Introduction
Diabetes mellitus is a common disease characterized
by dysregulated blood glucose homeostasis, and has reached
pandemic-proportion levels worldwide (1). Diabetic nephropathy (DN), identified
as a life-threatening microvascular complication of diabetes, is
the leading cause of end-stage renal disease (ESRD) (2). DN is caused by excessive precipitation
of extracellular matrix (ECM) proteins, which results in glomerular
hypertrophy and expansion, effacement of podocyte foot processes,
and inflammatory cell infiltration (3). Podocytes are highly differentiated
epithelial cells with extensive foot processes, and their
progressive injury plays a vital role in the deterioration of
glomerular function and albuminuria (4,5).
Emerging evidence shows that different mechanisms
contribute to the pathogenic progression of DN, including oxidative
stress, excessive accumulation of advanced glycation end-products
(AGEs), and inflammation (6). AGE
induces tissue injury through alterations in the extracellular
matrix architecture and interactions with the receptor for advanced
glycation end products (RAGE) (7).
RAGE is expressed on normal podocytes; however, its expression is
abnormally upregulated in DN. Excessive ROS generation is
implicated in the activation of NF-κB, which upregulates RAGE
expression, resulting in chronic inflammation, aggravation of
cellular dysfunction, and increased tissue damage (8,9). The
transcription and production of pro-inflammatory cytokines (such as
TNF-α and IL-1β) in podocytes contribute to the development of
DN.
Autophagy is the major mechanism by which podocytes
regulate cellular homeostasis, enabling the cells to degrade
oxidatively damaged or surplus organelles in autolysosomes
(10). Autophagy disorders play a
critical role in a wide range of human pathologies. Moreover,
autophagy plays a dual function in maintaining cellular
homeostasis, as both defective autophagy and excessive autophagy
are associated with cellular dysfunctions preceding cell death
(11). Various detrimental factors,
including oxidative stress, mitochondrial dysfunction, and
inflammatory reactions can lead to autophagic cell injury (12). In addition, podocytes have higher
basal levels of autophagy under physiological conditions,
suggesting that autophagy is an important mechanism by which
podocytes maintain homeostasis (13). Although several cell types
demonstrate a basal level of autophagy, autophagy can be further
activated to promote cellular survival in response to various forms
of cellular stress, such as oxidative stress or hypoxia (14).
Glucose variability represents a novel latent risk
factor for diabetes mellitus that consists of two aspects, namely,
the extent of blood glucose fluctuations and the time intervals
during which these fluctuations occur (15). Evidence suggests that acute glucose
fluctuation (AGF) may be more harmful than sustained hyperglycemia
regarding the risk of developing diabetic complications (16). Moreover, studies have shown that
intermittent hyperglycemia exposure induces more severe oxidative
stress than constant exposure to hyperglycemia (17,18).
AGF has been confirmed to accelerate renal damage in diabetic rats
(19). However, its impact on rat
podocytes is poorly understood.
Although our previous study revealed that
fluctuating hyperglycemia induces more severe inflammatory damage
than hyperglycemia in diabetic rats, the associated mechanism
remains unknown (20). In this
study, the effects of AGF were evaluated in rat podocytes in order
to elucidate further potential mechanisms of injury caused by
glucose variability.
Materials and methods
Cell culture and treatment
A rat podocyte cell line was obtained from Beijing
Wormhole Space Information Technology Co., Ltd. Rat podocytes were
cultured in DMEM/F12 culture medium (Hyclone; Cytiva) supplemented
with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified incubator with 5% CO2.
Cells were divided into three groups: i) Normal
glucose group (NG), cells incubated in culture medium with 17.5 mM
D-glucose; ii) high-glucose group (HG), cells incubated in culture
medium with 33 mM D-glucose; and iii) AGF, 17.5 and 33 mM D-glucose
culture medium were alternated every 3 h twice daily, with the
final incubation carried out overnight in 33 mM D-glucose culture
medium. The cells were maintained at the required conditions for 72
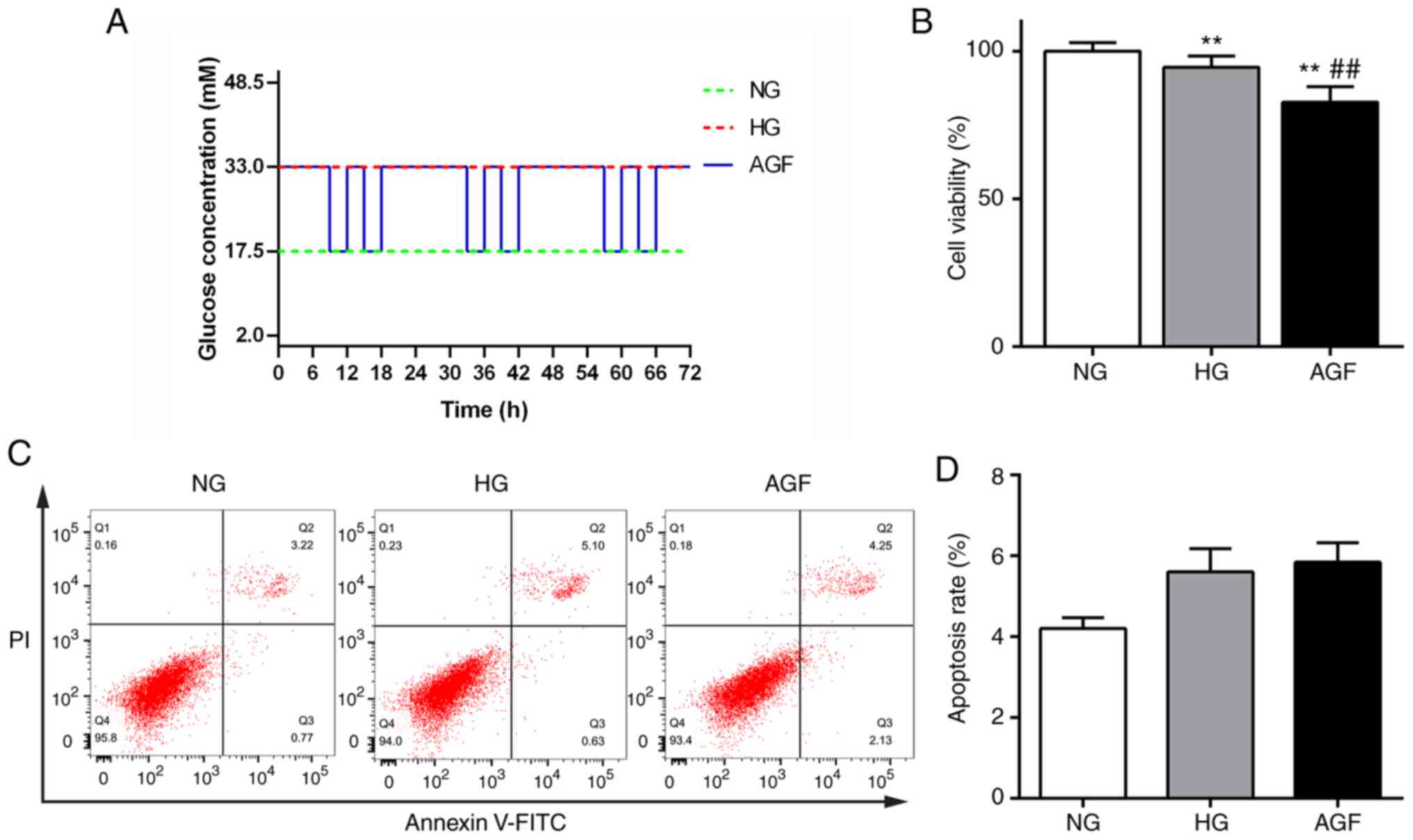
h, as shown in Fig. 1A.
