Cancer Biol. 47:168–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y and Zhou BP: New insights of
epithelial-mesenchymal transition in cancer metastasis. Acta
Biochim Biophys Sin (Shanghai). 40:643–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amano S, Akutsu N, Matsunaga Y, Nishiyama
T, Champliaud MF, Burgeson RE and Adachi E: Importance of balance
between extracellular matrix synthesis and degradation in basement
membrane formation. Exp Cell Res. 271:249–262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liao CL, Chu YL, Lin HY, Chen CY, Hsu MJ,
Liu KC, Lai KC, Huang AC and Chung JG: Bisdemethoxycurcumin
suppresses migration and invasion of human cervical cancer HeLa
cells via inhibition of NF-ĸB, MMP-2 and −9 pathways. Anticancer
Res. 38:3989–3997. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu S, Yang D, Beckford J and Alachkar H:
Upregulation of the EMT marker vimentin is associated with poor
clinical outcome in acute myeloid leukemia. J Transl Med.
16:1702018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gou Y, Zhai F, Zhang L and Cui L: RUNX3
regulates hepatocellular carcinoma cell metastasis via targeting
miR-186/E-cadherin/EMT pathway. Oncotarget. 8:61475–61486. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao G, Cai C, Yang T, Qiu X, Liao B, Li
W, Ji Z, Zhao J, Zhao H, Guo M, et al: MicroRNA-221 induces cell
survival and cisplatin resistance through PI3K/Akt pathway in human
osteosarcoma. PLoS One. 8:e539062013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo YS, Zhao R, Ma J, Cui W, Sun Z, Gao B,
He S, Han YH, Fan J, Yang L, et al: βig-h3 promotes human
osteosarcoma cells metastasis by interacting with integrin α2β1 and
activating PI3K signaling pathway. PLoS One. 9:e902202014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou CH, Lin FL, Tong KB, Hou SM and Liu
JF: Transforming growth factor alpha promotes osteosarcoma
metastasis by ICAM-1 and PI3K/Akt signaling pathway. Biochem
Pharmacol. 89:453–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aizawa J, Sakayama K, Kamei S, Kidani T,
Yamamoto H, Norimatsu Y and Masuno H: Effect of troglitazone on
tumor growth and pulmonary metastasis development of the mouse
osteosarcoma cell line LM8. BMC Cancer. 10:512010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsubaki M, Satou T, Itoh T, Imano M, Ogaki
M, Yanae M and Nishida S: Reduction of metastasis, cell invasion,
and adhesion in mouse osteosarcoma by YM529/ONO-5920-induced
blockade of the Ras/MEK/ERK and Ras/PI3K/Akt pathway. Toxicol Appl
Pharmacol. 259:402–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Lau GK, Chen L, Dong SS, Lan HY,
Huang XR, Li Y, Luk JM, Yuan YF and Guan XY: Interleukin 17A
promotes hepatocellular carcinoma metastasis via NF-kB induced
matrix metalloproteinases 2 and 9 expression. PLoS One.
6:e218162011. View Article : Google Scholar : PubMed/NCBI
|