Introduction
Adenoid cystic carcinoma (ACC) is one of the most
common types of cancers of the salivary gland. A previous study
showed that ACC is more frequently found in exocrine glands, such
as the lacrimal gland, nasal cavity, tracheobronchial tree and
prostate (1). ACC grows slowly
and is usually diagnosed at the advanced stage of the disease
(2). However, ACC may expand and
gradually metastasize along the nerves, which affects its resection
and the success of clinical surgery (3,4).
In addition, drug resistance of ACC is commonly noted for various
chemotherapeutic drugs, which affects the efficacy of treatment and
causes discomfort to patients with this disease (5).
Cluster of differentiation (CD)133 was initially
identified as a potential marker of tumor stem cells (6). Previous studies have shown that
CD133 can regulate cell proliferation and differentiation and
promote tumor growth and metastasis (7,8).
CD133 may be used as a marker of various stem and tumor cells and
can mediate cell signaling transduction. CD133 activates the
PI3K/AKT, AKT/Wnt and other signaling pathways and affects the
behavior of CD133+ cells, thereby playing a major role
in cancer therapy (9,10). In addition, CD133 is also involved
in the regulation of tumor resistance. Long-term chemotherapy leads
to a significant increase in CD133 expression (11). Targeting CD133 can reverse drug
resistance in colorectal cancer via the AKT/NF-κB/multidrug
resistance protein (MDR)1 pathway (12). In gastric cancer, inhibition of
CD133 overcomes 5-fluorouracil (5-FU) resistance by inhibiting the
PI3K/AKT/mTOR signaling pathway and the autophagy of
CD133+ gastric cancer cells (10). Therefore, CD133 can affect the
cellular resistance of tumor cells. However, to the best of the
authors' knowledge, the effects of CD133 on cell drug resistance in
ACC have not been reported to date.
It has been shown that CD133 is highly expressed in
ACC cells (13). In tumor samples
from patients with ACC, the positive expression of CD133 is
correlated with vasculogenic mimicry formation, local recurrence,
distant metastasis and poor prognosis and it has been demonstrated
that CD133+ tumor stem cell-like cells promote the
migration and invasion of ACC cells and induce vasculogenic mimicry
formation (14). However, the
effects of CD133 on the sensitivity of ACC cells to drugs and its
precise mechanism remain to be elucidated.
In the present study, the association between the
expression levels of CD133 in ACC and the sensitivity of cancer
cells to chemotherapeutic drugs was assessed and the mechanism
involved in this process was investigated. These results may
provide a theoretical basis for improving the efficacy of ACC
chemotherapeutic treatment.
Materials and methods
Cell culture
The ACC cell line KOA-1 was purchased from The Cell
Bank of Type Culture Collection of The Chinese Academy of Sciences.
The cells were incubated in DMEM supplemented with 10% FBS (both
from Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. The drug-resistant cell strains KOA-1/5-FU and
KOA-1/PYM (pingyangmycin) were purchased from Shanghai Gefan
Biotechnology Co., Ltd. The KOA-1/5-FU and KOA-1/PYM cell lines
were generated by an intermittent and stepwise method (15). In brief, the parental KOA-1 cells
were initially incubated with 5-FU and PYM (the concentration of
5-FU and PYM used were the IC50) for 4 days, and then
cultured in drug-free medium for 3–4 days at 37°C until the
recovery of normal cell viability. The 5-FU and PYM treatment was
performed six times during the induction period, and then 5-FU and
PYM -resistant clones were harvested. Drug resistance was
maintained in KOA-1/5-FU and KOA-1/PYM cells by supplementation of
culture media with 5-FU and PYM at a final concentration of 2
nmol/l.
Western blotting
Total protein from cells were extracted using RIPA
lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.).
The protein concentration was determined with the application of a
BCA assay kit (Beyotime Institute of Biotechnology). Afterwards,
10% SDS-PAGE gels were prepared to separate proteins (30 µg each
lane), and then the latter were transferred onto polyvinylidene
difluoride (PVDF) membranes (EMD Millipore). Then, the PVDF
membrane was fixed with 5% skimmed milk powder at room temperature
for 1.5 h. PVDF membranes were incubated with primary antibodies,
including anti-CD133 (1:1,000; cat. no. ab222782; Abcam), anti-MDR1
(1:1,000; cat. no. ab235954; Abcam), anti-MRP1 (1:1,000; cat. no.
ab3368; Abcam) and anti-GAPDH (1:1,000; cat. no. ab8245; Abcam),
and were removed the second day. In addition, goat anti-mouse IgG
antibody (1:5,000, cat. no. AP127F; Sigma-Aldrich; Merck KGaA) were
prepared for incubation with PVDF membranes at room temperature for
1.5 h. The protein bands were visualized and analyzed using a
chemiluminescence system (Bio-Rad Laboratories, Inc.) and
semi-quantified using ImageJ software (version 1.46; National
Institutes of Health).
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay (Dojindo Molecular Technologies,
Inc.) was used to measure cell viability. The cells were seeded at
a density of 1×104 cells per well into 96-well plates.
The cells were transfected and treated with 5-FU or PYM. Then, a
total of 10 µl CCK-8 solution was added to the cells, which were
incubated at 37°C for 4 h. The absorbance was subsequently measured
at 450 nm using a microplate reader (Molecular Devices, LLC).
Wound healing assay
Cell migration was determined using a wound healing
assay. Briefly, transfected cells were plated in 12-well plates at
a density of 1×105 cells/well. Once cells reached 80%
confluence, the medium was replaced with serum-free DMEM and cells
were incubated at 37°C overnight before initiating the experiment.
Subsequently, a wound was created on the surface of the cell
monolayer using a 200-µl pipette tip. The cells were then rinsed
twice with serum-free medium in order to remove free-floating cells
and debris. An inverted light microscope (magnification, ×200;
BX51; Olympus Corporation) was used to monitor cells at the edges
of the scratch. The percentage of wound closure was determined
according to the following equation: [(Ai-At)/Ai] ×100, where Ai
represented the initial area of the wound at 0 h and At represented
the area of the wound after 24 h.
