Introduction
Gastric cancer (GC) is the third leading cause of
cancer-related death (after lung and colorectal cancer) worldwide
and is the most common and fast-growing malignancy of the digestive
system with a high mortality (1,2). It is
estimated that >1,000,000 new cases of GC were diagnosed (5.7%
of the overall cancer incidence) and 800,000 deaths occurred (8.2%
of all cancer mortalities) in 2018 worldwide (3). Furthermore, according to previous
studies, patients with GC regularly exhibit partial metastasis at
diagnosis or during chemotherapy (4,5).
Despite advances in GC diagnostic techniques, surgery and
neoadjuvant chemoradiotherapy, the 5-year survival rate remains
poor in China, particularly at advanced stages (6,7). Owing
to the complexity of the pathogenesis of GC, its underlying
molecular mechanisms remain unclear. CBX2 is widely considered to
be a tumor-promoting gene (8,9). Due
to its significant association with large tumor size and lymph node
metastasis, it has been reported that CBX2 is highly expressed in
breast cancer tissues compared with adjacent normal tissues and may
serve as a novel biomarker for predicting the prognosis of patients
with breast cancer (10).
Additionally, CBX2 has been demonstrated to be upregulated in
patients with metastatic castration-resistant prostate cancer,
which was closely associated with a poor prognosis (11). Furthermore, high CBX2 expression is
associated with poor clinical outcomes in hepatocellular carcinoma,
with CBX2 serving regulatory effects on proliferation and apoptosis
via the phosphorylation of yes-associated protein (YAP) in
hepatocellular carcinoma cells (12). However, the precise function of CBX2
in GC remains unclear.
As a transcription co-activator of the Hippo family,
YAP is a mechanotransduction protein that serves a key role in
proliferation, differentiation and organ size (13–15).
Emerging evidence has determined that abnormal YAP activation is
involved in the growth of certain tumors, including liver and
prostate cancer (16,17). A previous study demonstrated that
CBX2 knockdown suppressed hepatocellular carcinoma cell
proliferation and promoted apoptosis by increasing the
phosphorylation of YAP (12). YAP1
overexpression has also been demonstrated to promote the
proliferation and migration of bladder cancer cells (18). In addition, increasing evidence has
revealed that the aberrant activation of Wnt/β catenin signaling
serves a key role in promoting the tumorigenesis and progression of
cancer (19). More importantly, YAP
has the ability to induce the nuclear localization and accumulation
of β-catenin (20). It is therefore
hypothesized that CBX2 knockdown may inhibit the expression of β
catenin signaling by promoting the phosphorylation of YAP and its
translocation into the nuclear cytoplasm, which may further inhibit
the proliferation, invasion and migration of GC cells.
The aim of the present study was to investigate the
biomolecular role and underlying mechanism of CBX2 in GC
progression. Thus, GC cell lines were transfected with short
hairpin (sh)RNA-CBX2, which was constructed to regulate the
expression of CBX2. It was determined that CBX2 knockdown by shRNA
inhibited GC cell proliferation, invasion and migration. Thus, CBX2
may serve as an effective target for clinical GC therapy. The
present study aimed to investigate the biomolecular role of CBX2
and its underlying mechanism in the pathogenesis of GC.
Materials and methods
Cell culture and transfection
Normal gastric GES-1 cells, a mouse gastric
carcinoma cell line (MFC) and various human GC cell lines
including, HGC-27, AGS and MKN-45 were obtained from the American
Type Culture Collection. Each cell line was maintained in DMEM/F12
1:1 medium (HyClone; Cytiva) supplemented with 10% FBS (HyClone;
Cytiva) and incubated at 37°C with 5% CO2, as previously
described (21,22). Cells were split every 3 days and
were diluted 1 day before each experiment. The expression of CBX2
in GC cell lines was analyzed using the Cancer Cell Line
Encyclopedia (CCLE; portals.broadinstitute.org/ccle).
shRNA-CBX2, shRNA-dickkopf-related protein 1 (DKK1)
and negative control (NC) shRNA plasmids cloned into the PLL3.79
lentiviral vector were constructed by Shanghai GenePharma Co., Ltd.
Lentiviruses (10 µg/ml) were transduced into MFC cells
(2×104/well) for 36 h. The sequences of shRNA-CBX2 and
shRNA-DKK1 used were as follows: shRNA-CBX2-1,
5′-GCTGGTCCTCCAAACATAACA-3′; shRNA-CBX2-2,
5′-GGCCTTCCAGAAGAAGGAACA-3′; shRNA-DKK1-1,
5′-GCTCTCATGGACTAGAAATAT-3′; shRNA-DKK1-2,
5′-GGAATAAGTACCAGACCATTG-3′; and shRNA-NC,
5′-TTCTCCGAACGTGTCACGT-3′. YAP-overexpression plasmids and control
overexpression plasmids cloned into the pCDNA3.1 vector were
provided by Shanghai GenePharma Co., Ltd. MFC cells
(2×104/well) were transfected with 100 pmol pcDNA-3.1
vectors using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) for 36 h according to the manufacturer's
protocol. shRNA-CBX2-1 and shRNA-DKK1-1 was selected for further
experimentation. Cells were divided into control (without
treatment), shRNA-NC (infected with unrelated sequence), shRNA-CBX2
(infected with shRNA-CBX2) and shRNA-CBX2 + YAP groups (infected
with shRNA-CBX2 and transfected with YAP plasmids). At 36 h
post-transfection, cells were harvested for western blotting or
reverse transcription-quantitative PCR (RT-qPCR) to detect the
efficiency of infection or transfection.