In a separate experiment, rat podocytes were
pre-treated with 5 mM N-Acetyl-L-cysteine (NAC) or 10 µM
pyrrolidine dithiocarbamate (PDTC) (both Beyotime Institute of
Biotechnology) for 30 min and then treated with the aforementioned
conditions for 72 h, as shown in Fig.
1A.
Cell viability assays
The effects of AGF treatment on cell viability were
measured using a Cell Counting Kit-8 (Beyotime Institute of
Biotechnology). Rat podocytes were seeded into 96-well plates
(Corning, Inc.) at a density of 3×104 cells/ml with 100
µl culture medium per well, then cultured for 24 h. After 72 h of
NG, HG or AGF treatment, the culture medium was replaced with 100
µl fresh culture medium, and 10 µl CCK-8 solution was added to each
well. After incubating the cells for 1 h, the absorbance was
detected at 450 nm.
Cell apoptosis
Cell apoptosis was assessed with an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) detection
kit (Dojindo Molecular Technologies, Inc.). Rat podocytes were
plated in six-well plates (Corning, Inc.) at a density of
3×105 cells/ml with 1 ml culture medium per well, then
cultured for 24 h. After 72 h of treatment, the rat podocytes were
digested with 0.25% trypsin and gently washed with PBS. The
single-cell suspension was incubated with 5 µl Annexin V-FITC and 5
µl PI in 100 µl binding buffer solution for 15 min at room
temperature in the dark. Next, 400 µl Annexin V binding buffer was
added, and apoptotic cells were detected using a FACSCanto II flow
cytometer (BD Biosciences) within 1 h. The apoptosis rates were
analyzed using FlowJo software (version 10.0.7r2; FlowJo LLC).
MRFP-GFP-LC3 assay
The adenovirus encoding mRFP-GFP-LC3 was purchased
from Hanbio Biotechnology Co., Ltd. (cat. no. HBAD-1007). To
monitor autophagic flux, rat podocytes were grown to 80%
confluence, and transfected with adenoviral particles at 50 MOI in
serum-free medium at 37°C for 24 h. After 72-h NG, HG or AGF
treatment, the cells were fixed with 4% paraformaldehyde for 30 min
and the cell nuclei was stained with DAPI solution for 10 min at
room temperature. Fluorescent images were captured with a confocal
microscope (Carl Zeiss, Inc.). Images were acquired using a
microscopic system (magnification, ×630). GFP fluorescence is
quenched under acidic conditions, and an increase in both yellow
and red spots represent enhanced autophagic flux. The number of
autolysosomes (red dots) and autophagosomes (yellow dots) was
quantified to evaluate autophagic flux using Image-J software
(version 1.51j8; National Institutes of Health).
Measurement of cellular ROS
levels
The level of intracellular ROS in the rat podocytes
was assessed using a 2′,7′-Dichlorofluorescin diacetate (DCFH-DA)
fluorescent probe (cat. no. D6470; Beijing Solarbio Science &
Technology Co., Ltd.). Following 72 h NG, HG or AGF treatment, the
podocytes were stained with 10 µM DCFH-DA for 60 min at room
temperature in the dark. The rat podocytes were digested with 0.25%
pancreatin and washed with PBS. The mean fluorescence intensity was
analyzed by FACSCanto II flow cytometry (BD Biosciences, Inc.). A
total of 10,000 events were collected for each group, and the
relative ROS levels were analyzed using FlowJo software (version
10.0.7r2; FlowJo LLC).
Western blotting
The cells were resuspended in RIPA lysis buffer
(Beyotime Institute of Biotechnology). The protein concentrations
were determined using a BCA kit (Beyotime Institute of
Biotechnology) and an equal amount of total protein (30 µg) was
separated by 10 or 12% SDS-PAGE and transferred to PVDF membranes
(EMD Millipore). The membranes were blocked in 5% non-fat milk in
TBST buffer for 30 min and incubated with the primary antibodies
overnight at 4°C. The following primary antibodies were used:
anti-TNF-α antibody (1:500; cat. no. 11948T; Cell Signaling
Technology, Inc.), anti-LC3B antibody (1:1,000; cat. no. 3868s;
Cell Signaling Technology, Inc.), anti-Beclin-1 antibody (1:1,000;
cat. no. 3495s; Cell Signaling Technology, Inc.), anti-RAGE
antibody (1:1,000; cat. no. sc-80652; Santa Cruz Biotechnology,
Inc.), anti-phosphorylated (p)-NF-κB p65 antibody (1:1,000; cat.
no. 3033; Cell Signaling Technology, Inc.), anti-NF-κB p65 antibody
(1:1,000; cat. no. 4765; Cell Signaling Technology, Inc.),
anti-p-IκB-α antibody (1:1,000; cat. no. 9246s; Cell Signaling
Technology, Inc.), anti-IκB-α antibody (1:1,000; cat. no. 9242s;
Cell Signaling Technology, Inc.), anti-IL-1β antibody (1:500; cat.
no. 12703T; Cell Signaling Technology, Inc.), and anti-GAPDH
antibody (1:1,000; cat. no. 5174; Cell Signaling Technology Inc.).
The next day, the membranes were then probed with corresponding
HRP-conjugated goat anti-rabbit IgG (1:2,000; cat. no. 14708; Cell
Signaling Technology, Inc.) and anti-mouse IgG (1:2,000; cat. no.
7076; Cell Signaling Technology, Inc.) secondary antibodies for 90
min at room temperature. The bands were visualized using an ECL
reagent (Biological Industries, Inc.) and recorded on X-ray film.
The densitometry of each band was analyzed using ImageJ software
(version 1.51j8; National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from rat podocytes from each
group using RNAiso reagent (Beijing Solarbio Science &
Technology Co., Ltd.). The concentration of total RNA was
quantified using a NanoDrop™ 2000 spectrometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). The RNA was reverse
transcribed to synthesize cDNA using a PrimeScript™ RT reagent kit
(Takara Bio, Inc.; 16°C for 30 min, 42°C for 30 min and 85°C for 5
min). The amplification of cDNA was performed with a TB Green PCR
kit (Beijing Solarbio Science & Technology Co., Ltd.). qPCR was
carried out on a LightCycler® 480 system (Roche
Diagnostics). The primers were designed by Sangon Biotech Co. Ltd.