Transwell assay
The invasion assay was performed by precoating the
upper chambers of 24-well Transwell plates (8-µm pore size) with
Matrigel (Sigma-Aldrich; Merck KGaA), according to the
manufacturer's instructions. Cell suspensions were then added to
the Transwell chambers at a density of 1–1.5×106
cells/ml. The lower chamber was filled with 0.5 ml DMEM containing
FBS. The cells were incubated for 24 h at 37°C, then the cells
remaining in the upper chamber were removed using cotton swabs. The
invasive cells in the lower chamber were fixed with
paraformaldehyde for 15 min at room temperature and stained with
0.1% crystal violet for 30 min at room temperature. Finally, the
cells were counted using a light microscope (magnification,
×200).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells (3×104
cells/well) using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and an Invitrogen SuperScript™ III Reverse
Transcriptase kit (Thermo Fisher Scientific, Inc.) was used for
first-strand cDNA synthesis. The mRNA levels were quantified in
triplicate using a real-time PCR system with the Brilliant
SYBR-Green qPCR Kit (Stratagene; Agilent Technologies, Inc.). The
aforementioned steps were performed following the manufacturer's
instructions. The sequences of the primers were as follows: CD133
(sense, 5′-GGATTATTCTATGCTGTGTCCTG-3′ and antisense,
5′-TGCCACAAAACCATAGAAGAT-3′), MDR1 (sense,
5′-CTCGTGCCCTTGTTAGACAGCCTCAT-3′ and antisense,
5′-GATGCGTGCCATGCTCCTTGACTCT-3′), multidrug resistance-associated
protein (MRP)1 (sense, 5′-GAGTGGCGGTGATGGTCCTCATGGT-3′ and
antisense, 5′-CACGGCTGACAGGTAGGCAGACTTCT-3′), GAPDH (sense,
5′-AGTGGTGGACCTGACCTGCCGTCTA-3′ and antisense,
5′-GGAGGAGTGGGTGTCGCTGTTGAAGT-3′). The following PCR thermocycling
conditions were used: 10 min at 95°C; followed by 47 cycles at 95°C
for 30 sec, 60°C for 30 sec and 72°C for 60 sec. Expression was
determined by the 2−ΔΔCq method (16). The experiment was repeated three
times.
Immunofluorescence (IF) staining
The cells were seeded at a density of
4×104 cells/well into 6-well plates and incubated
overnight. The cells were treated with the indicated
concentrations, fixed using 4% formaldehyde for 30 min at 25°C and
treated with 3% bovine serum albumin (BSA; Thermo Fisher
Scientific, Inc.) in phosphate buffered saline for 30 min. The
coverslips were incubated with rabbit anti-MDR1 (1:200; cat. no.
ab235954; Abcam) and anti-MRP1 antibodies (1:200; cat. no. ab3368;
Abcam) at 4°C overnight diluted in 3% BSA. Subsequently, an
immunofluorescent antibody (1:500; cat. no. A-21072; Alexa Fluor;
Invitrogen; Thermo Fisher Scientific, Inc.) was incubated with the
cells at 37°C for 2 h. DAPI was added to cells and incubation was
performed in the dark for 5 min at room temperature. The cells were
visualized using a fluorescence microscope equipped with
appropriate filter sets (magnification, ×200; Zeiss GmbH). Negative
controls contained cells without conjugated antibodies. The
integrated optical density values, which represented the staining
intensity, were calculated for the acquired images using Image-Pro
Plus 6.0 (Media Cybernetics, Inc.).
Small interfering RNA (siRNA)
transfection
siRNAs were synthesized and purified by Shanghai
GenePharma Co., Ltd. The transfection of siRNA CD133#1 and siRNA
CD133#2 was performed using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions at a final concentration of 100 nmol/l.
The transfection of siRNA negative control (NC) was performed using
the same conditions. The sequences used were: CD133#1 siRNA,
5′-GGCAGAUAGCAAUUUCAAGGACUTG-3′; CD133#2 siRNA,
5′-GGCUUGGAAUUAUGAAUUGCCUGCA-3′; and siRNA-NC,
5′-UUCUCCGAACGUGUCACGU-3′. Follow-up experiments were conducted 48
h after transfection.
Statistical analysis
SPSS version 21.0 (IBM Corp.) was used to perform
all statistical analyses and the data are presented as the mean ±
standard deviation. Statistical comparisons between groups were
made using a one-way ANOVA followed by a Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was repeated three times.
Results
Drug-resistant cell lines exhibit
significantly increased survival and invasive ability
The CCK-8 assay was used to detect the effects of
5-FU and PYM on the survival rate of KOA-1 cells and the
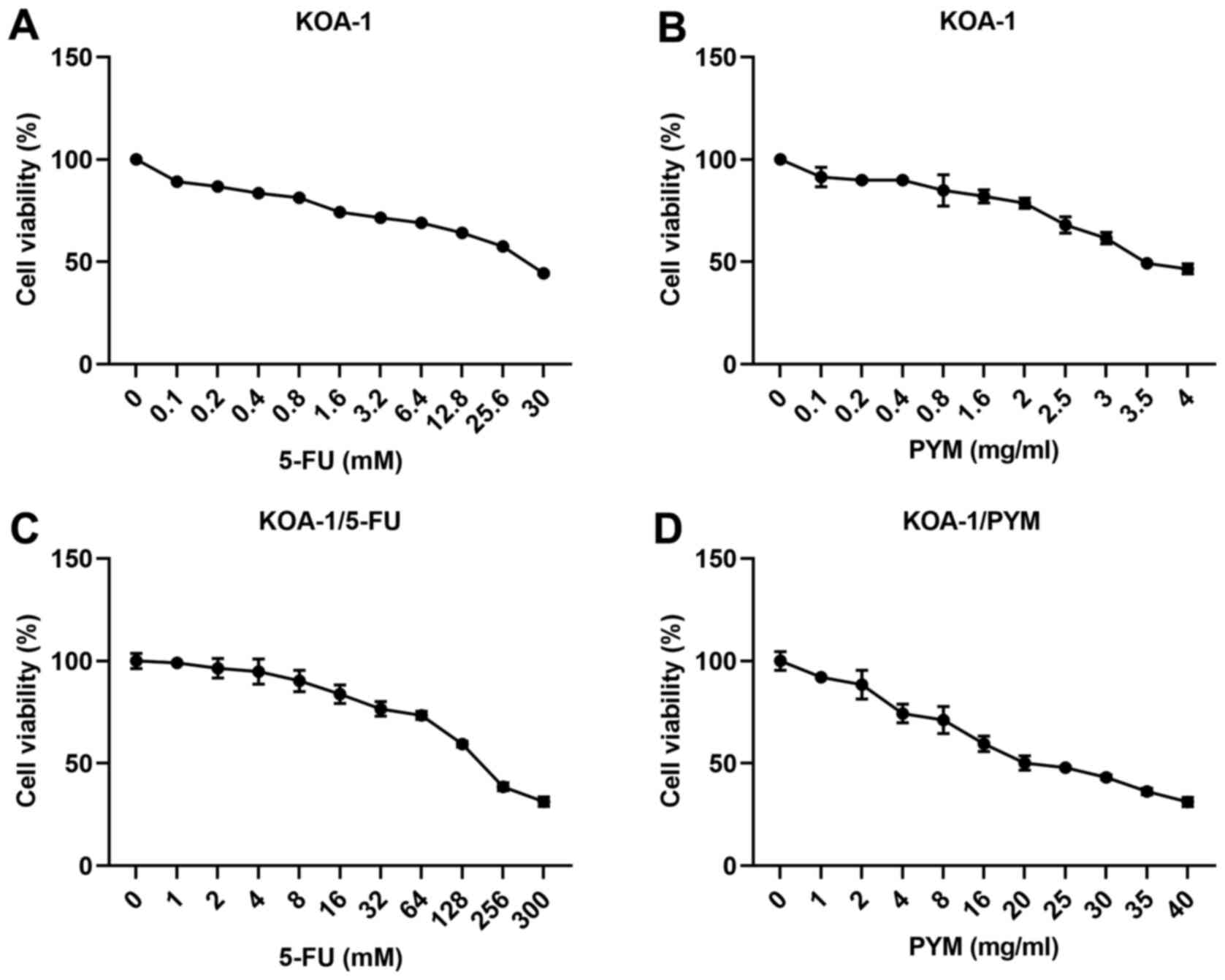
corresponding drug-resistant cell types. As shown in Fig. 1A-D, the IC50 value of
5-FU in KOA-1 cells was estimated to be 25 mM, while that of 5-FU
in KOA-1/5FU cells was estimated to be 150 mM, indicating a
significant increase in the drug resistance of KOA-1/5-FU and
KOA-1/PYM cells. The effects of PYM on KOA-1 cells and the
corresponding drug-resistant cell types were consistent with the
results noted following treatment of the cells with 5-FU.
