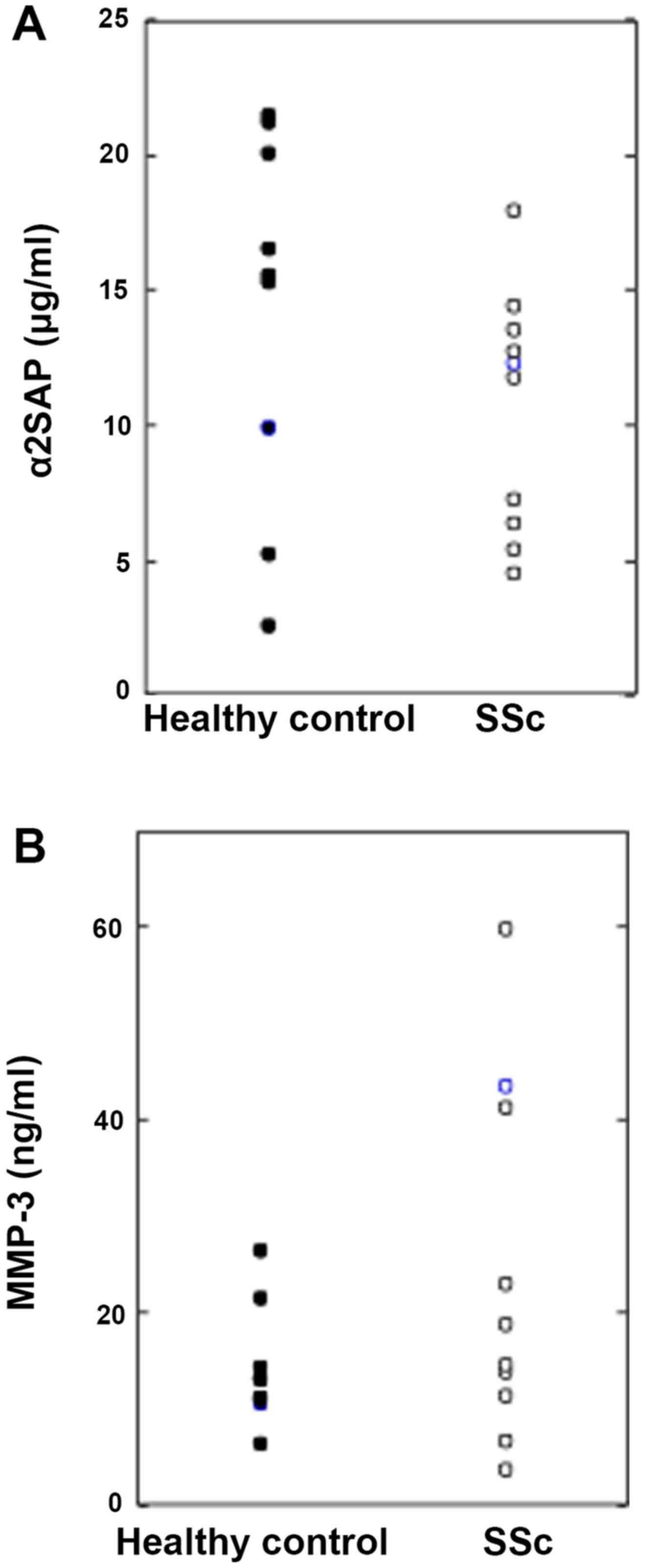
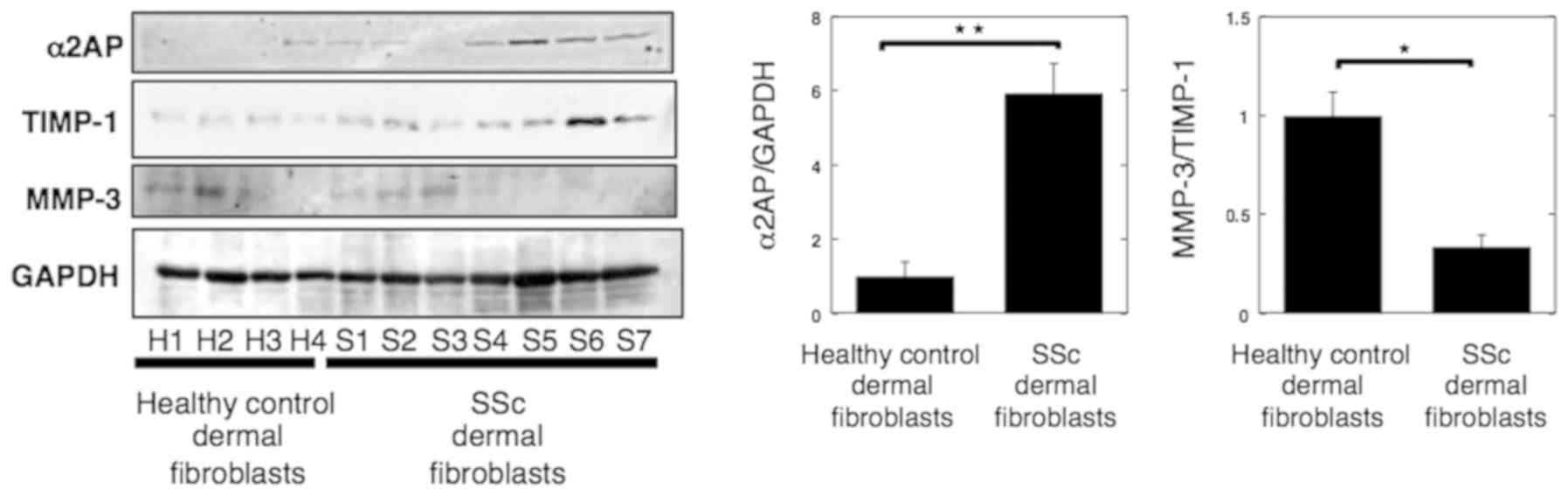
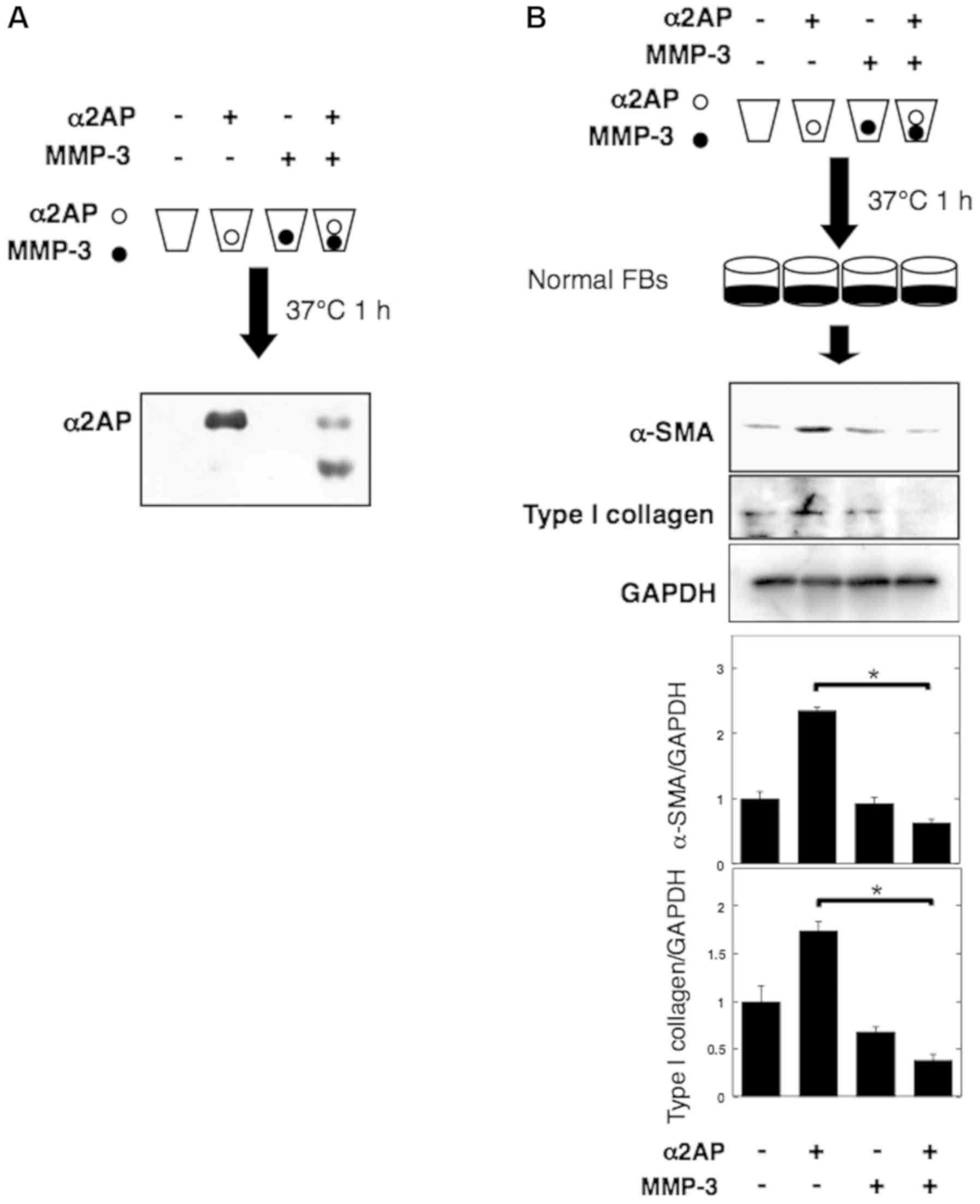
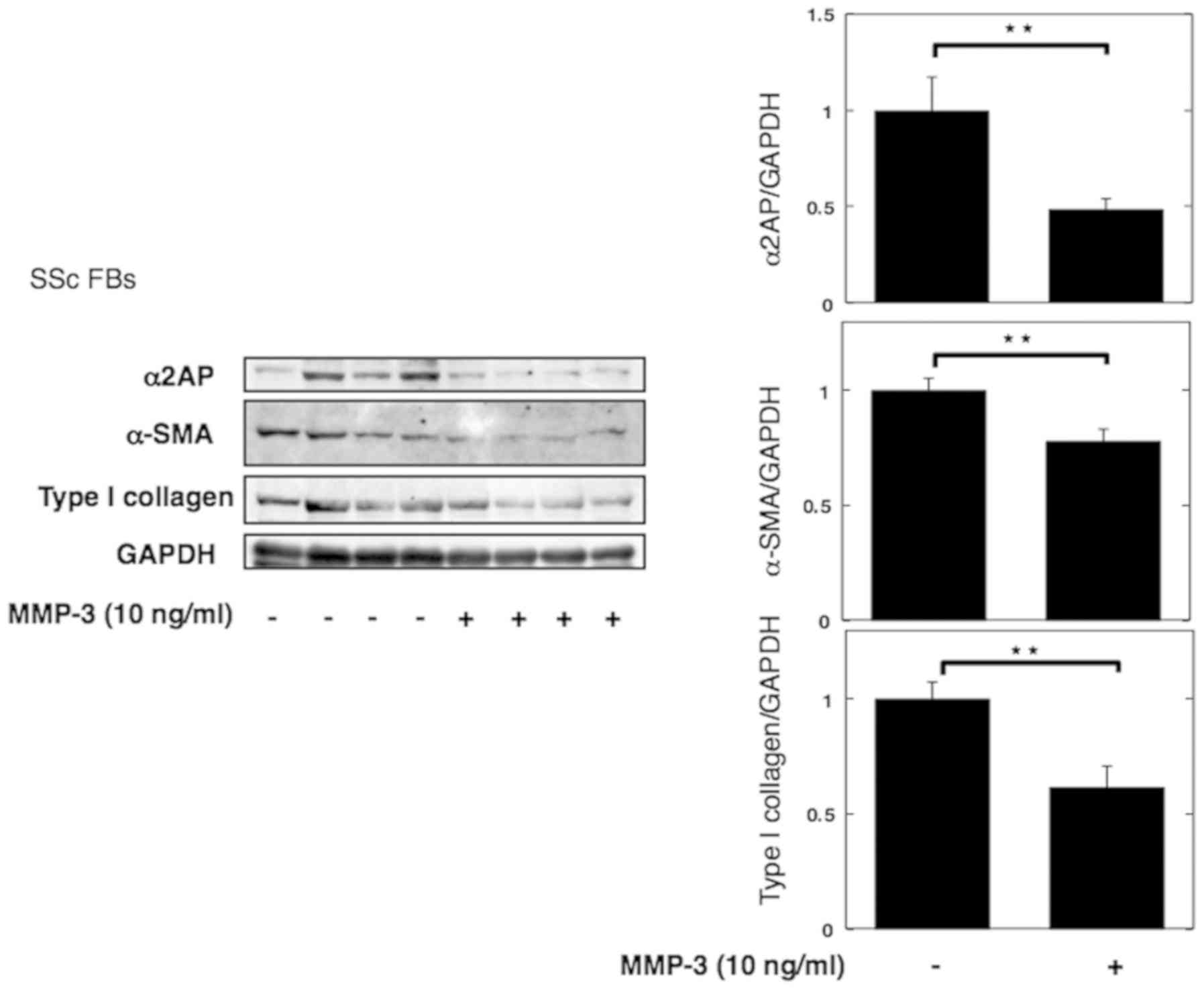
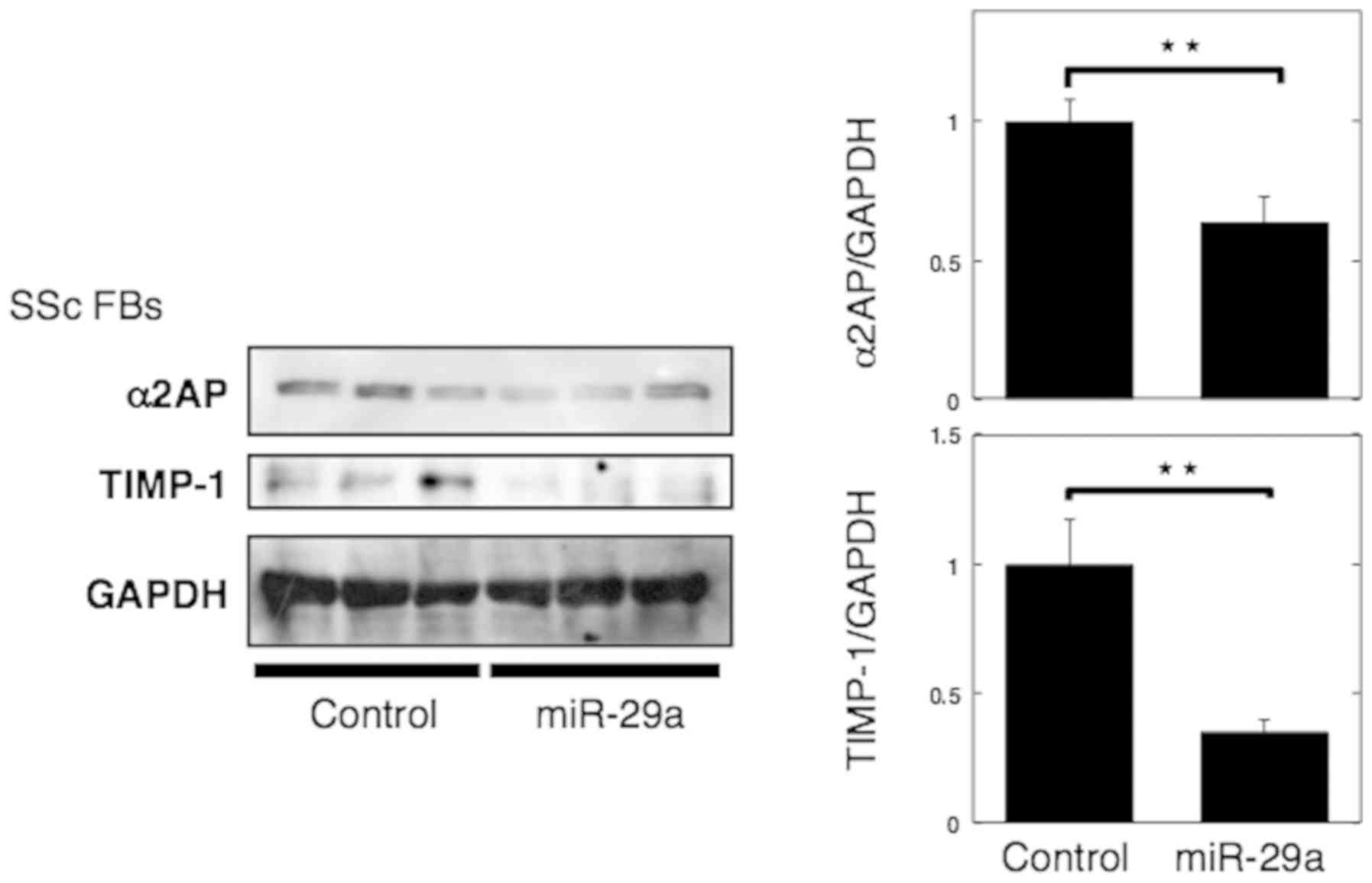
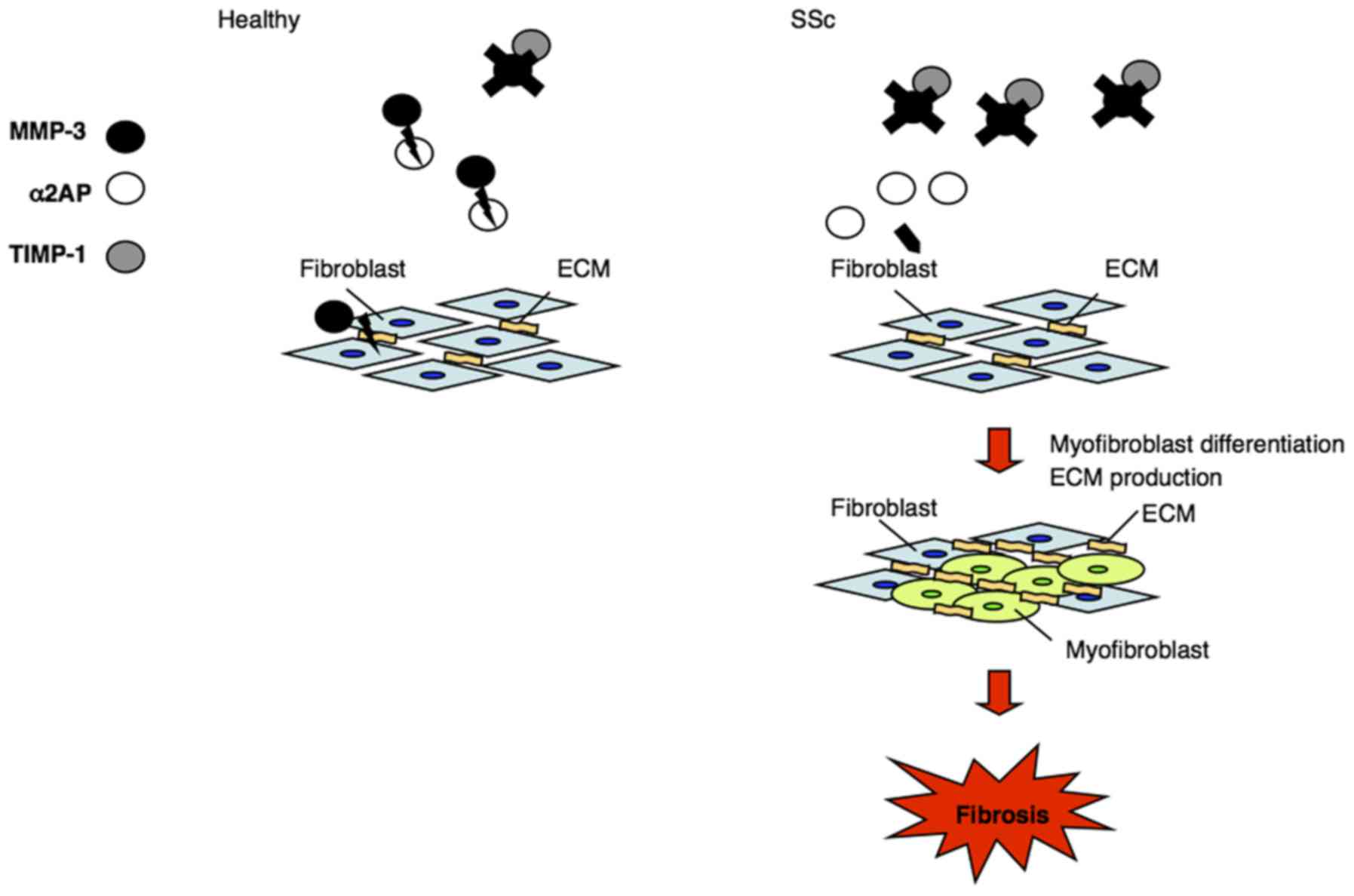
|
1
|
Gilbane AJ, Denton CP and Holmes AM:
Scleroderma pathogenesis: A pivotal role for fibroblasts as
effector cells. Arthritis Res Ther. 15:30012013. View Article : Google Scholar
|
|
2
|
Collen D: Identification and some