RT-qPCR
Total RNA (1 µg) from cultured cells was extracted
using TRIzol® reagent (Takara Bio, Inc.) and synthesized
into cDNA using a Reverse Transcription kit (Thermo Fisher
Scientific, Inc.), according to manufacturer's protocol. RT-qPCR
was performed using Roche SYBR-Green PCR kits (Roche Diagnostics)
and carried out using an Applied Biosystems™ 7500 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used for qPCR:
Predenaturation at 95°C for 10 min; followed by 40 cycles of 95°C
for 10 sec, 60°C for 1 min and 60°C for 1 min; and 4°C for
preservation. The relative quantitation of target gene expression
was calculated using the 2−ΔΔCq method (23) and normalized to the expression of
GAPDH. The primer sequences were as follows: CBX2 forward,
5′-CTGTGTCAAGGGCAGTGCTA-3′ and reverse, 5′-ATACGTGCTCGATGAGGCTG-3′;
DKK1 forward, 5′-GGAAGGAAACCCACCAACTT-3′ and reverse,
5′-TCAATGCAGTACAGGCGAAG-3′; YAP forward, 5′-GGATTTCTGCCTTCCCTGAA-3′
and reverse, 5′-GATAGCAGGGCGTGAGGAAC-3′; and GAPDH forward,
5′-CTGGGCTACACTGAGCACC-3′ and reverse,
5′-AAGTGGTCGTTGAGGGCAATG-3′.
Western blotting
According to the manufacturer's protocol, nuclear
and cytosolic cellular proteins were obtained using the nuclear
protein extraction agent and cytoplasmic protein extraction agent,
respectively (both purchased from Beyotime Institute of
Biotechnology). A BCA assay was employed to determine the protein
concentration. Subsequently, equal quantities of protein (25 µg)
were separated via 10% SDS-PAGE, and subsequently transferred onto
PVDF membranes (EMD Millipore). After blocking with 5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) for 2 h at room temperature,
membranes were incubated at 4°C overnight with the following
primary antibodies: Anti-CBX2 (1:1,000; cat. no. ab235305; Abcam),
β-catenin (1:1,000; cat. no. 8480; Cell Signaling Technology,
Inc.), c-myc (1:1,000; cat. no. ab32072; Abcam), cyclin D1
(1:1,000; cat. no. 55506; Cell Signaling Technology, Inc.), MMP7
(1:1,000; cat. no. 71031; Cell Signaling Technology, Inc.), MMP13
(1:1,000; cat. no. 94808; Cell Signaling Technology, Inc.),
phosphorylated (P)-YAP (1:500; cat. no. 13619; Cell Signaling
Technology, Inc.), YAP (1:1,000; cat. no. 14710; Cell Signaling
Technology, Inc.) and GAPDH (1:1,000; cat. no. 5174; Cell Signaling
Technology, Inc.). Subsequently, membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000;
cat. no. DC03L; Sigma-Aldrich; Merck KGaA) or goat anti-mouse IgG
(1:5,000; cat. no. DC02L; Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature. Samples were visualized using the Tanon-5200
Chemiluminescence Imager (Tanon Science and Technology Co., Ltd.)
with an ECL western blotting substrate (EMD Millipore). Band
intensity was semi-quantified using ImageJ (v1.8.0; National
Institutes of Health) and relative protein levels were normalized
to GAPDH (1:1,000; cat. no. 3683; Cell Signaling Technology, Inc.),
β-actin (1:1,000; cat. no. 5125; Cell Signaling Technology, Inc.)
or LAMINB (1:1,000; cat. no. 24209; Cell Signaling Technology,
Inc.). All experiments were performed in triplicate.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay was performed to determine cell
viability. MFC cells (2×103 cells/well) were seeded into
a 96-well plate and incubated at 37°C. At 0, 24, 48 and 72 h, the
absorbance of cells was measured at 450 nm after addition of 10 µl
CCK-8 reagent (Dojindo Molecular Technologies, Inc.) for 1 h.
Experiments were performed six times for each sample.
Colony formation assay
MFC cells transfected with or without shRNA CBX2 and
DKK1 were seeded into a 35-mm petri dish (1×103/well).
After culture for 10–14 days, the resulting cell colonies were
fixed with 4% paraformaldehyde and stained with 0.1% crystal violet
solution for 20 min at room temperature. Colonies with diameters
>0.5 mm were imaged and counted using a digital camera (Nikon
Corporation).
Wound healing assay
MFC cells were seeded into six-well plates at a
density of 5×105 cells/well. The monolayer was scratched
with a 100-µl pipette tip once cells had reached 90% confluence.
Floating cells were subsequently removed by washing with PBS and
plates were maintained in serum-free medium at 37°C. Finally,
transfected MFC cells were imaged using a fluorescence microscope
(Olympus Corporation) at 0 and 48 h after wounding. The width of
the wound was measured to estimate the migration of MFC cells. The
recovered wound area (%) at the indicated time point (48 h) was
calculated according to the following formula: [(wound width at 0
h) - (wound width at 48 h)]/wound width at 0 h.
Transwell assay
Transfected MFC cells were suspended in serum-free
medium (5×105 cells/ml) and added to the upper chamber
of the Transwell instrument, which was pre-coated with Matrigel at
37°C for 4 h. The lower chamber was filled with culture medium
containing 20% FBS. After incubation for 24 h at 37°C, cells on the
bottom of the membrane were fixed with 4% formaldehyde solution for
20 min at 37°C, stained with 0.1% crystal violet for 30 min at room
temperature and washed twice with PBS. The number of cells from
five different fields were captured and counted using an inverted
microscope (Olympus Corporation).