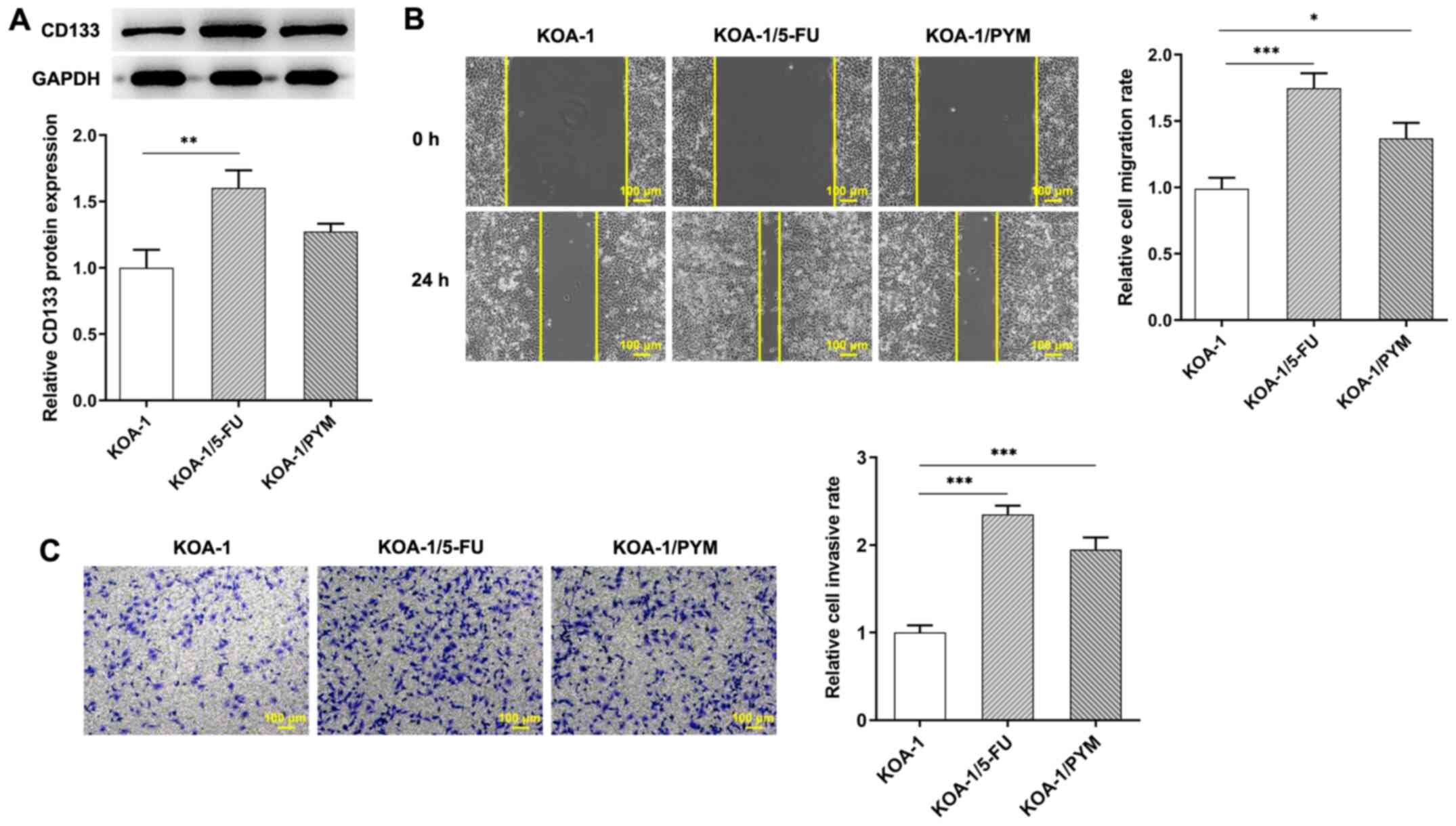
Subsequently, western blotting was used to detect the expression of
CD133 and CD133 was increased in KOA-1/5-FU and KOA-1/PYM cells
compared with that of KOA-1 cells (Fig. 2A) the wound healing and Transwell
assay was used to detect the migration and invasive ability of the
cells. The results indicated that the migration and invasive
activity of KOA-1/5-FU and KOA-1/PYM cells was significantly
increased compared with that of KOA-1 cells, suggesting that cell
resistance and the migration and invasive activity of KOA-1/5-FU
and KOA-1/PYM-resistant cell lines were significantly increased
(Fig. 2B and C). The results
showed that drug-resistant cell lines exhibit significantly
increased survival, migration and invasive ability.