The reaction conditions included an initial denaturation at 95°C
for 10 min, followed by 40 cycles at 95°C for 15 sec and at 60°C
for 60 sec. The primer sequences were as follows: GAPDH forward,
5′-GACATGCCGCCTGGAGAAAC-3′ and reverse, 5′-AGCCCAGGATGCCCTTTAGT-3′;
IL-1β forward, 5′-CTCACAGCAGCATCTCGACAAGAG-3′ and reverse,
5′-TCCACGGGCAAGACATAGGTAGC-3′; TNF-α forward,
5′-TCCACGGGCAAGACATAGGTAGC-3′ and reverse,
5′-GCTCCTCCGCTTGGTGGTTTG-3′; RAGE forward,
5′-CTGCCTCTGAACTCACAGCCAATG-3′ and reverse,
5′-TCCTGGTCTCCTCCTTCACAACTG-3′. GAPDH was used as a housekeeping
gene control. Relative gene expression were measured using the
2−∆∆Cq method (21).
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons between two groups were analyzed using Student's
t-test, and multiple comparisons were analyzed using one-way ANOVA
followed by Tukey's post hoc test. All graphs in this study were
generated with GraphPad Prism 6.0 (GraphPad Software. Inc.).
P<0.05 was considered to indicate the final version of the
manuscript.
Results
Impact of acute glucose fluctuations
on rat podocyte viability and apoptosis
The effect of AGF treatment on cell viability was
measured using a CCK-8 kit. The viability of cells in the HG group
significantly decreased compared with that of the NG group
(P<0.01). Moreover, cell viability in the AGF group was
significantly reduced compared with the NG and HG groups
(P<0.01). These data indicated that AGF significantly inhibited
the viability of rat podocytes (Fig.
1B). Changes in apoptosis were detected by flow cytometry, and
no significant difference between the groups was observed
(P>0.05; Fig. 1C and D).
Acute glucose fluctuation promotes rat
podocyte autophagy
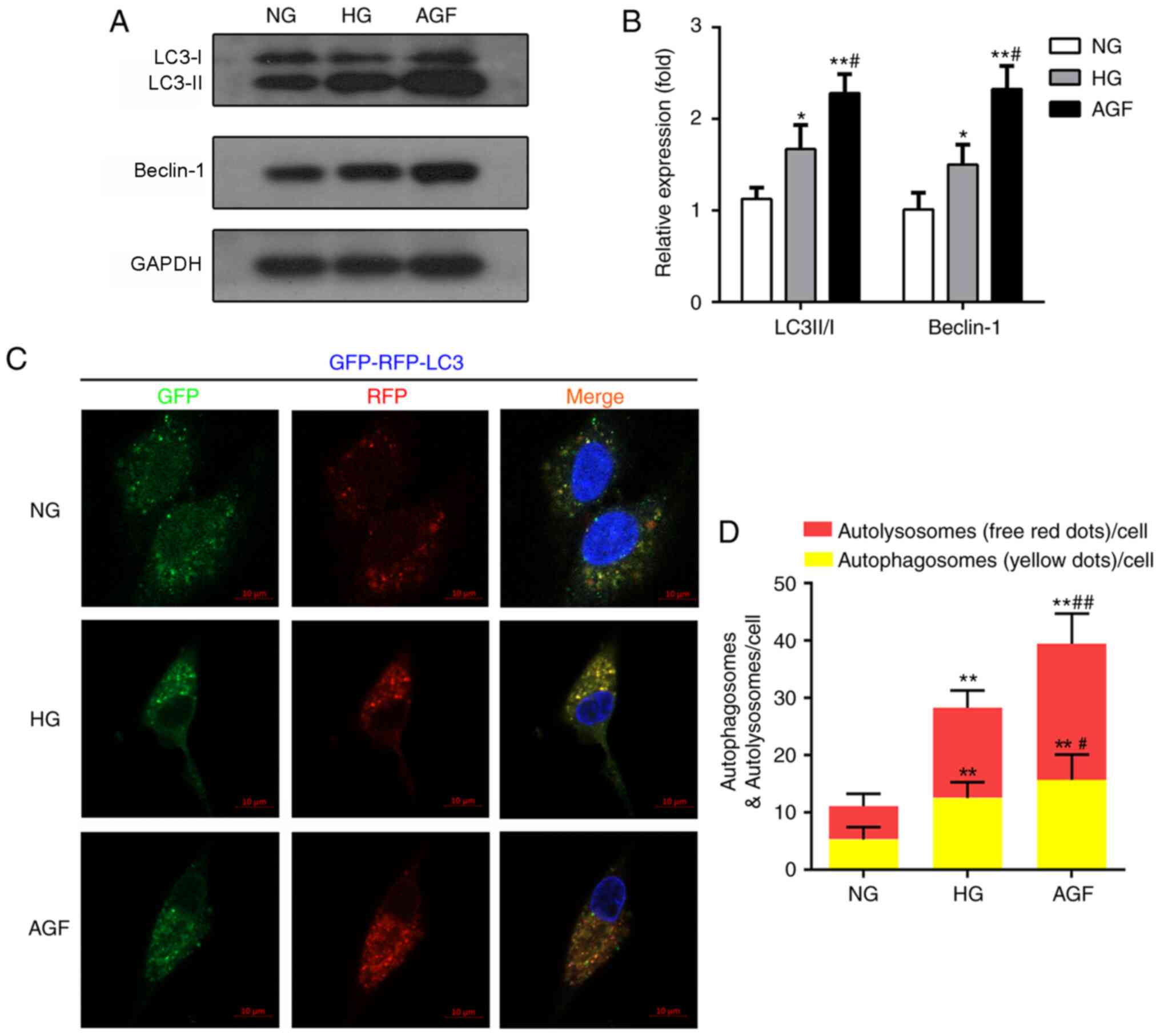
Autophagy is also involved in the maintenance of
podocyte function and cell homeostasis (22). To define the AGF-induced alterations
in autophagy, the expression levels of autophagy biomarkers
(LC3II/I and Beclin-1) were evaluated by western blotting. The AGF
group exhibited enhanced LC3II/I and Beclin-1 expression levels,
compared with the NG (P<0.01) and HG groups (P<0.05; Fig. 2A and B). To further confirm the
effects of AGF on autophagy flux, rat podocytes were transfected
with an mRFP-GFP-LC3B adenovirus vector, then treated with NG, HG
or AGF and observed by confocal microscopy. Compared with the NG
group, HG group exhibited a significant increase in the abundance
of yellow and red puncta (P<0.01), suggesting an increase in
autophagy in HG-treated cells. The number of yellow and red puncta
was further enhanced by AGF compared with the HG group (P<0.05;
Fig. 2C and D).