Statistical analysis
Data are presented as the mean ± SEM. Statistical
analysis was performed using SPSS version 23.0 (IBM Corp.) and
GraphPad Prism 5.0 software (GraphPad Software, Inc.). Differences
among multiple groups were analyzed using one-way ANOVA followed by
Bonferroni post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
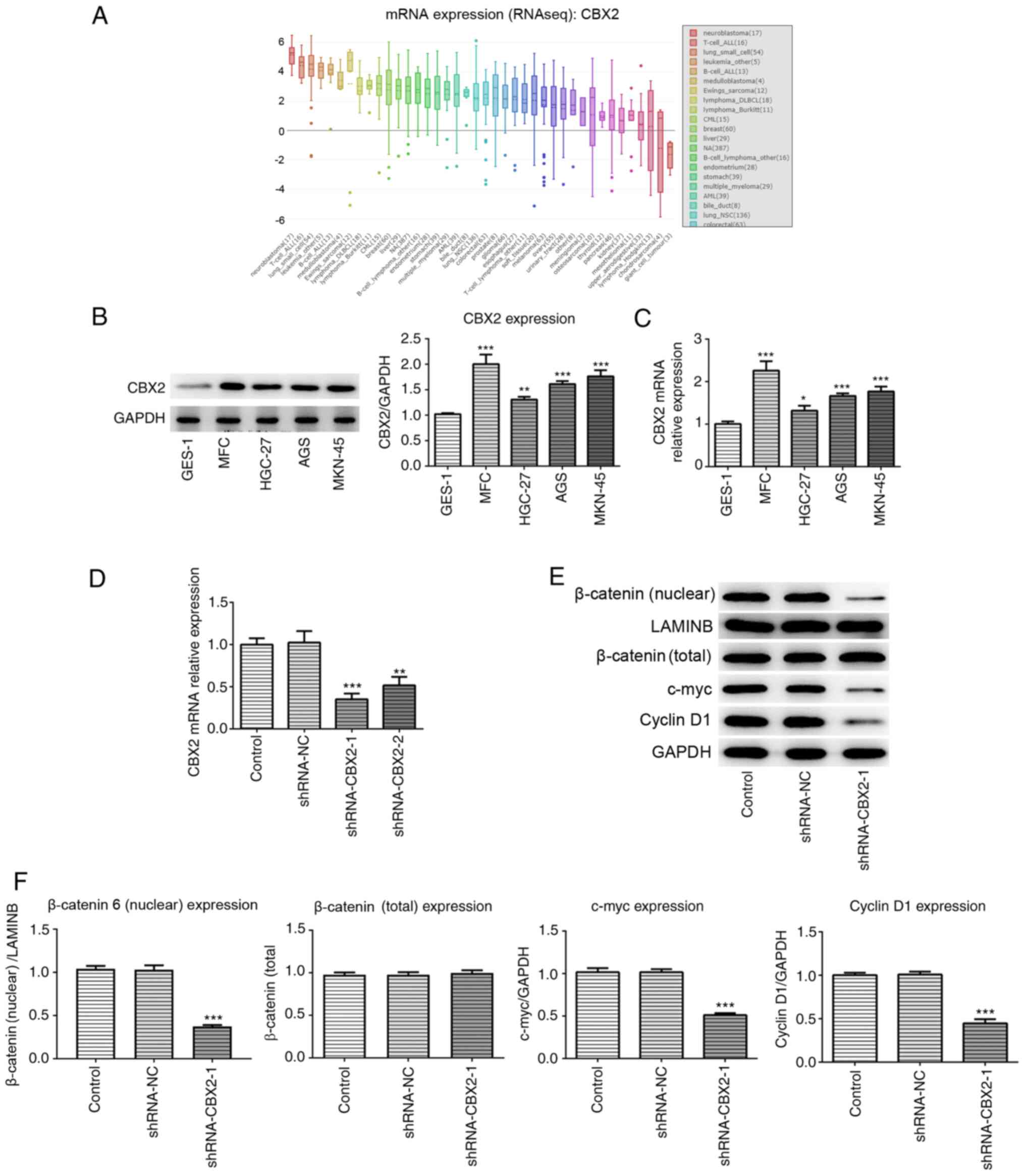
CBX2 is overexpressed in GC cell
lines
By searching the CCLE, the results demonstrated that
CBX2 expression was increased in GC cell lines compared with normal
gastric cells (Fig. 1A). To confirm
whether CBX2 expression was increased in GC cell lines, RT-qPCR and
western blotting were performed. In the present study, normal
gastric GES-1 cells and GC cell lines, including MFC, HGC-27, AGS
and MKN-45, were selected to investigate the role of CBX2 in GC.
The results demonstrated that CBX2 expression was significantly
elevated in GC cells compared with GES-1 cells (Fig. 1B and C). As the highest level of
CBX2 was observed in MFC cells, this cell line was selected for
further experimentation. The results indicated that upregulated
CBX2 expression may be involved in GC pathogenesis.
CBX2 knockdown inhibits the activation
of the β-catenin signaling pathway
To investigate the role of CBX2 and its underlying
mechanism in GC pathogenesis, the effect of CBX2 on the β-catenin
signaling pathway was analyzed. shRNA-CBX2 was constructed to
downregulate CBX2 expression. The results of RT-qPCR revealed that
the knockdown effect of shRNA-CBX2-1 was greater than that of
shRNA-CBX2-2. Therefore, shRNA-CBX2-1 was used for further
experimentation (Fig. 1D). Western
blotting was subsequently performed to detect the expression of
β-catenin and its various downstream proteins, including c-myc and
cyclin D1. The results demonstrated that the expression of c-myc,
cyclin D1 and nuclear β-catenin in the shRNA-CBX2-1 group were
significantly decreased compared with the control, while total
β-catenin expression showed no significant difference (Fig. 1E and F). The results revealed that
CBX2 knockdown inhibited the activation of the β-catenin signaling
pathway.