CD133 expression is associated with the
drug sensitivity of tumor cells
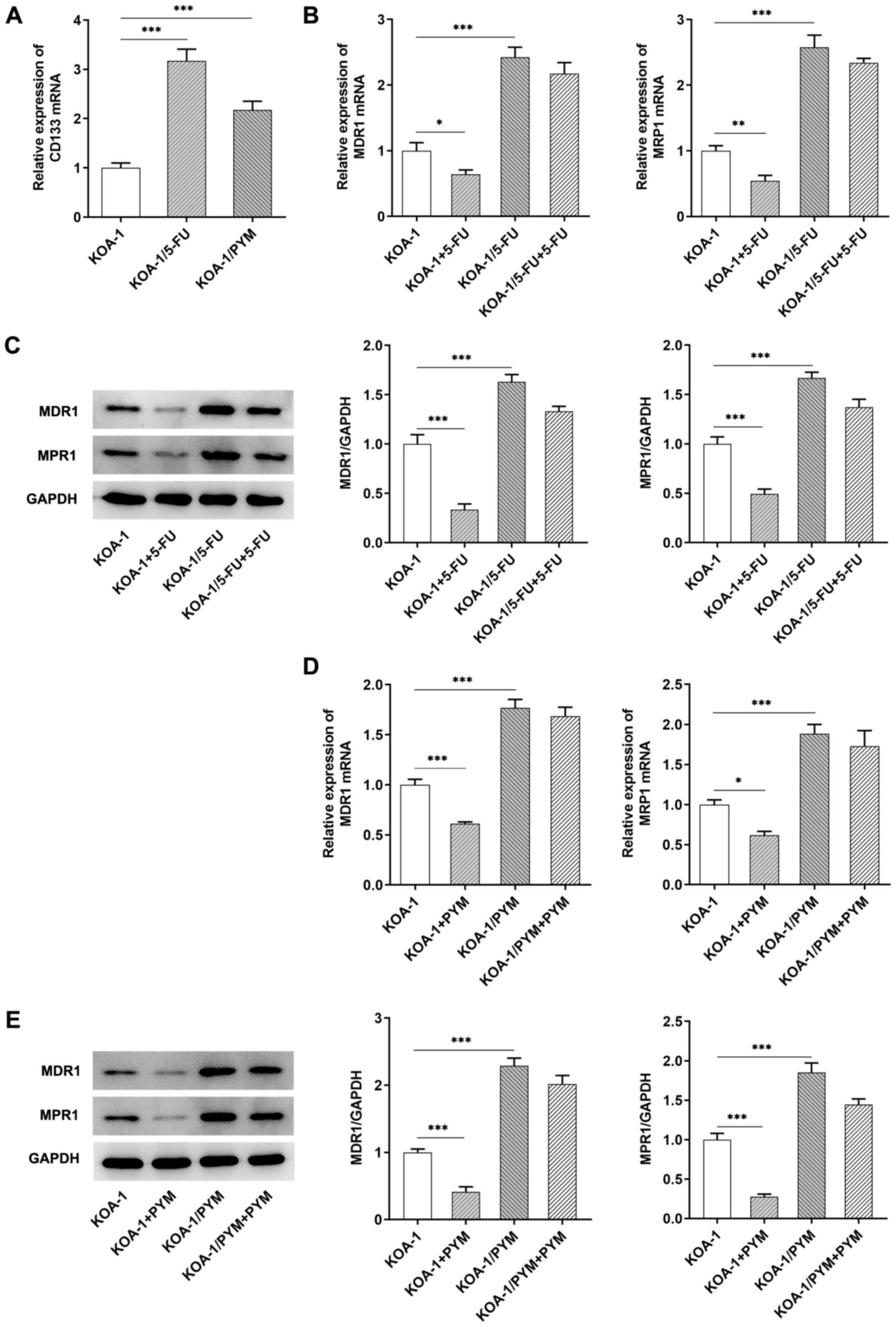
Subsequently, RT-qPCR analysis was used to detect
the expression levels of CD133 in KOA-1 cells and their
drug-resistant cell types. CD133 expression levels were
significantly increased in drug-resistant cell lines compared with
those noted in KOA-1 cells (Fig.
3A). Subsequently, RT-qPCR and western blotting was used to
detect the expression levels of the multidrug resistant proteins,
MDR1 and MRP1, in the corresponding cell lines. The results
indicated that the expression levels of MDR1 and MRP1 were
significantly increased in KOA-1/5-FU and KOA-1/PYM cells compared
with those of KOA-1 cells. The expression levels of MDR1 and MRP1
in KOA-1 cells were significantly decreased following treatment
with 5-FU and PYM. However, the expression levels of MDR1 and MRP1
in KOA-1/5-FU and KOA-1/PYM cells treated with 5-FU and PYM,
respectively, were not significantly altered, indicating that the
drug resistance of KOA-1/5-FU (Fig.
3B and C) and KOA-1/PYM (Fig. 3D
and E) cell lines was significantly increased. The expression
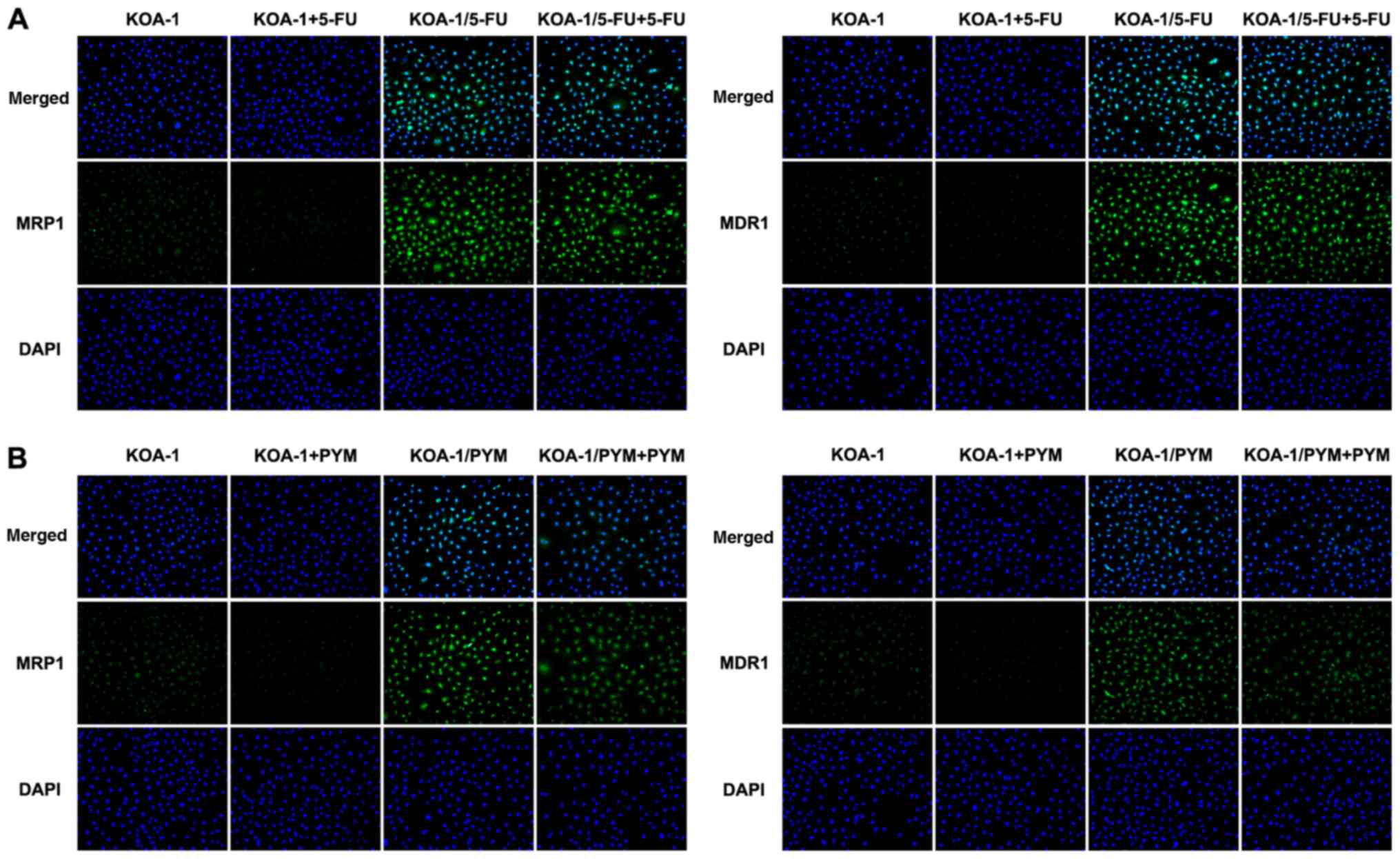
levels of MDR1 and MRP1 were also detected by IF and the results
were consistent with the results of the RT-qPCR assays (Fig. 4A and B). These results suggested a
possible association between CD133 expression and the drug
sensitivity of KOA-1 cells.