Acute glucose fluctuation promotes
IL-1β and TNF-α generation in rat podocytes
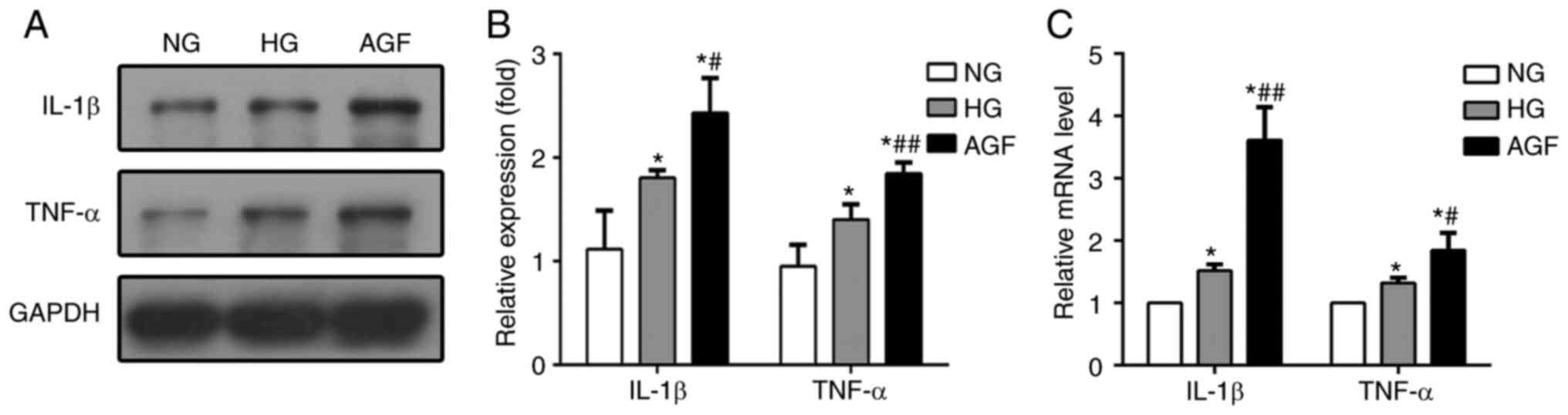
In our previous study, AGF aggravated inflammatory
lesions in diabetic rats (19). To
test whether AGF could elevate the generation of inflammatory
lesions in vitro, we determined the mRNA and protein
expression of levels of TNF-α and IL-1β in treated rat podocytes.
The expression of both of these pro-inflammatory cytokines were
higher in the HG and AGF group compared with the NG group
(P<0.05), and significantly increased in the AGF group compared
with the HG group, both at the mRNA and protein levels (P<0.05;
Fig. 3). These results indicated
that AGF aggravated the inflammatory response in rat podocytes.
Acute glucose fluctuation increases
ROS levels and activates the RAGE/NF-κB signaling pathway in rat
podocytes
TNF-α plays a central role in mediating renal injury
though the induction of ROS production (14). After 72 h of AGF treatment, the
levels of intracellular ROS in rat podocytes was significantly
increased, compared with the NG and HG groups (P<0.05; Fig. 4A and B).
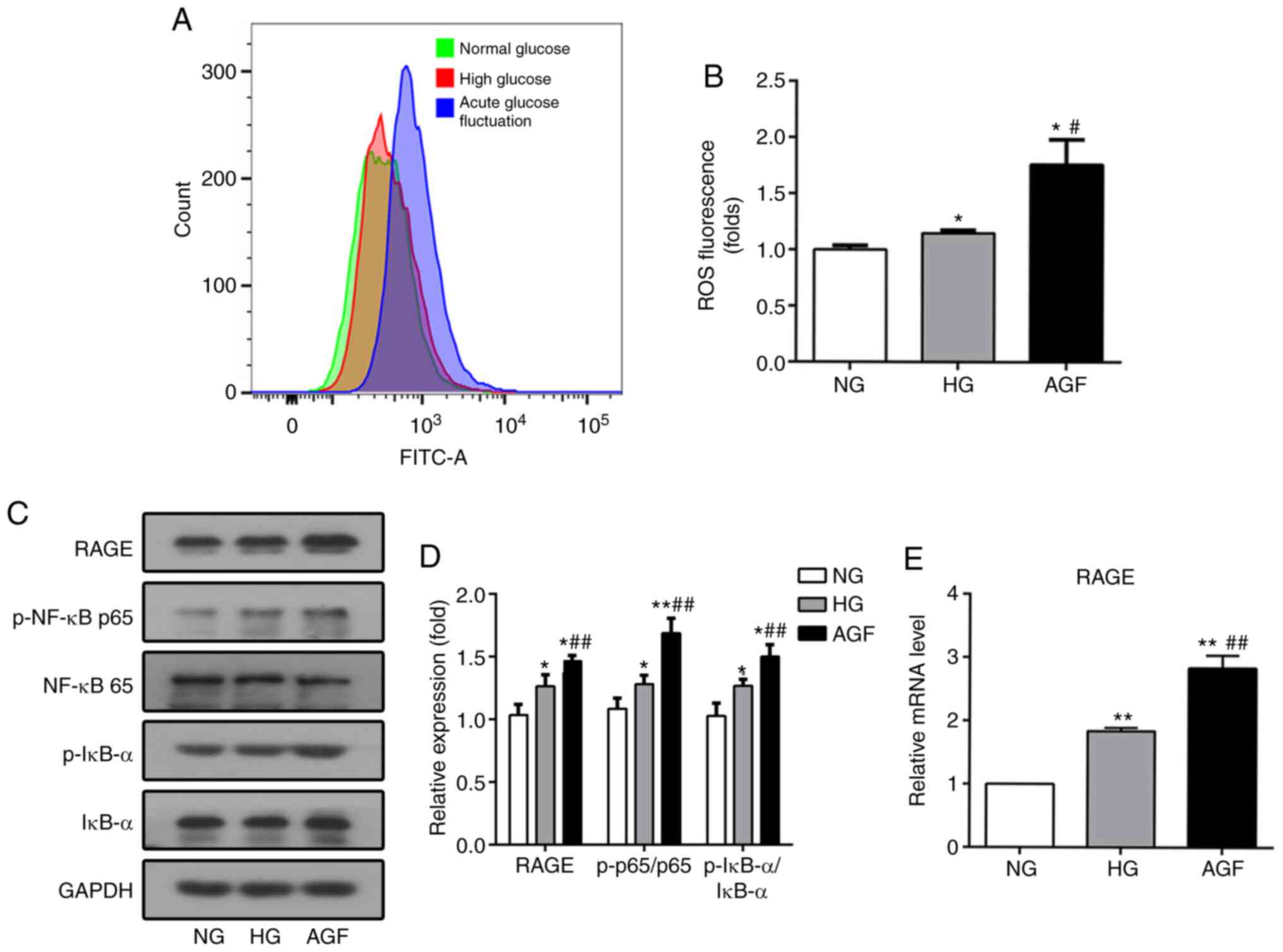 | Figure 4.AGF increases the level of
intracellular ROS and RAGE expression, as well as enhances the
phosphorylation of p-NF-κB p65 and p-IκB-α expression in rat
podocytes. (A and B) Flow cytometry analysis of intracellular ROS
production. (C and D) The level of RAGE, p-NF-κB p65, NF-κB p65,
p-IκB-α, and IκB-α were examined by western blotting. (E) The level
of RAGE mRNA was examined by qRT-PCR. Data are presented as the
mean ± SD. n=3. *P<0.05; **P<0.01 vs. NG;
#P<0.05, ##P<0.01 vs. HG. AGF, acute
glucose fluctuation; NG, normal glucose; HG, high glucose; FITC,
fluorescein isothiocyanate; RAGE, receptor for advanced glycation
end products. |
In addition, inflammatory stimulation can increase
the levels of RAGE transcription via the binding of NF-κB to the
RAGE promoter region, which results in increased RAGE expression
(23). To confirm whether AGF can
regulate the RAGE/NF-κB signaling pathway, the expression levels of
RAGE, p-NF-κB p65, NF-κB p65, p-IκBα, and IκBα were determined. AGF
induced a significant increase in RAGE expression compared with the
NG or HG group (P<0.05; Fig. 4C and
E). Phosphorylation of NF-κB p65 and IκBα was significantly
upregulated following AGF treatment compared with the NG or HG
groups (P<0.05; Fig. 4C and D).