properties of a new fast-reacting plasmin inhibitor in human
plasma. Eur J Biochem. 69:209–216. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanno Y, Ishisaki A, Kawashita E, Kuretake
H, Ikeda K and Matsuo O: uPA attenuated LPS-induced inflammatory
osteoclastogenesis through the plasmin/PAR-1/Ca(2+)/CaMKK/AMPK
axis. Int J Biol Sci. 12:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Menoud PA, Sappino N, Boudal-Khoshbeen M,
Vassalli JD and Sappino AP: The kidney is a major site of
alpha(2)-antiplasmin production. J Clin Invest. 97:2478–2484. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanno Y: The role of fibrinolytic
regulators in vascular dysfunction of systemic sclerosis. Int J Mol
Sci. 20:6192019. View Article : Google Scholar
|
|
6
|
Kanno Y, Hirade K, Ishisaki A, Nakajima S,
Suga H, Into T, Matsushita K, Okada K, Matsuo O and Matsuno H: Lack
of alpha2-antiplasmin improves cutaneous wound healing via
over-released vascular endothelial growth factor-induced
angiogenesis in wound lesions. J Thromb Haemost. 4:1602–1610. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanno Y, Kuroki A, Okada K, Tomogane K,
Ueshima S, Matsuo O and Matsuno H: Alpha2-antiplasmin is involved
in the production of transforming growth factor beta1 and fibrosis.
J Thromb Haemost. 5:2266–2273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanno Y, Kawashita E, Minamida M, Kaneiwa
A, Okada K, Ueshima S, Matsuo O and Matsuno H: Alpha2-antiplasmin
is associated with the progression of fibrosis. Am J Pathol.
176:238–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanno Y, Kawashita E, Kokado A, Okada K,
Ueshima S, Matsuo O and Matsuno H: Alpha2-antiplasmin regulates the
development of dermal fibrosis in mice by prostaglandin F(2α)
synthesis through adipose triglyceride lipase/calcium-independent
phospholipase A(2). Arthritis Rheum. 65:492–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanno Y, Kawashita E, Kokado A, Kuretake
H, Ikeda K, Okada K, Seishima M, Ueshima S, Matsuo O and Matsuno H:
α2AP mediated myofibroblast formation and the development of renal
fibrosis in unilateral ureteral obstruction. Sci Rep. 4:59672014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanno Y, Ishisaki A, Kuretake H, Maruyama
C, Matsuda A and Matsuo O: α2-antiplasmin modulates bone formation
by negatively regulating osteoblast differentiation and function.