Knockdown of CD133 expression in
drug-resistant cell lines inhibits cell viability and invasion
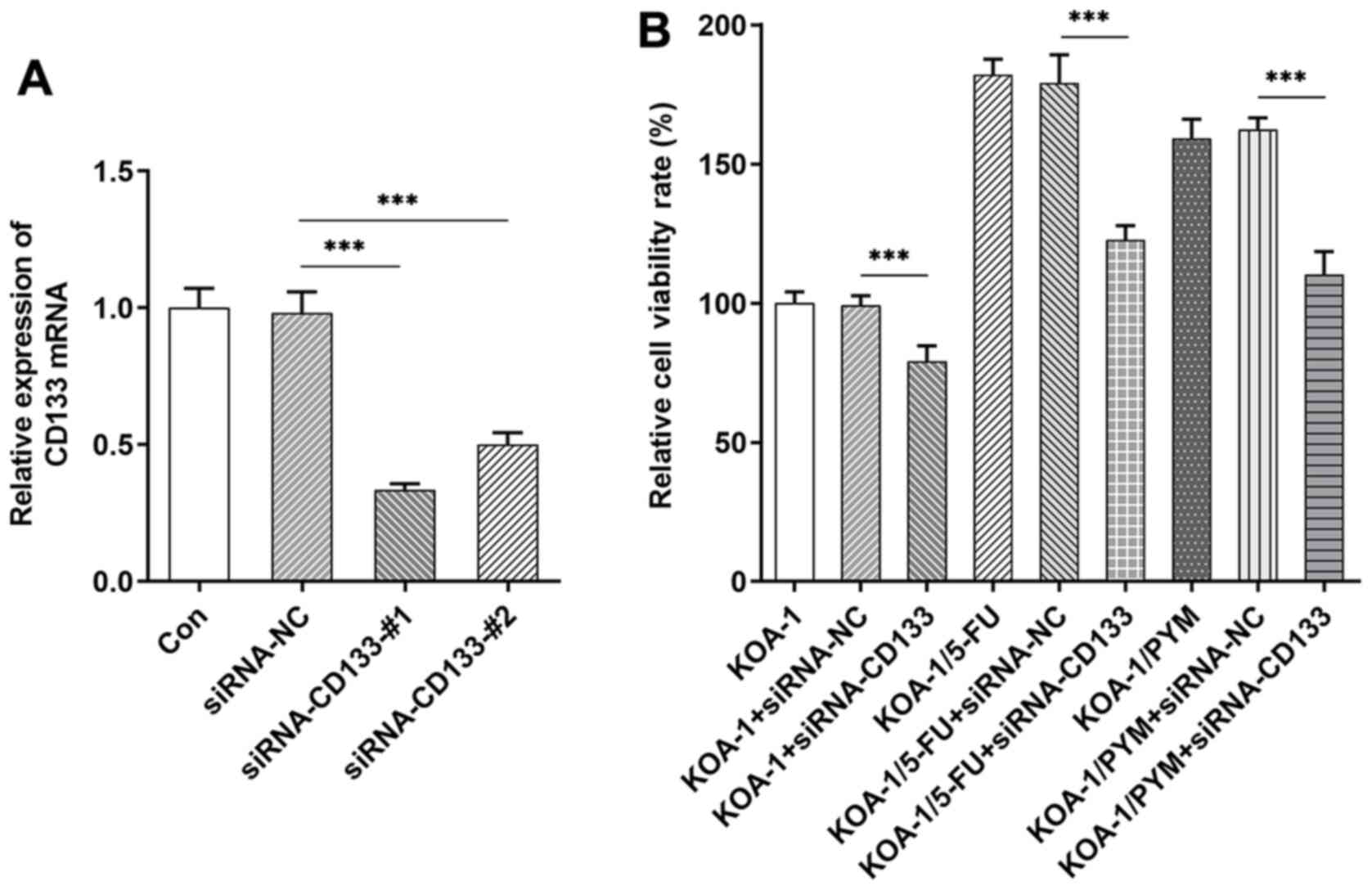
Cell transfection was used to knockdown the
expression of CD133 in KOA-1 cells and a RT-qPCR assay was used to
detect the transfection efficiency, as shown in Fig. 5A. siRNA CD133#1 was selected for
subsequent experiments. The siRNA CD133 plasmid was transfected
into KOA-1, KOA-1/5-FU and KOA-1/PYM cells and a CCK-8 assay was
used to detect cell viability. The data indicated that the
viability of drug-resistant cell lines was higher than that of
KOA-1 cells. Following inhibition of CD133 expression, the
viability of KOA-1 cells and the drug-resistant cell lines,
KOA-1/5-FU and KOA-1/PYM, were significantly decreased. Notably,
the decrease observed in KOA-1/5-FU and KOA-1/PYM cells was higher
than that of KOA-1 cells (Fig.