This finding suggested that could AGF activate the NF-κB signaling
pathway in rat podocytes.
Downregulation of intracellular ROS
levels inhibits the NF-κB/RAGE signaling pathway in rat
podocytes
NAC, a ROS inhibitor, was used to further confirm
whether ROS affects the NF-κB/RAGE signaling pathway in response to
AGF. NAC pretreatment reversed the effect of AGF treatment, as cell
viability was found to be significantly higher compared with that
of the AGF group (P<0.01; Fig.
5A). Moreover, the level of AGF-induced intracellular ROS was
significantly reduced following pretreatment with NAC (P<0.01;
Fig 5B and C). Subsequent western
blot and RT-qPCR analysis demonstrated that NAC significantly
inhibited the phosphorylation of NF-κB p65 and IκBα (P<0.05) and
decreased RAGE expression induced by AGF (P<0.05; Fig. 5D-F). These results confirm the role
of ROS in the NF-κB/RAGE signaling pathway.
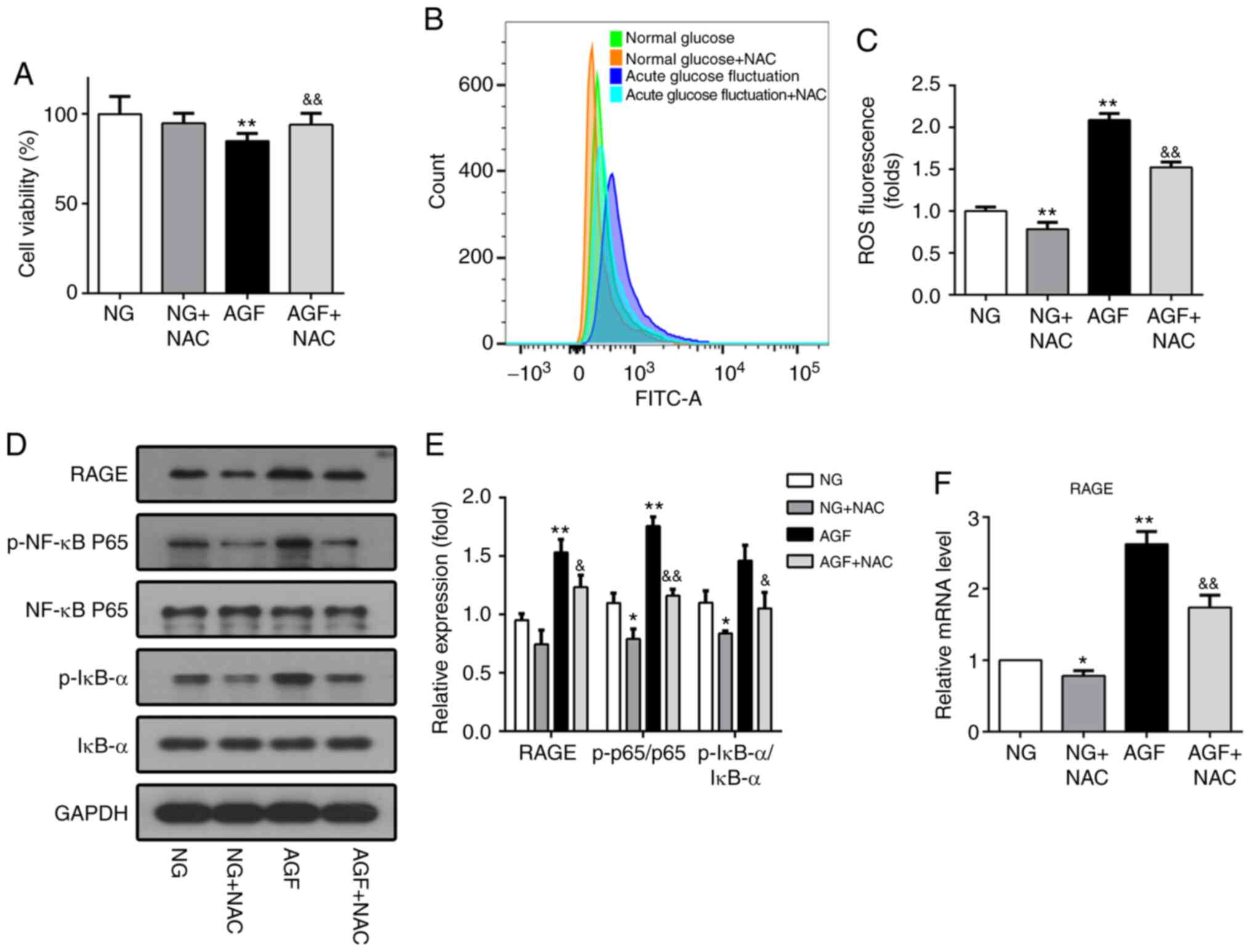 | Figure 5.Downregulation of intracellular ROS
levels inhibits activation of the NF-κB/RAGE signaling pathway in
rat podocytes. (A) Cell viability was measured using a
Cell-Counting-Kit-8 assay. (B and C) ROS generation induced by AGF
in rat podocytes with or without 5 mM NAC pretreatment. (D and E)
Protein levels of RAGE, p-NF-κB p65, NF-κB p65, p-IκB-α, and IκB-α.
(F) mRNA levels of RAGE. Data are presented as the mean ± SD. n=3.