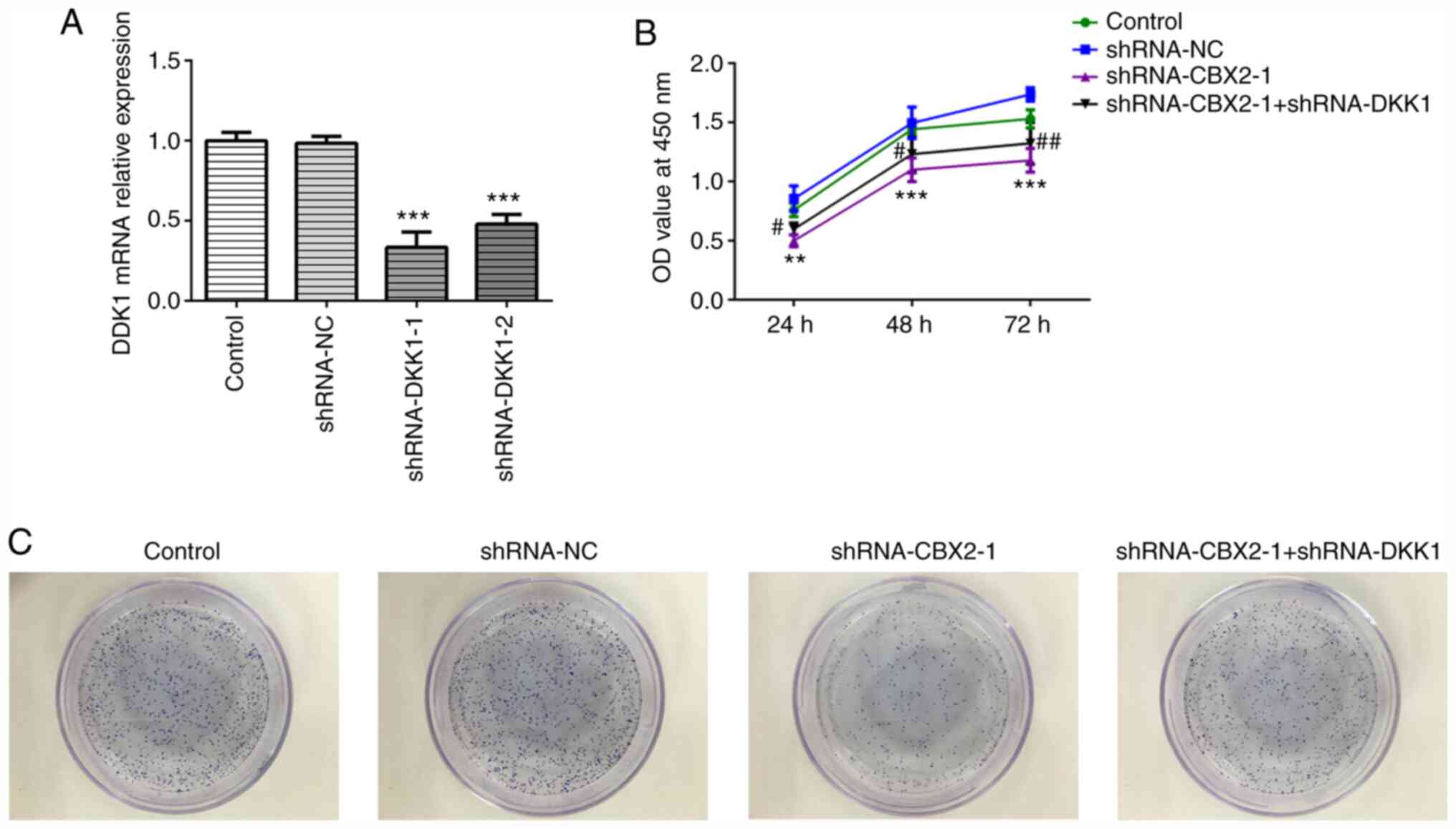
CBX2 knockdown inhibits GC cell
proliferation by inactivating the β-catenin signaling pathway
To confirm whether the β-catenin signaling pathway
is involved in the role of CBX2 in GC, shRNA-DKK1 was synthesized.
As DKK1 is a negative regulator of Wnt/β-catenin signaling
(24), DKK1 was silenced to
activate β-catenin signaling. Due to its higher transfection
efficiency, shRNA-DKK1-1 was selected for further use, as
determined by RT-qPCR (Fig. 2A). A
CCK-8 assay was carried out to evaluate the viability of MFC cells
transfected with shRNA-CBX2-1 or shRNA-DKK1-1 at the indicated time
points (0, 24, 48 and 72 h). As presented in Fig. 2B, cells transfected with
shRNA-CBX2-1 demonstrated a lower viability compared with the
control, whereas cells transfected with shRNA-CBX2-1 and
shRNA-DKK1-1 demonstrated increased viability compared with
shRNA-CBX2-1 transfected cells. A colony formation assay was
subsequently performed to determine MFC cell proliferation. As
presented in Fig. 2C, MFC cell
proliferation was suppressed by shRNA-CBX2-1 transfection, which
was subsequently abolished by shRNA-DKK1-1 transfection. These data
suggested that CBX2 knockdown inhibited the proliferation of GC
cells by inactivating the β-catenin signaling pathway.
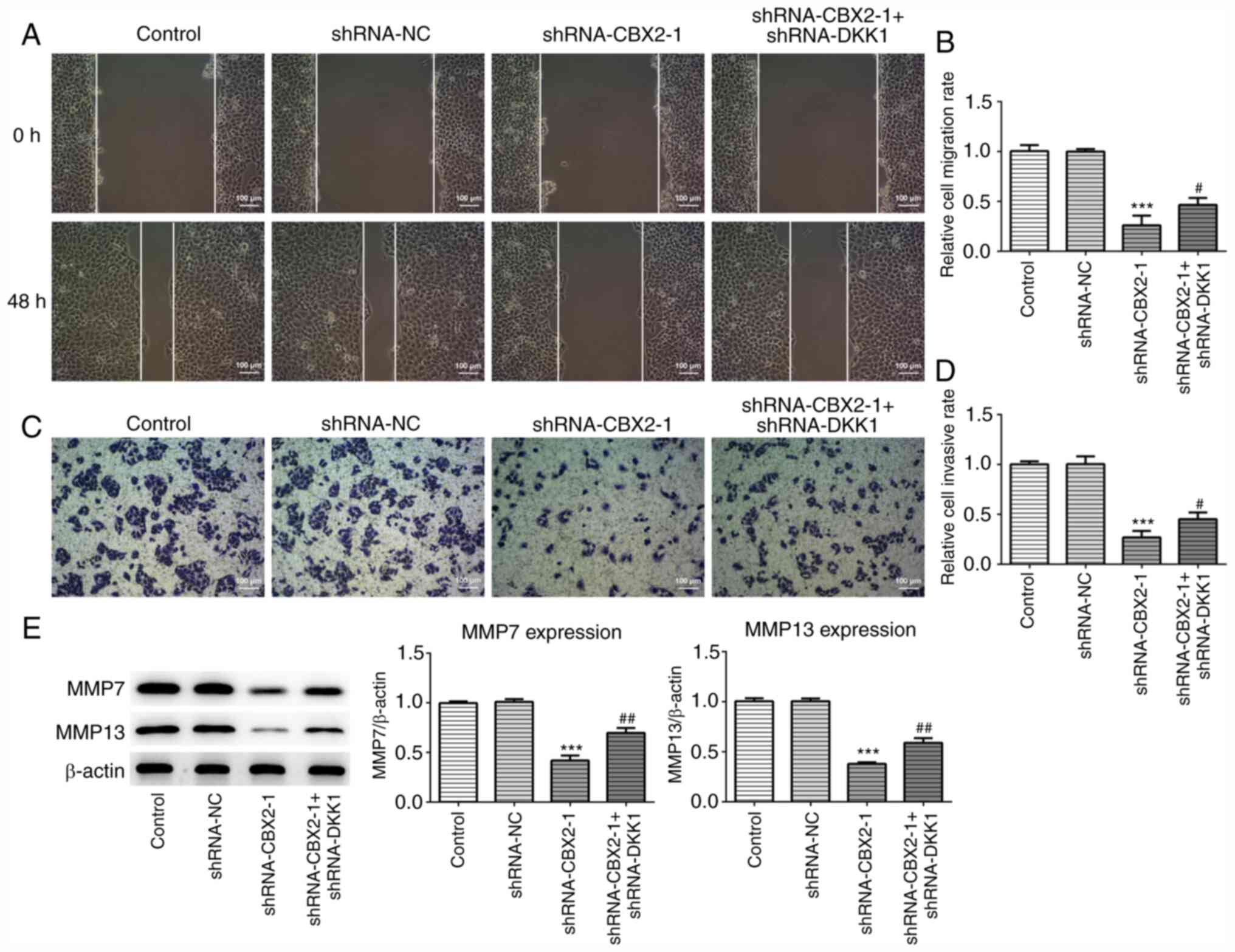
CBX2 knockdown inhibits the migration
and invasion of GC cells by inactivating the β-catenin signaling
pathway
To further confirm the role of CBX2 in GC, wound
healing and Transwell assays were performed to assess migration and