Int J Mol Med. 40:854–858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawashita E, Kanno Y, Asayama H, Okada K,
Ueshima S, Matsuo O and Matsuno H: Involvement of α2-antiplasmin in
dendritic growth of hippocampal neurons. J Neurochem. 126:58–69.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou Y, Okada K, Okamoto C, Ueshima S and
Matsuo O: Alpha2-antiplasmin is a critical regulator of angiotensin
II-mediated vascular remodeling. Arterioscler. Thromb Vasc Biol.
28:1257–1262. 2008. View Article : Google Scholar
|
|
14
|
Jinnin M, Ihn H, Yamane K, Asano Y, Yazawa
N and Tamaki K: Plasma plasmin-alpha2-plasmin inhibitor complex
levels are increased in systemic sclerosis patients with pulmonary
hypertension. Rheumatology (Oxford). 42:240–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawakami M, Kawagoe M, Harigai M, Hara M,
Hirose T, Hirose W, Norioka K, Suzuki K, Kitani A and Nakamura H:
Elevated plasma levels of alpha 2-plasmin inhibitor-plasmin complex
in patients with rheumatic diseases. Possible role of fibrinolytic
mechanism in vasculitis. Arthritis Rheum. 32:1427–1433. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanno Y, Shu E, Kanoh H and Seishima M:
The antifibrotic effect of α2AP neutralization in systemic
sclerosis dermal fibroblasts and mouse models of systemic
sclerosis. J Invest Dermatol. 136:762–769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanno Y, Shu E, Kanoh H, Matsuda A and
Seishima M: α2AP regulates vascular alteration by inhibiting VEGF
signaling in systemic sclerosis: The roles of α2AP in vascular
dysfunction in systemic sclerosis. Arthritis Res Ther. 19:222017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Q, Jin M, Yang F, Zhu J, Xiao Q and
Zhang L: Matrix metalloproteinases: Inflammatory regulators of cell
behaviors in vascular formation and remodeling. Mediators Inflamm.
Jun 12–2013.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Van Hove I, Lemmons K, Van de Velde S,
Verslegers M and Moons L: Matrix metalloproteinase-3 in the central
nervous system: A look on the bright side. J Neurochem.
123:203–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishijima C, Hayakawa I, Matsushita T,
Komura K, Hasegawa M, Takehara K and Sato S: Autoantibody against
matrix metalloproteinase-3 in patients with systemic sclerosis.
Clin Exp Immunol. 138:357–363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jinnin M, Ihn H, Asano Y, Yamane K, Yazawa
N and Tamaki K: Serum matrix metalloproteinase-3 in systemic
sclerosis. Arch Dermatol Res. 296:25–29. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Young-Min SA, Beeton C, Laughton R,
Plumpton T, Bartram S, Murphy G, Black C and Cawston TE: Serum
TIMP-1, TIMP-2, and MMP-1 in patients with systemic sclerosis,
primary Raynaud's phenomenon, and in normal controls. Ann Rheum
Dis. 60:846–851. 2001.PubMed/NCBI
|
|
23
|
Lijnen HR, Van Hoef B and Collen D:
Inactivation of the serpin alpha(2)-antiplasmin by stromelysin-1.
Biochim Biophys Acta. 1547:206–213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonaventura P, Lamboux A, Albarède F and
Miossec P: Regulatory effects of zinc on cadmium-induced
cytotoxicity in chronic inflammation. PLoS One. 12:e01808792017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciechomska M, O'Reilly S, Suwara M,
Bogunia-Kubik K and van Laar JM: miR-29a reduces TIMP-1 production
by dermal fibroblasts via targeting TGF-β activated kinase 1
binding protein 1, implications for systemic sclerosis. PLoS One.
9:e1155962014. View Article : Google Scholar : PubMed/NCBI
|