*P<0.05, **P<0.01 vs. NG; &P<0.05,
&&P<0.01 vs. HG. AGF, acute glucose
fluctuation; NG, normal glucose; HG, high glucose; FITC,
fluorescein isothiocyanate; RAGE, receptor for advanced glycation
end products; ROS, reactive oxygen species; NAC,
N-Acetyl-L-cysteine; p, phosphorylated. |
Inhibition of NF-κB by PDTC reduces the AGF-induced
increase in RAGE expression in rat podocytes. PDTC is an inhibitor
of the NF-κB signaling pathway. To further investigate the
relationship between AGF-induced increases in RAGE expression and
activation of the NF-κB signal pathway, PDTC was used to inhibit
the NF-κB signal pathway in rat podocytes. The effect of PDTC on
cell viability was first measured. The AGF+PDTC group exhibited
significantly improved cell viability compared with that of the AGF
group (P<0.05; Fig. 6A). Next,
the level of RAGE expression, as well as the ratio of p-NF-κB
p65/NF-κB p65 and p-IκB-a/IκBα were measured. The results showed
that PDTC significantly inhibited the phosphorylation of NF-κB p65
and IκB-α (P<0.05), as well as the upregulation of RAGE protein
expression induced by AGF (P<0.05; Fig. 6B-D). These data indicated that
activation of the NF-κB signaling pathway was required for
AGF-induced cell damage and increased RAGE expression levels in rat
podocytes.
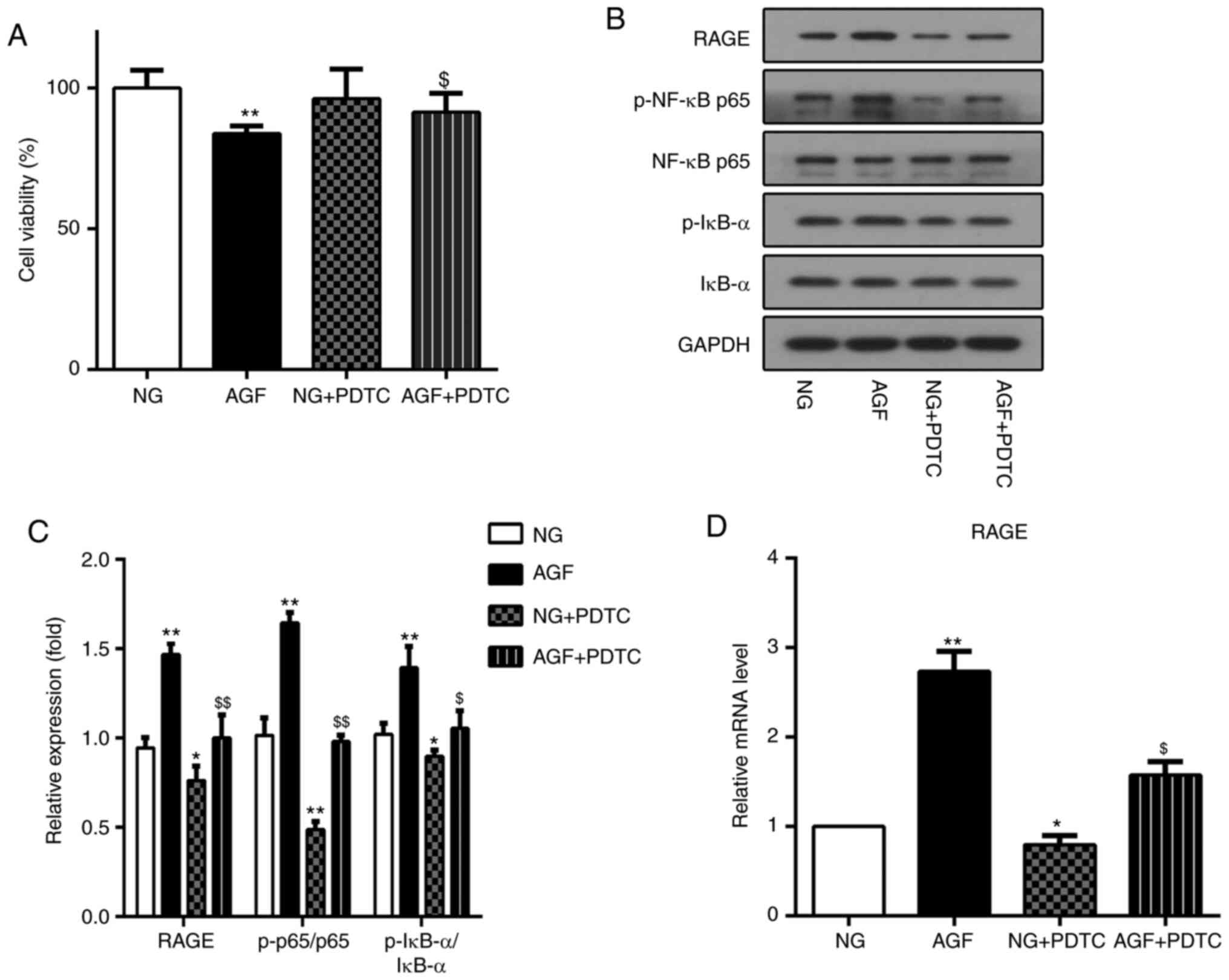 | Figure 6.Inhibition of NF-κB by PDTC reduced
acute glucose fluctuation-induced increases in RAGE expression in
rat podocytes. (A) Cell viability was measured using a CCK-8 assay.
(B and C) Protein levels of RAGE, p-NF-κB p65, NF-κB p65, p-IκB-α
and IκB-α. (D) mRNA levels of RAGE. Data are presented as the mean
± SD. n=3. *P<0.05, **P<0.01 vs. NG group;
$P<0.05, $$P<0.01 vs. AGF. AGF, acute
glucose fluctuation; NG, normal glucose; HG, high glucose; FITC,
fluorescein isothiocyanate; RAGE, receptor for advanced glycation
end products; PTDC, pyrrolidinedithiocarbamate; p,
phosphorylated. |
Discussion
DN pathogenesis is highly complex and poorly
understood. Hyperglycemia represents a major contributor in the
occurrence of DN; however, current therapies remain limited and
cannot completely prevent DN progression. Increasing evidence
suggests that AGF induces more severe renal damage than sustained
hyperglycemia (19,24). To confirm the role of AGF
experimentally, our previous study involved changing the blood
glucose levels of diabetic rats acutely through the injection of
glucose and insulin (20). In this
study, an in vitro model of AGF-induced podocyte injury. To
simulate changes in the blood glucose of diabetic rat podocytes
in vitro, the glucose concentration of the culture medium
was altered at different time points.