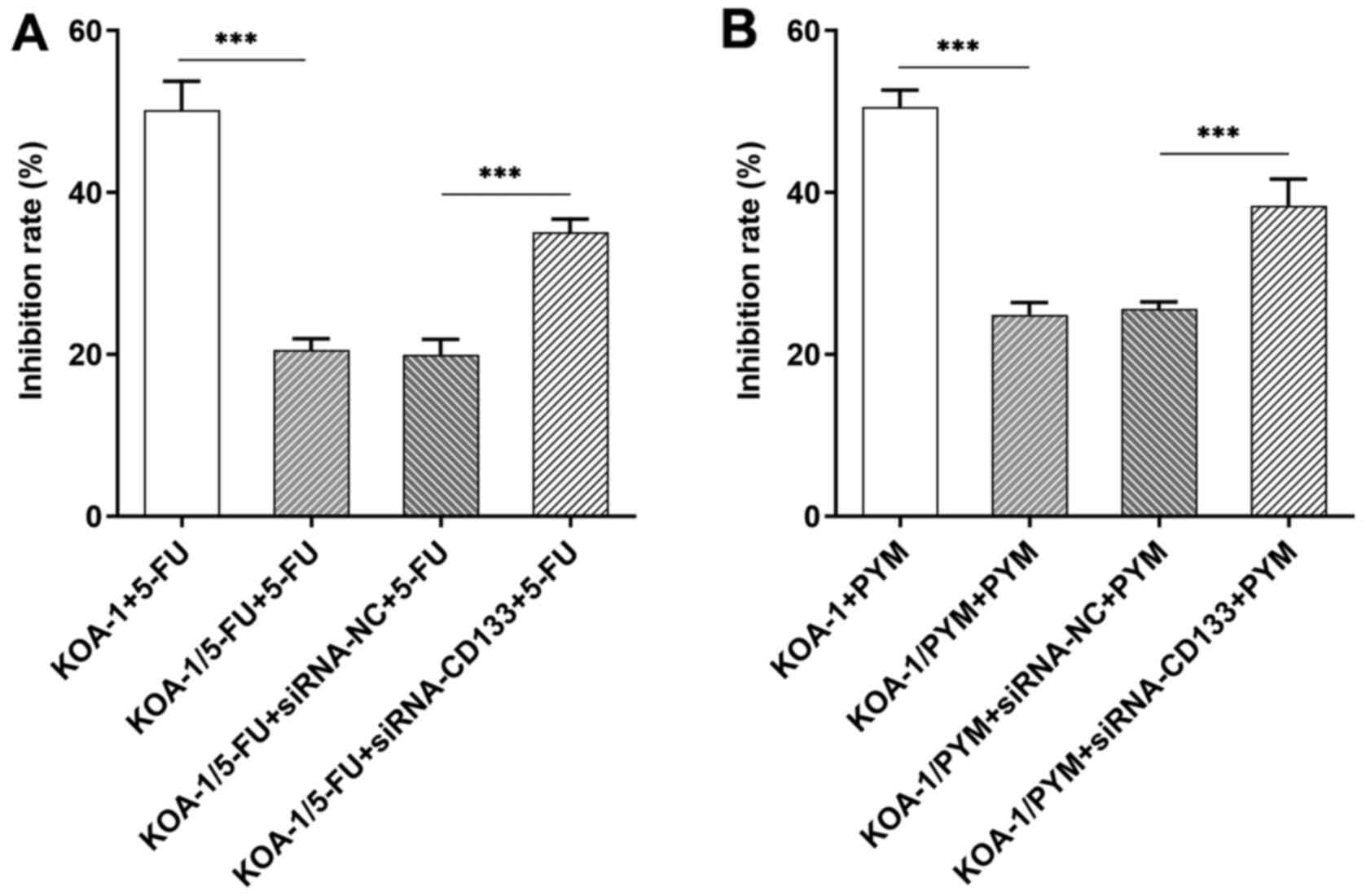
5B). Subsequently, a CCK-8 assay was used to detect cell
viability following addition of the chemotherapeutic compounds,
5-FU and PYM. The IC50 of 5-FU on KOA-1/5-FU cells was
lower than that on KOA-1 cells. Following interference of the
expression of CD133, the inhibitory rate of 5-FU on KOA-1/5-FU
cells (KOA-1/5-FU + siRNA CD133) was significantly increased
compared with that of the corresponding negative control cells
(KOA-1/5-FU + siRNA NC cells; Fig.
6A). The inhibitory rate of PYM was consistent with that of
5-FU (Fig. 6B). Wound healing and
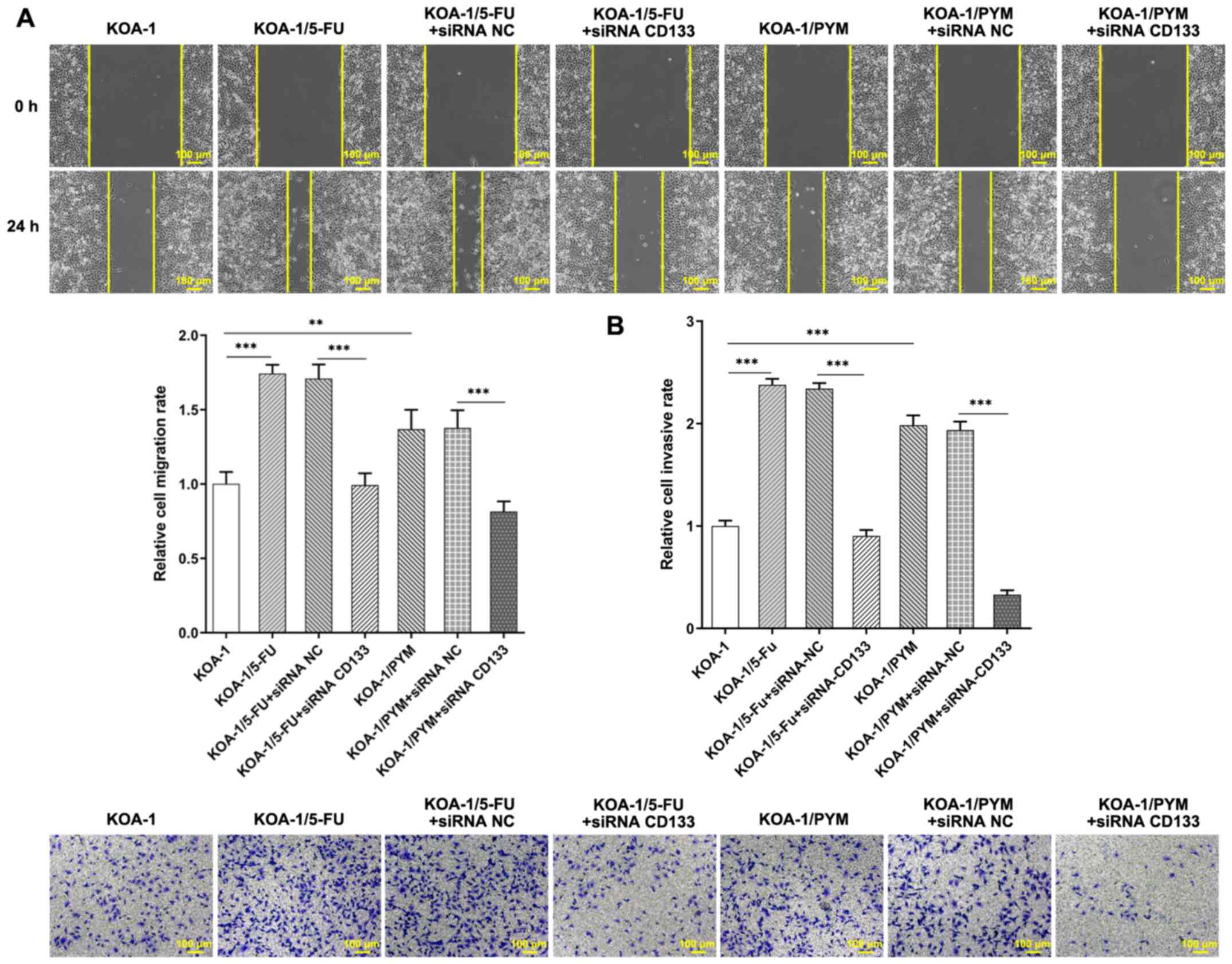
Transwell assays were used to detect the migration and invasive
ability of the cells following inhibition of CD133 expression.