invasion, respectively. As presented in Fig. 3A-D, the migration and invasion of
MFC cells transfected with shRNA-CBX2-1 was significantly inhibited
compared with the control, whereas shRNA-CBX2-1 + shRNA-DKK1-1
co-transfection reversed this effect. It has been demonstrated that
MMP7 and MMP13 are significantly upregulated in malignant tumors,
and are associated with tumor invasion and metastasis (25). As presented in Fig. 3E, the expression of MMP7 and MMP13
were significantly downregulated in MFC cells transfected with
shRNA-CBX2-1 compared with controls. Furthermore, transfection with
shRNA-DKK1-1 inhibited the CBX2 knockdown-induced suppression of
MMP7 and MMP13 expression. The results indicated that CBX2
knockdown inhibited the migration and invasion of GC cells by
inactivating the β-catenin signaling pathway.
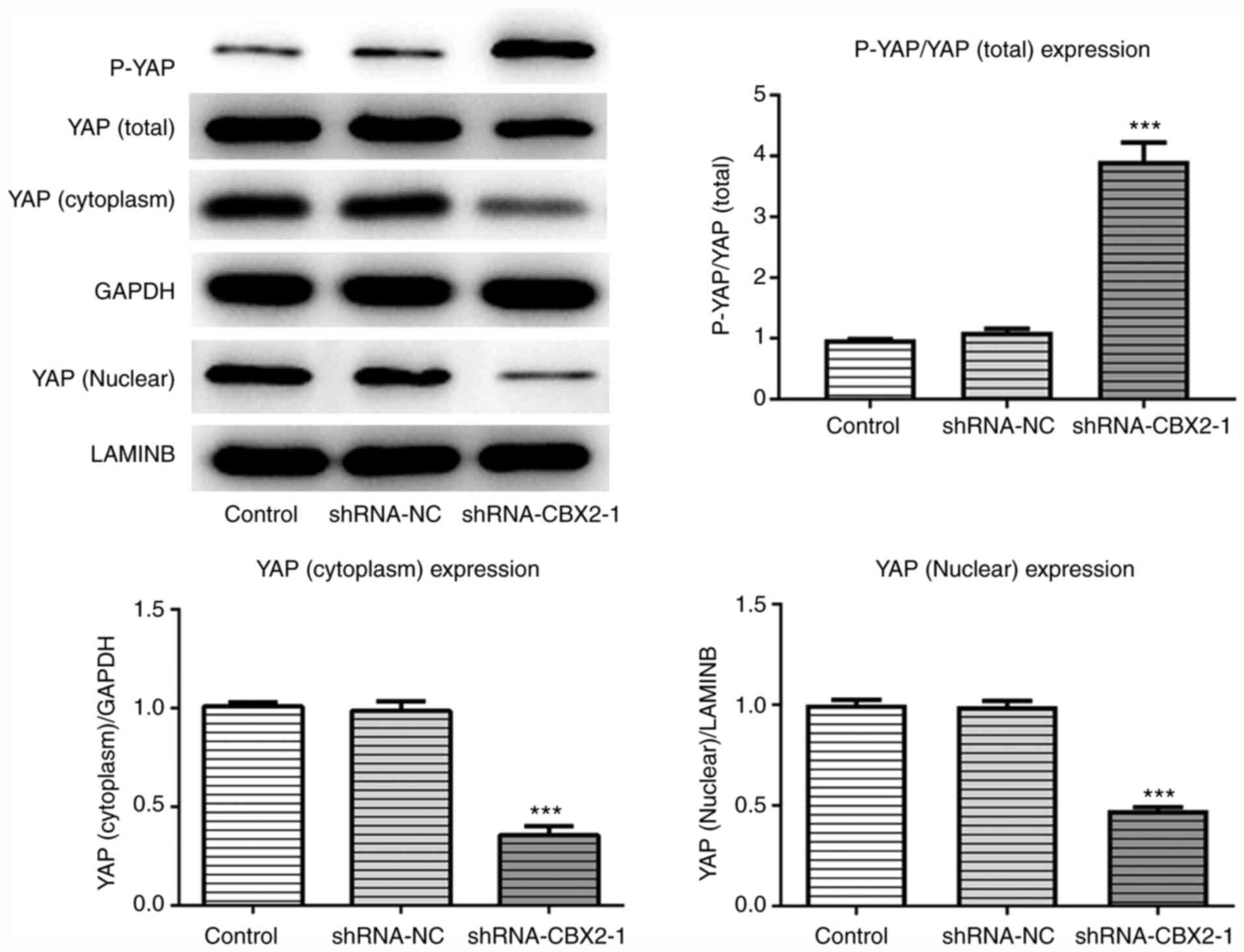
CBX2 knockdown promotes YAP
phosphorylation and its nuclear cytoplasm translocation
The present study determined that CBX2 promoted the
proliferation, invasion and migration of GC cells by inactivating
the β-catenin pathway. However, the specific molecular mechanism
underlying this effect remains unclear. To determine the underlying
mechanism associated with the effect of CBX2 in GC, the expression
of P-YAP, cytoplasmic YAP and nuclear YAP were detected via western
blotting. The results demonstrated that the phosphorylation of YAP
in the shRNA-CBX2-1 group was significantly upregulated compared
with the control, whereas the expression levels of cytoplasmic and
nuclear YAP were downregulated (Fig.