Podocytes play an important role in the maintenance
of the glomerular filtration barrier, and the decrease in podocyte
number is associated with the progression of proteinuria (25). Therefore, the effect of glucose
fluctuation on cell proliferation and apoptosis was examined. The
results showed that there was a significant inhibition in cellular
proliferation following AGF treatment. A decrease in viable cells
was observed after 72 h of exposure to changes in the glucose
concentration in the culture medium. However, the apoptosis rate
measured by flow cytometry was not statistically significant, which
is similar to the results of a study using human umbilical vein
endothelial cells, which found that AGF failed to induce cell
apoptosis (26). Extensive studies
have indicated that apoptosis and proliferation are mediated by
increased levels of ROS (27). ROS
plays a dual role in tissue injury, as moderate levels of ROS
promote cell survival and proliferation, whereas excessive ROS
production can directly induce necrocytosis (28). In the present study, AGF generated
more severe oxidative stress with increased level of intracellular
ROS compared to hyperglycemia (Fig.
4).
Current evidence suggests that autophagy may mediate
resistance to apoptosis (29).
Similar to apoptosis, autophagy also plays a vital role in cellular
proliferation and survival, and dysregulated autophagy activation
has been described in several diseases (30). It is well-established that Beclin-1
and LC3 are important proteins in autophagy (31). The present results indicated that
hyperglycemia caused an increase in the LC3-II/LC3-I ratio and a
significant increase in the level of Beclin-1, demonstrating
enhanced autophagy. As a cytoprotective function, autophagy is
accompanied by ROS production (32). Podocytes may respond to higher ROS
levels resulting from AGF by triggering autophagy protective
mechanisms to prevent apoptosis. Therefore, AGF induces more
autophagy in rat podocytes.
Additionally, various inflammatory responses were
involved in AGF-induced podocyte injury. AGF significantly
increased the generation of inflammatory lesions, including the
production of IL-1β and TNF-α, consistent with the findings of our
previous study (20). TNF-α has
been confirmed to induce intracellular ROS production, but the
detailed mechanisms remain unclear (33). A previous study indicated that an
increase in ROS and TNF-α, in addition to inducing tissue damage,
also contributes to the activation of NF-κB (34). An increase in the NADPH oxidase
complex through NF-κB activation ultimately leads to enhanced ROS
production and further NF-κB activation (35). Ultimately, such activation generates
a vicious cycle that contributes to the progressive loss of renal
function (36). AGF could also
augment inflammatory responses through the activation of the NF-κB
signaling pathway.
RAGE, the specific receptor for AGEs, plays a vital
role in oxidative stress. Interactions between RAGE and AGEs
activate various signaling pathways and subsequently induce
oxidative stress and an inflammatory response, which leads to the
pathological development of DN (37). Due to long-term hyperglycemia,
diabetic patients accumulate a larger number of AGEs in
vivo, and an excessive amount can increase RAGE expression. In
addition, TNF-α is potent inducer of RAGE expression, and
ROS-mediated RAGE induction occurs via NF-κB activation (38). Thus, we investigated whether AGF
could upregulate RAGE expression. As expected, hyperglycemia
increased the level of RAGE expression in rat podocytes, which was
further promoted by AGF. This effect is likely related to higher
levels of ROS, and the downstream transcription factor, NF-κB.
In conclusion, the present study demonstrates that
acute glucose fluctuation induces rat podocyte injury by
aggravating oxidative stress, enhancing the inflammatory response,
and activating the RAGE/NF-κB signaling pathway. Therefore, the
maintenance of a stable range of blood glucose fluctuation may help
delay the development of DN.
Acknowledgements
Not applicable.
Funding
This work was supported by The National Natural
Science Foundation of China (grant no. 81973767).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH and HW developed the aim of the study,
participated in its design and coordination, and helped draft the
manuscript. WF, YL, and HL contributed to the acquisition and
interpretation of the data. WC provided critical review and
substantially revised the manuscript. ZH and HW had seen all the
raw data and confirmed the authenticity of data shown in the
present manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raja P, Maxwell AP and Brazil DP: The
Potential of Albuminuria as a Biomarker of Diabetic Complications.
Cardiovasc Drugs Ther. Jul 17–2020.(Epub ahead of print). doi:
10.1007/s10557-020-07035-4. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lagies S, Pichler R, Bork T, Kaminski MM,
Troendle K, Zimmermann S, Huber TB, Walz G, Lienkamp SS and
Kammerer B: Impact of Diabetic Stress Conditions on Renal Cell
Metabolome. Cells. 8:82019. View Article : Google Scholar
|
|
3
|
Kato M and Natarajan R: Epigenetics and
epigenomics in diabetic kidney disease and metabolic memory. Nat
Rev Nephrol. 15:327–345. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bork T, Liang W, Yamahara K, Lee P, Tian
Z, Liu S, Schell C, Thedieck K, Hartleben B, Patel K, et al:
Podocytes maintain high basal levels of autophagy independent of
mtor signaling. Autophagy. 16:1932–1948. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nishi H and Nangaku M: Podocyte
lipotoxicity in diabetic kidney disease. Kidney Int. 96:809–812.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Antony V, Wang Y, Wu G and Liang
G: Pattern recognition receptor-mediated inflammation in diabetic
vascular complications. Med Res Rev. 40:2466–2484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsieh CF, Liu CK, Lee CT, Yu LE and Wang
JY: Acute glucose fluctuation impacts microglial activity, leading
to inflammatory activation or self-degradation. Sci Rep. 9:8402019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang R, Loux T, Tang D, Schapiro NE,
Vernon P, Livesey KM, Krasinskas A, Lotze MT and Zeh HJ III: The
expression of the receptor for advanced glycation endproducts
(RAGE) is permissive for early pancreatic neoplasia. Proc Natl Acad
Sci USA. 109:7031–7036. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chtourou Y, Aouey B, Kebieche M and Fetoui
H: Protective role of naringin against cisplatin induced oxidative
stress, inflammatory response and apoptosis in rat striatum via
suppressing ROS-mediated NF-κB and P53 signaling pathways. Chem
Biol Interact. 239:76–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang Q, Zhan L, Cao H, Li J, Lyu Y, Guo
X, Zhang J, Ji L, Ren T, An J, et al: Increased mitochondrial
fission promotes autophagy and hepatocellular carcinoma cell
survival through the ROS-modulated coordinated regulation of the
NFκB and TP53 pathways. Autophagy. 12:999–1014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim KA, Shin YJ, Akram M, Kim ES, Choi KW,
Suh H, Lee CH and Bae ON: High glucose condition induces autophagy
in endothelial progenitor cells contributing to angiogenic
impairment. Biol Pharm Bull. 37:1248–1252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saha S, Panigrahi DP, Patil S and Bhutia
SK: Autophagy in health and disease: A comprehensive review. Biomed
Pharmacother. 104:485–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lenoir O, Jasiek M, Hénique C, Guyonnet L,
Hartleben B, Bork T, Chipont A, Flosseau K, Bensaada I, Schmitt A,
et al: Endothelial cell and podocyte autophagy synergistically
protect from diabetes-induced glomerulosclerosis. Autophagy.