Inhibition of CD133 expression significantly reduced the migration
and cell invasive ability of KOA-1/5-FU and KOA-1/PYM cells
(Fig. 7A and B). In addition, it
was found that after 5-FU administration, the migration and
invasion rate of KOA-1/5-FU cells was significantly higher than
that of KOA-1 cells. After the expression of CD133 was inhibited
and 5-FU was administered, the migration and invasion rate of
KOA-1/5-FU+siRNA CD133 cells was significantly decreased compared
with KOA-1/5-FU +siRNA NC cells (Fig.
8A and B). The results indicated that inhibition of CD133
expression in drug-resistant cell lines significantly reduced the
migratory and invasive abilities of drug-resistant cell lines. The
results showed that the chemotherapeutic agents had a strong
inhibitory effect on the viability and invasion ability of the
knockdown CD133 resistant cell lines and that inhibiting the
expression of CD133 could increase the sensitivity of ACC
drug-resistant cells to chemotherapeutic agents.
Knockdown of CD133 expression in
drug-resistant cell lines enhances cell sensitivity to
chemotherapeutic drugs through inhibiting MDR1 and MRP1
A RT-qPCR assay was used to detect the expression
levels of CD133 in cells. The expression levels of CD133 in
KOA-1/5-FU + siRNA CD133 cells were significantly decreased
compared with those in the KOA-1/5-FU + siRNA NC group (Fig. 9A). The expression levels of MDR1
and MRP1 proteins were also decreased (Fig. 9B). The expression pattern of these
proteins in KOA-1/PYM cells was consistent with that noted in
KOA-1/5-FU cells (Fig. 9C). The
expression levels of MDR1 and MRP1 proteins were detected by IF and
the results were consistent with the results of the RT-qPCR assay
(Fig. 10A and B). It was
hypothesized that the knockdown of CD133 expression in
drug-resistant cell lines may increase the cellular sensitivity to
5-FU and PYM through inhibiting MDR1 and MRP1.
Discussion
At present, the treatment of ACC mainly consists of
surgical resection supplemented by chemotherapy. 5-FU and PYM are
often used as chemotherapeutic drugs for the treatment of ACC
(17,18). However, repeated or long-term use
of 5-FU and PYM is likely to cause cellular resistance in tumors,
leading to a decline in chemotherapy efficacy and uncontrolled
tumor growth (19). In Bi et
al (20), CD133+
glioma stem cells isolated from the tumor tissue of glioma patients
showed high expression of MDR1 and MRP1, suggesting that
CD133+ brain tumor stem cells can be used as the root of
multi-drug resistance and the key therapeutic target of glioma
chemotherapy. In the present study, the data indicated that the
migratory and invasive abilities of KOA-1/5-FU/PYM-resistant cell
lines were significantly increased. The expression of MDR1 and MRP1
decreased, and the sensitivity to the drugs increased. The results
indicated that the administration of chemotherapeutic drugs did not
inhibit tumor growth following the development of cellular
resistance. Therefore, it is imperative to identify effective and
less toxic drugs for patients with tumors that have developed drug
resistance.
CD133 is considered as an important marker of tumor
stem cells and has been widely used in subpopulation sorting and
their consequent identification (21). Accumulating evidence has shown
that CD133 is involved in the regulation of specific biological
characteristics of several tumor cells, including chemotherapy
resistance (22,23). Previous studies have shown that
CD133 is associated with the development of chemotherapy resistance
in lung, stomach, colon and liver cancers (24). CD133 expression has been used as a
marker of tumor stem cells in small cell lung cancer and it has
been shown that it is significantly associated with drug resistance
and potent tumorigenicity. Further studies have shown that the
proportion of CD133+ cells in small cell lung cancer is
significantly increased following chemotherapy (25). A higher proportion of stem cells
in acute myeloid leukemia at diagnosis is associated with a higher
recurrence rate and the worse prognosis of patients following
chemotherapy (26). In addition,
in vivo tumor transplantation experiments using standard
5-FU-based chemotherapy regimens indicate that CD133+
and CD24+ stem cells are enriched in patients with
non-small cell lung cancer (27)
and hepatocellular carcinoma (28), respectively. However, the
association between CD133 expression and ACC drug resistance has
not been previously reported.
In the present study, 5-FU and PYM were used to
treat the ACC cell line, KOA-1, in order to establish 5-FU and PYM
drug-resistant cell lines. A significant increase was noted in
CD133 expression in ACC-resistant cell lines. Inhibition of CD133
expression in drug-resistant cell lines inhibited the migratory and
invasive abilities of drug-resistant cells and enhanced their
sensitivity to chemotherapeutic drugs. These results suggested that
CD133 serves an important role in the regulation of human ACC drug
resistance and these are the main factors affecting the high
morbidity and mortality in patients with ACC.
MDR1 and MRP1 are multi-drug resistant proteins, and
their expressions can reflect the sensitivity of tumor cells to
chemotherapy drugs (29). A
previous study has shown that compared with CD133(−) glioblastoma
stem cells, the protein expressions of MDR1 and MRP1 in CD133(+)
cells are significantly increased (16). In the present study, the
expression of CD133 was significantly increased in drug-resistant
cell lines. When the expression of CD133 was inhibited, the
expressions of MDR1 and MRP1 were also inhibited, which reduced the
viability and migratory ability of drug-resistant cell lines, and
increased the sensitivity of drug-resistant cell lines to
drugs.
The present study contains certain limitations. The
results were not confirmed by in vivo studies. The role of
CD133 will be examined in the future in an ACC animal model and the
associated mechanism will be further investigated. Only one ACC
cell line was used in the present study, which may not be widely
representative. The laboratory of the authors will conduct
verification experiments in other ACC cell lines in the future. The
present study proved the sensitivity of chemotherapeutic drugs by
using only CCK-8 to detect cell viability, without verifying cell
apoptosis, which is also one of its limitations. Future studies
will further detect the cell apoptosis to consolidate the
conclusions of the present study. It might be also worth showing
whether in the parental KOA-1 cells, inhibition of CD133 itself
could result in inhibition of invasion ability, and compare with
the drug-resistant lines. In addition, the present study only
detected that CD133 has a regulatory effect on the drug resistance
of 5-FU and PYM, but whether it has a regulatory effect on the drug
resistance of all other drugs is still unknown. The present study
detected endogenous CD133 expression, but the induction experiment
of exogenous CD133 at different concentrations has not been carried
out yet.
Overall, the present study demonstrated that
knockdown of CD133 expression in drug-resistant cell lines enhances
cell sensitivity to chemotherapeutic drugs through inhibiting MDR1
and MRP1. The data provide a theoretical basis for the prevention
of drug resistance in ACC tumors.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Chongqing City
Committee of Science and Technology and Social Undertakings
Livelihood Security Science and Technology Innovation Projects
(grant no. cstc2016shmszx0770).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW, LZ and YS wrote the manuscript and analyzed the
data. GY and YZ performed the experiments and supervised the study.