4). The results revealed that CBX2 exerted a regulatory effect
on YAP expression.
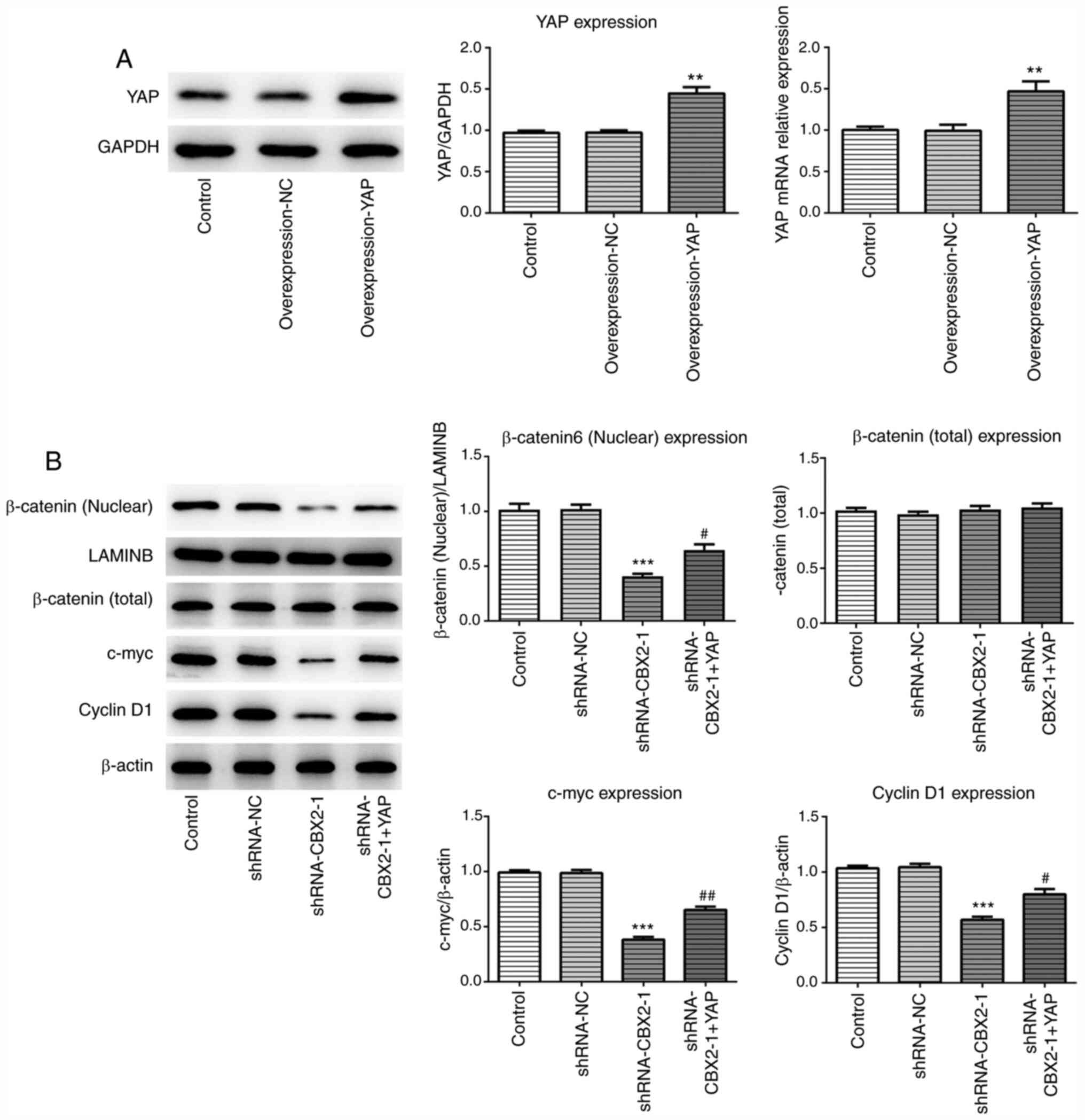
YAP overexpression reverses the
inhibitory effect of CBX2 knockdown on the β-catenin signaling
pathway
To further investigate the molecular mechanism
underlying the effect of CBX2 and GC, a YAP overexpression plasmid
was constructed. The expression of YAP was analyzed via RT-qPCR and
western blotting. As presented in Fig.
5A, YAP expression was increased in MFC cells transfected with
overexpression-YAP plasmids compared with control cells.
Additionally, western blotting was performed to evaluate the
expression of β-catenin and its downstream proteins, including
c-myc and cyclin D1, after transfection with or without
shRNA-CBX2-1 and overexpression-YAP plasmids. The results
demonstrated that shRNA-CBX2-1 transfection led to a significant
reduction in the expression of the aforementioned proteins, which
was consistent with Fig. 1D. As
predicted, YAP overexpression attenuated the suppressive effect of
CBX2 knockdown on protein expression (Fig. 5B). Therefore, these data indicated
that CBX2 promoted GC pathogenesis by activating the YAP/β-catenin
pathway.
Discussion
The incidence of digestive tract tumors has
significantly increased following the improvement of human living
standards, due to the change of diet and habits (26). GC is the most common and aggressive
form of digestive tract cancer worldwide (27). There are great limitations on the
understanding of GC etiology and pathogenesis, owing to the
multifaceted process and complex molecular mechanism that
contribute to the tumorigenesis and progression of GC. Hence, it is
of great importance to investigate the underlying mechanisms of GC
pathogenesis and to determine effective molecular targets for GC
therapy.