11:1130–1145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishida K, Watanabe H, Ogaki S, Kodama A,
Tanaka R, Imafuku T, Ishima Y, Chuang VT, Toyoda M, Kondoh M, et
al: Renoprotective effect of long acting thioredoxin by modulating
oxidative stress and macrophage migration inhibitory factor against
rhabdomyolysis-associated acute kidney injury. Sci Rep.
5:144712015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Z, Sun B, Huang S, Zhu C and Bian M:
Glycemic variability: Adverse clinical outcomes and how to improve
it? Cardiovasc Diabetol. 19:1022020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monnier L, Colette C and Owens DR:
Glycemic variability: The third component of the dysglycemia in
diabetes. Is it important? How to measure it? J Diabetes Sci
Technol. 2:1094–1100. 2008.PubMed/NCBI
|
|
17
|
Ceriello A, Esposito K, Piconi L, Ihnat
MA, Thorpe JE, Testa R, Boemi M and Giugliano D: Oscillating
glucose is more deleterious to endothelial function and oxidative
stress than mean glucose in normal and type 2 diabetic patients.
Diabetes. 57:1349–1354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ying C, Wang S, Lu Y, Chen L, Mao Y, Ling
H, Cheng X and Zhou X: Glucose fluctuation increased mesangial cell
apoptosis related to AKT signal pathway. Arch Med Sci. 15:730–737.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ying C, Zhou X, Chang Z, Ling H, Cheng X
and Li W: Blood glucose fluctuation accelerates renal injury
involved to inhibit the AKT signaling pathway in diabetic rats.
Endocrine. 53:81–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Deng J, Chen L, Ding K and Wang Y:
Acute glucose fluctuation induces inflammation and neurons
apoptosis in hippocampal tissues of diabetic rats. J Cell Biochem
jcb. 29523:2019.
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Zhu Q, Zheng R, Yan J, Wei M, Fan Y,
Deng Y and Zhong Y: Puerarin Attenuates Diabetic Nephropathy by
Promoting Autophagy in Podocytes. Front Physiol. 11:732020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uchida T, Shirasawa M, Ware LB, Kojima K,
Hata Y, Makita K, Mednick G, Matthay ZA and Matthay MA: Receptor
for advanced glycation end-products is a marker of type I cell
injury in acute lung injury. Am J Respir Crit Care Med.
173:1008–1015. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang ZY, Miao LF, Qian LL, Wang N, Qi MM,
Zhang YM, Dang SP, Wu Y and Wang RX: Molecular Mechanisms of
Glucose Fluctuations on Diabetic Complications. Front Endocrinol
(Lausanne). 10:6402019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nicholas SB, Basgen JM and Sinha S: Using
stereologic techniques for podocyte counting in the mouse: Shifting
the paradigm. Am J Nephrol. 33 (Suppl 1):1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo J, Sang Y, Yin T, Wang B, Yang W, Li
X, Li H and Kang Y: miR-1273g-3p participates in acute glucose
fluctuation-induced autophagy, dysfunction, and proliferation
attenuation in human umbilical vein endothelial cells. Am J Physiol
Endocrinol Metab. 310:E734–E743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amidi S, Hashemi Z, Motallebi A, Nazemi M,
Farrokhpayam H, Seydi E and Pourahmad J: Identification of
(Z)-2,3-Diphenylacrylonitrileas Anti-Cancer Molecule in Persian
Gulf Sea Cucumber Holothuria parva. Mar Drugs. 15:152017.
View Article : Google Scholar
|
|
28
|
Tang S, Hou Y, Zhang H, Tu G, Yang L, Sun
Y, Lang L, Tang X, Du YE, Zhou M, et al: Oxidized ATM promotes
abnormal proliferation of breast CAFs through maintaining
intracellular redox homeostasis and activating the PI3K-AKT,
MEK-ERK, and Wnt-β-catenin signaling pathways. Cell Cycle.
14:1908–1924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma H, Su L, Yue H, Yin X, Zhao J, Zhang S,
Kung H, Xu Z and Miao J: HMBOX1 interacts with MT2A to regulate
autophagy and apoptosis in vascular endothelial cells. Sci Rep.
5:151212015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Irace C, Misso G, Capuozzo A, Piccolo M,
Riccardi C, Luchini A, Caraglia M, Paduano L, Montesarchio D and
Santamaria R: Antiproliferative effects of ruthenium-based
nucleolipidic nanoaggregates in human models of breast cancer in
vitro: Insights into their mode of action. Sci Rep. 7:452362017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu T, Xia Y, Li J, Li S, Feng J, Wu L,
Zhang R, Xu S, Cheng K, Zhou Y, et al: Shikonin Attenuates
Concanavalin A-Induced Acute Liver Injury in Mice via Inhibition of
the JNK Pathway. Mediators Inflamm. 2016:27483672016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li P, Zheng X, Shou K, Niu Y, Jian C, Zhao
Y, Yi W, Hu X and Yu A: The iron chelator Dp44mT suppresses
osteosarcoma's proliferation, invasion and migration: In vitro and
in vivo. Am J Transl Res. 8:5370–5385. 2016.PubMed/NCBI
|
|
33
|
Zhao W, Feng H, Guo S, Han Y and Chen X:
Danshenol A inhibits TNF-α-induced expression of intercellular
adhesion molecule-1 (ICAM-1) mediated by NOX4 in endothelial cells.
Sci Rep. 7:129532017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Amaral LSB, Souza CS, Volpini RA, Shimizu
MHM, de Bragança AC, Canale D, Seguro AC, Coimbra TM, de Magalhães
ACM and Soares TJ: Previous Exercise Training Reduces Markers of
Renal Oxidative Stress and Inflammation in Streptozotocin-Induced
Diabetic Female Rats. J Diabetes Res. 2018:61703522018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmelzer C, Lorenz G, Rimbach G and
Döring F: In Vitro Effects of the Reduced Form of Coenzyme Q(10) on
Secretion Levels of TNF-alpha and Chemokines in Response to LPS in
the Human Monocytic Cell Line THP-1. J Clin Biochem Nutr. 44:62–66.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guijarro C and Egido J: Transcription
factor-kappa B (NF-kappa B) and renal disease. Kidney Int.
59:415–424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cai W, Li J, Xu JX, Liu Y, Zhang W, Xiao
JR, Zhu LY and Liu JY: Association of 2184AG Polymorphism in the
RAGE Gene with Diabetic Nephropathy in Chinese Patients with Type 2
Diabetes. J Diabetes Res. 2015:3102372015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mukherjee TK, Mukhopadhyay S and Hoidal
JR: The role of reactive oxygen species in TNFalpha-dependent
expression of the receptor for advanced glycation end products in
human umbilical vein endothelial cells. Biochim Biophys Acta.
1744:213–223. 2005. View Article : Google Scholar : PubMed/NCBI
|