LZ searched the literature and revised the manuscript for important
intellectual content. JW and LZ confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bradley PJ: Adenoid cystic carcinoma
evaluation and management: Progress with optimism! Curr Opin
Otolaryngol Head Neck Surg. 25:147–153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coca-Pelaz A, Rodrigo JP, Bradley PJ,
Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A,
Haigentz M Jr, Takes RP, et al: Adenoid cystic carcinoma of the
head and neck - An update. Oral Oncol. 51:652–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castelnuovo P and Turri-Zanoni M: Adenoid
cystic carcinoma. Adv Otorhinolaryngol. 84:197–209. 2020.PubMed/NCBI
|
|
4
|
Gao M, Hao Y, Huang MX, Ma DQ, Luo HY, Gao
Y, Peng X and Yu GY: Clinicopathological study of distant
metastases of salivary adenoid cystic carcinoma. Int J Oral
Maxillofac Implants. 42:923–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma B, Liang LZ, Liao GQ, Liang YJ, Liu HC,
Zheng GS and Su YX: Inhibition of autophagy enhances cisplatin
cytotoxicity in human adenoid cystic carcinoma cells of salivary
glands. J Oral Pathol Med. 42:774–780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu J, Tang Z, Gong W, Zhang M and Quan Z:
Isolation and identification of tumor-initiating cell properties in
human gallbladder cancer cell lines using the marker cluster of
differentiation 133. Oncol Lett. 14:7111–7120. 2017.PubMed/NCBI
|
|
7
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li C, Wang C, Xing Y, Zhen J and Ai Z:
CD133 promotes gallbladder carcinoma cell migration through
activating Akt phosphorylation. Oncotarget. 7:17751–17759. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manoranjan B, Chokshi C, Venugopal C,
Subapanditha M, Savage N, Tatari N, Provias JP, Murty NK, Moffat J,
Doble BW, et al: A CD133-AKT-Wnt signaling axis drives glioblastoma
brain tumor-initiating cells. Oncogene. 39:1590–1599. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu R, Zhao G, Yang Y, Jiang Z, Cai J and
Hu H: Inhibition of CD133 overcomes cisplatin resistance through
inhibiting PI3K/AKT/mTOR signaling pathway and autophagy in
CD133-positive gastric cancer cells. Technol Cancer Res Treat. Jan
1–2019.(Epub ahead of print). doi: 10.1177/1533033819864311.
View Article : Google Scholar
|
|
11
|
Fayi MA, Alamri A and Rajagopalan P:
IOX-101 Reverses drug resistance through suppression of
Akt/mTOR/NF-κB signaling in cancer stem cell-like, sphere-forming
NSCLC cell. Oncol Res. 28:177–189. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan Z, Liang X, Zhan Y, Wang Z, Xu J, Qiu
Y, Wang J, Cao Y, Le VM, Ly HT, et al: Targeting CD133 reverses
drug-resistance via the AKT/NF-κB/MDR1 pathway in colorectal
cancer. Br J Cancer. 122:1342–1353. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Panaccione A, Zhang Y, Ryan M, Moskaluk
CA, Anderson KS, Yarbrough WG and Ivanov SV: MYB fusions and CD
markers as tools for authentication and purification of cancer stem
cells from salivary adenoid cystic carcinoma. Stem Cell Res (Amst).
21:160–166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang SS, Gao XL, Liu X, Gao SY, Fan YL,
Jiang YP, Ma XR, Jiang J, Feng H, Chen QM, et al: CD133+
cancer stem-like cells promote migration and invasion of salivary
adenoid cystic carcinoma by inducing vasculogenic mimicry
formation. Oncotarget. 7:29051–29062. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Panayotopoulou EG, Müller AK, Börries M,
Busch H, Hu G and Lev S: Targeting of apoptotic pathways by SMAC or
BH3 mimetics distinctly sensitizes paclitaxel-resistant triple
negative breast cancer cells. Oncotarget. 8:45088–45104. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cherifi F, Rambeau A, Johnson A, Florescu
C, Géry B, Babin E and Thariat J: Systemic treatments of metastatic
or locally recurrent adenoid cystic carcinoma of the head and neck,
a systematic review. Bull Cancer. 106:923–938. 2019.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi W, Guo J, Wu S, Su B, Zhang L, Pan J
and Zhang J: Synergistic effect of nanosecond pulsed electric field
combined with low-dose of pingyangmycin on salivary adenoid cystic
carcinoma. Oncol Rep. 31:2220–2228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bi CL, Fang JS, Chen FH, Wang YJ and Wu J:
Chemoresistance of CD133(+) tumor stem cells from human brain
glioma. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 32:568–573. 2007.(In
Chinese). PubMed/NCBI
|
|
21
|
Attia S, Atwan N, Arafa M and Shahin RA:
Expression of CD133 as a cancer stem cell marker in invasive
gastric carcinoma. Pathologica. 111:18–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barzegar Behrooz A, Syahir A and Ahmad S:
CD133: Beyond a cancer stem cell biomarker. J Drug Target.
27:257–269. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aghajani M, Mansoori B, Mohammadi A,
Asadzadeh Z and Baradaran B: New emerging roles of CD133 in cancer
stem cell: Signaling pathway and miRNA regulation. J Cell Physiol.
234:21642–21661. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jia Q, Zhang X, Deng T and Gao J: Positive
correlation of Oct4 and ABCG2 to chemotherapeutic resistance in
CD90(+)CD133(+) liver cancer stem cells. Cell Reprogram.
15:143–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sarvi S, Mackinnon AC, Avlonitis N,
Bradley M, Rintoul RC, Rassl DM, Wang W, Forbes SJ, Gregory CD and
Sethi T: CD133+ cancer stem-like cells in small cell
lung cancer are highly tumorigenic and chemoresistant but sensitive
to a novel neuropeptide antagonist. Cancer Res. 74:1554–1565. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Rhenen A, Feller N, Kelder A, Westra
AH, Rombouts E, Zweegman S, van der Pol MA, Waisfisz Q,
Ossenkoppele GJ and Schuurhuis GJ: High stem cell frequency in
acute myeloid leukemia at diagnosis predicts high minimal residual
disease and poor survival. Clin Cancer Res. 11:6520–6527. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bertolini G, Roz L, Perego P, Tortoreto M,
Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S,
et al: Highly tumorigenic lung cancer CD133+ cells
display stem-like features and are spared by cisplatin treatment.
Proc Natl Acad Sci USA. 106:16281–16286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee TK, Castilho A, Cheung VC, Tang KH, Ma
S and Ng IO: CD24(+) liver tumor-initiating cells drive
self-renewal and tumor initiation through STAT3-mediated NANOG
regulation. Cell Stem Cell. 9:50–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong FB, Deng QM, Deng HQ, Dong CC, Li L,
He CG, Wang XT, Xu S and Mai W: Siva 1 regulates multidrug
resistance of gastric cancer by targeting MDR1 and MRP1 via the
NF-κB pathway. Mol Med Rep. 22:1558–1566. 2020. View Article : Google Scholar : PubMed/NCBI
|