CBX2, a member of chromobox family, is an essential
regulator of cancer progression and the cell cycle (28). It has been reported that CBX2 exerts
an oncogenic effect on osteosarcoma progression and promotes
osteosarcoma cell proliferation and metastasis (29). A previous study demonstrated that
CBX2 knockdown markedly suppressed the proliferation of
hepatocellular carcinoma cells and promoted apoptosis (12). In the present study, increased CBX2
expression was observed in GC cell lines compared with normal
gastric cells, indicating that CBX may serve an important role in
GC onset and development. Additionally, CBX2 knockdown suppressed
the proliferation, migration and invasion of GC cells, which was
consistent with the results of the aforementioned studies. Thus,
CBX may serve as a novel molecular target.
Growing evidence has demonstrated that the
Wnt/β-catenin signaling pathway is downregulated in normal cells,
and significantly upregulated in cancer cells, indicating that is
has an important role in cancer cell proliferation and apoptosis
(30,31). The present study revealed that CBX2
knockdown inhibited nuclear localization and β-catenin
accumulation, and suppressed the expression of its downstream
proteins, including c-myc and cyclin D1, suggesting that CBX2
promoted tumorigenesis and progression in GC via the β-catenin
pathway. To confirm whether the β-catenin pathway was involved in
the role of CBX2 in GC, the expression of DKK1, which is a negative
regulator of β-catenin signaling (32), was silenced. DKK1 knockdown
inhibited the suppressive effect of CBX2 knockdown on GC cell
proliferation, migration and invasion, suggesting that CBX2
promoted GC cell proliferation and metastasis by activating the
β-catenin pathway. However, further experiments are required to
investigate the specific mechanism of the role of CBX2 in GC.
YAP is a downstream effector of the Hippo pathway,
which mediates the phosphorylation of YAP (33). P-YAP binds to other proteins and
cannot translocate into the cell nucleus, and is subsequently
degraded by ubiquitination in the cytoplasm, thereby leading to the
inactivation of YAP (34).
According to a previous study, YAP served a crucial role in organ
growth and cell proliferation (35,36).
Furthermore, YAP was reported to contribute to tumor initiation,
progression and metastasis (37).
Tang et al (38)
demonstrated that the expression of β-catenin was markedly reduced
after YAP downregulation in laryngeal cancer cells. Wang et
al (39) also reported that
P-YAP contributed to the degradation of β-catenin in colorectal
cancer cells. Thus, YAP may represent an effective target in
anticancer therapy. In the present study, YAP overexpression
plasmids were transfected into GC cells to induce YAP upregulation.
The results demonstrated that CBX2 knockdown elevated P-YAP levels
and inhibited YAP expression in the cytoplasm and nucleus,
suggesting that CBX2 knockdown promoted YAP translocation, causing
the degradation of cytoplasmic YAP. In addition, YAP inhibition
suppressed the expression of β-catenin and its downstream signaling
molecules, including c-myc and cyclin D1 (24), which is consistent with the results
of the present study. The current study revealed that YAP
overexpression reversed the inhibitory effect of CBX2 knockdown on
the β-catenin pathway. The results indicated that CBX2 regulated
the GC cell cycle, migration and invasion via the YAP/β-catenin
pathway. The present study provided evidence to support that CBX2
may be an effective target for GC treatment. However, the present
study had several limitations. For example, FBS was used in culture
medium contained a mixture of growth factors, which may have
affected the present results. Additionally, further studies will be
performed to fully elucidate the specific mechanisms underlying the
role of CBX2 in GC progression.
In summary, the present study demonstrated that CBX2
was significantly upregulated in GC cells. CBX2 knockdown led to
the suppression of the β-catenin pathway, and the promotion of YAP
phosphorylation and translocation. The results suggested that CBX2
promoted GC cell proliferation, migration and invasion by
activating the YAP/β-catenin pathway. CBX2 may represent a novel
and promising therapeutic target for GC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QLG designed the experiment and drafted the
manuscript. MMZ, BXL and LY performed the experiments and analyzed
the data. QLG and MMZ reviewed the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin J, Wu X, Li S, Li C and Guo Z: Impact
of environmental factors on gastric cancer: A review of the
scientific evidence, human prevention and adaptation. J Environ Sci
(China). 89:65–79. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohara H, Ishibashi Y, Yoshimura S,
Yamazaki R, Hatao F, Koshiishi T, Morita Y and Imamura K:
Intratumoral pseudoaneurysm within a liver metastasis of gastric
cancer: A case report. Surg Case Rep. 6:392020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujita I, Toyokawa T, Makino T, Matsueda
K, Omote S and Horii J: Small early gastric cancer with synchronous
bone metastasis: A case report. Mol Clin Oncol. 12:202–207.
2020.PubMed/NCBI
|
|
6
|
Zong L, Abe M, Seto Y and Ji J: The
challenge of screening for early gastric cancer in China. Lancet.
388:26062016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao J, Zhang H, Liu C, Chen S, Qian R and
Zhao K: miR-450b-3p inhibited the proliferation of gastric cancer
via regulating KLF7. Cancer Cell Int. 20:472020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wheeler LJ, Watson ZL, Qamar L, Yamamoto
TM, Post MD, Berning AA, Spillman MA, Behbakht K and Bitler BG:
CBX2 identified as driver of anoikis escape and dissemination in
high grade serous ovarian cancer. Oncogenesis. 7:922018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clermont PL, Sun L, Crea F, Thu KL, Zhang
A, Parolia A, Lam WL and Helgason CD: Genotranscriptomic
meta-analysis of the Polycomb gene CBX2 in human cancers: Initial
evidence of an oncogenic role. Br J Cancer. 111:1663–1672. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen WY, Zhang XY, Liu T, Liu Y, Zhao YS
and Pang D: Chromobox homolog 2 protein: A novel biomarker for
predicting prognosis and Taxol sensitivity in patients with breast
cancer. Oncol Lett. 13:1149–1156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clermont PL, Crea F, Chiang YT, Lin D,
Zhang A, Wang JZ, Parolia A, Wu R, Xue H, Wang Y, et al:
Identification of the epigenetic reader CBX2 as a potential drug
target in advanced prostate cancer. Clin Epigenetics. 8:162016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao J, Tian Y, Wang C, Jiang K, Li R, Yao
Y, Zhang R, Sun D, Liang R, Gao Z, et al: CBX2 Regulates
Proliferation and Apoptosis via the Phosphorylation of YAP in
Hepatocellular Carcinoma. J Cancer. 10:2706–2719. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo T, Mohan K, Iglesias PA and Robinson
DN: Molecular mechanisms of cellular mechanosensing. Nat Mater.
12:1064–1071. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Low BC, Pan CQ, Shivashankar GV,
Bershadsky A, Sudol M and Sheetz M: YAP/TAZ as mechanosensors and
mechanotransducers in regulating organ size and tumor growth. FEBS
Lett. 588:2663–2670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maugeri-Saccà M and De Maria R: The Hippo
pathway in normal development and cancer. Pharmacol Ther.
186:60–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen T, Li Y, Zhu S, Yu J, Zhang B, Chen
X, Zhang Z, Ma Y, Niu Y and Shang Z: YAP1 plays a key role of the
conversion of normal fibroblasts into cancer-associated fibroblasts
that contribute to prostate cancer progression. J Exp Clin Cancer
Res. 39:362020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Yu Z, Chen SS, Li F, Lei CY, Chen
XX, Bao JM, Luo Y, Lin GZ, Pang SY, et al: The YAP1 oncogene
contributes to bladder cancer cell proliferation and migration by
regulating the H19 long noncoding RNA. Urol Oncol. 33:427.e1–e10.
2015. View Article : Google Scholar
|
|
19
|
Wu F, Xing T, Gao X and Liu F: miR 501 3p
promotes colorectal cancer progression via activation of Wnt/β
catenin signaling. Int J Oncol. 55:671–683. 2019.PubMed/NCBI
|
|
20
|
Rosenbluh J, Nijhawan D, Cox AG, Li X,
Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et
al: β-catenin-driven cancers require a YAP1 transcriptional complex
for survival and tumorigenesis. Cell. 151:1457–1473. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang W, Zheng X, Wang P and Guo S:
Deguelin exerts anticancer activity of human gastric cancer MGC-803
and MKN-45 cells in vitro. Int J Mol Med. 41:3157–3166.
2018.PubMed/NCBI
|
|
22
|
Han MY, Nie JW, Li YY, Zhu YZ and Wu G:
Downregulation of NGAL is required for the inhibition of
proliferation and the promotion of apoptosis of human gastric
cancer MGC-803 cells. Cell Physiol Biochem. 50:694–705. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang Q, Liu T, Yu C, Yang X, Shao Y, Shi
J, Ye X, Zheng X, Yan J, Xu D, et al: lncRNA TUG1 alleviates
cardiac hypertrophy by targeting miR-34a/DKK1/Wnt-β-catenin
signalling. J Cell Mol Med. 24:3678–3691. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gobin E, Bagwell K, Wagner J, Mysona D,
Sandirasegarane S, Smith N, Bai S, Sharma A, Schleifer R and She
JX: A pan-cancer perspective of matrix metalloproteases (MMP) gene
expression profile and their diagnostic/prognostic potential. BMC
Cancer. 19:5812019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ghelfi F, Tieri M, Gori S, Nicolis F,
Petrella MC, Filiberti A, Apolone G and Titta L: Do cancer patients
change their diet in the e-health information era? A review of the
literature and a survey as a proposal for the Italian population.
Food Res Int. 104:59–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao DL and Wu QL: Effect of inhibition to
Yes-related proteins-mediated Wnt/β-catenin signaling pathway
through miR-195-5p on apoptosis of gastric cancer cells. Eur Rev
Med Pharmacol Sci. 23:6486–6496. 2019.PubMed/NCBI
|
|
28
|
Morey L, Pascual G, Cozzuto L, Roma G,
Wutz A, Benitah SA and Di Croce L: Nonoverlapping functions of the
Polycomb group Cbx family of proteins in embryonic stem cells. Cell
Stem Cell. 10:47–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han Q, Li C, Cao Y, Bao J, Li K, Song R,
Chen X, Li J and Wu X: CBX2 is a functional target of miRNA let-7a
and acts as a tumor promoter in osteosarcoma. Cancer Med.
8:3981–3991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li F, Zhang L, Li W, Deng J, Zheng J, An
M, Lu J and Zhou Y: Circular RNA ITCH has inhibitory effect on ESCC
by suppressing the Wnt/β-catenin pathway. Oncotarget. 6:6001–6013.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang J, Liu Y, Mai X, Lu S, Jin L and Tai
X: STAT1-induced upregulation of LINC00467 promotes the
proliferation migration of lung adenocarcinoma cells by
epigenetically silencing DKK1 to activate Wnt/β-catenin signaling
pathway. Biochem Biophys Res Commun. 514:118–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moya IM, Castaldo SA, Van den Mooter L,
Soheily S, Sansores-Garcia L, Jacobs J, Mannaerts I, Xie J,
Verboven E, Hillen H, et al: Peritumoral activation of the Hippo
pathway effectors YAP and TAZ suppresses liver cancer in mice.
Science. 366:1029–1034. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Z, Du J, Wang S, Shao L, Jin K, Li
F, Wei B, Ding W, Fu P, van Dam H, et al: OTUB2 Promotes Cancer
Metastasis via Hippo-Independent Activation of YAP and TAZ. Mol
Cell. 73:7–21.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koo JH, Plouffe SW, Meng Z, Lee DH, Yang
D, Lim DS, Wang CY and Guan KL: Induction of AP-1 by YAP/TAZ
contributes to cell proliferation and organ growth. Genes Dev.
34:72–86. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Piccolo S, Dupont S and Cordenonsi M: The
biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev.
94:1287–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu H, Dai X, Cao X, Yan H, Ji X, Zhang H,
Shen S, Si Y, Chen J, Li L, et al: PRDM4 mediates YAP induced cell
invasion by activating leukocyte specific integrin β2 expression.
EMBO Rep. 19:e451802018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang X, Sun Y, Wan G, Sun J, Sun J and Pan
C: Knockdown of YAP inhibits growth in Hep-2 laryngeal cancer cells
via epithelial-mesenchymal transition and the Wnt/β-catenin
pathway. BMC Cancer. 19:6542019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang S, Ma K, Zhou C, Wang Y, Hu G, Chen
L, Li Z, Hu C, Xu Q, Zhu H, et al: LKB1 and YAP phosphorylation
play important roles in Celastrol-induced β-catenin degradation in
colorectal cancer. Ther Adv Med Oncol. 11:17588359198437362019.
View Article : Google Scholar : PubMed/NCBI
|