Introduction
Extravillous trophoblast cells (EVTs) are a group of
cells that serve a critical role in reproduction and development,
and are associated with obstetrical and gynecological diseases,
such as placenta accreta spectrum (PAS) (1). The migration of placental EVTs into
the uterine decidua facilitates the establishment of blood
circulation between mother and fetus at the early stage of
pregnancy and is modulated by EVT-decidual cell interaction
(2). It has been demonstrated that
excessive migration and invasion of EVTs is closely associated with
the incidence of PAS (3). Over the
past few decades, the incidence of PAS has not decreased despite
research efforts (4). Abnormal
invasion of EVTs and vascular generation are pivotal factors that
induce PAS (4). Therefore, further
research on EVTs is vital for developing preventative measures and
therapies for PAS.
The invasion and vascular remodeling of EVTs are
complex (5). The epidermal growth
factor, fibroblast growth factor (FGF), TGF-β, Wnt and NOTCH
pathways serve crucial roles in placentation and development of
trophoblasts (6,7). Furthermore, vascular endothelial
growth factor (VEGF) and platelet-derived growth factor (PDGF) are
closely associated with the process of vascular remodeling, and
thus, also participate in the regulation of EVTs and PAS (8,9). The
pigment epithelium-derived factor (PEDF) is a multifunctional
regulatory factor that participates in several important biological
processes, including differentiation, inflammation and angiogenesis
(10,11). It has previously been demonstrated
that upregulated levels of PEDF are involved in cancer progression
(12,13). Furthermore, PEDF has been reported
to be highly expressed in the vasculature of healthy pregnant
patients and placenta trophoblasts, which indicates its role during
placental blood vessel formation (14). Nevertheless, the specific role and
regulatory mechanisms of PEDF in PAS development are still
unclear.
Ferroptosis is a newly identified form of regulated
cell death that has drawn great attention in recent years (15). Ferroptosis is characterized by
iron-dependent lipid peroxidation and is implicated in the
regulation of various diseases, including neurodegenerative
diseases, cardiovascular diseases, carcinogenesis and reproductive
diseases (16–18). Several factors, especially
glutathione peroxidase 4 (GPX4) and solute carrier family 7 member
11 (SLC7A11), have been identified as suppressors of ferroptosis
via regulation of the transportation of cysteine and clearance of
lipid peroxides (19).
The present study assessed the effects of PEDF on
EVTs in PAS and explored the potential molecular mechanism.
Materials and methods
Cell lines
The extravillous trophoblast cell line HTR-8/SVneo
was purchased from the American Type Culture Collection and
maintained in RPMI-1640 medium (HyClone; Cytiva) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 µg/ml streptomycin. All cells were cultured in a 37°C incubator
with 5% CO2. For BML-284 treatment, the cells were
treated with 10 nM BML-284 (HY-19987; MedChemExpress) for 24 h
after overexpression treatment for 48 h in a 37°C incubator with 5%
CO2. For ferrostatin-1 (Fer-1) treatment, cells were
incubated with 1 µM ferrostatin-1 (HY-100579; MedChemExpress) for
24 h after overexpression treatment for 48 h in a 37°C incubator
with 5% CO2.
Cell transfection
The PEDF overexpression vector (OE-PEDF; 2 µg;
pcDNA3.1+), VEGF overexpression vector (OE-VEGF; 2 µg), empty
vector (pcDNA3.1+; 2 µg), small interfering RNA targeting PEDF
(siPEDF; 100 nM; sense, 5′-GGAUUUCUACUUGGAUGAA-3′ and antisense,
5′-UUCAUCCAAGUAGAAAUCC-3′) and small interfering RNA negative
control (siNC; 100 nM; sense, 5′-CUAACUAUCUCGAACGCAAdTdT-3′ and
antisense, 5′-UUGCGUUCGAGAUAGUUAGdTdT-3′) were synthesized by
Shanghai GenePharma Co., Ltd. Cell transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol at 37°C
for 48 h. PEDF overexpression vectors were mixed with Lipofectamine
2000 reagent alone in DMEM for 20 min or together with the VECF
overexpression vectors. After 48 h, the transfected cells were
further processed for the following experiments.
Cell proliferation
Cell viability was assessed using a Cell Counting
Kit-8 (CCK-8; Beyotime Institute of Biotechnology) and a
5-ethynyl-2′-deoxyuridine (EdU) assay kit (Beyotime Institute of
Biotechnology). For the CCK-8 assay, transfected cells were seeded
into a 96-well plate at a density of 5,000 cells/well. After
incubation in a 37°C incubator for 24 h, CCK-8 reagent was added
into each well and the cells were incubated for another 2 h at
37°C. The absorbance values were then measured at 450 nm. For EdU
assays, cells were seeded into 96-well plates (5×103
cells/well) and incubated for 24 h at 37°C, then fixed in 4%
paraformaldehyde at room temperature for 30 min and stained with
EdU reagent for 2 h at 37°C, followed by DAPI staining at room
temperature for 20 min. Images were captured under a fluorescence
microscope (Leica Microsystems GmbH).
Transwell assay
Transwell plate chambers (8 µm pore size) were
coated with Matrigel (BD Biosciences) at 37°C for 30 min before
use. For cell migration assays, cells (1×104) were
suspended in RPMI-1640 medium without FBS and seeded into the upper
chamber with no Matrigel, while complete culture medium (RPMI-1640
medium with 10% FBS) was added to the lower chamber. After
incubation for 24 h at 37°C, the cells were collected and stained
with 0.2% crystal violet in methanol for 10 min at room
temperature. The cell invasion was analyzed using the same method,
and the cells were added into upper chambers precoated with
Matrigel. Five random images were captured under a light microscope
(Leica Microsystems GmbH).
Wound healing assay
The wound healing assay was conducted to evaluate
the migration of cells. The cell monolayer (70–80% confluence as a
monolayer) in a 6-well plate was scratched with a 200-µl sterile
pipette tip, then washed with PBS and cultured with RPMI-1640
medium (HyClone; Cytiva) containing 0.1% FBS for 12 h. Images of
the width of the wound were captured at 0, 6 and 12 h after
scratching using a light microscope (Nikon Corporation). The width
of wounds was measured using ImageJ software (version 1.54;
National Institutes of Health).
Colony formation assay
For colony formation assays, HTR-8/SVneo cells were
seeded into 6-well plates at a density of 1,000 cells/well. Cells
were incubated in a 37°C cell incubator for 14 days. Following
fixation with 4% paraformaldehyde at room temperature for 30 min,
the colonies were stained with 0.1% crystal violet (MilliporeSigma)
at room temperature for 30 min and images were captured and counted
under a light microscope (Leica Microsystems GmbH). A cluster with
>50 cells was considered a colony. The number of colonies was
quantified using ImageJ software (Fiji Version, National Institutes
of Health).
Tube formation assay
HTR-8/SVneo cells in RPMI-1640 medium with 10% FBS
(HyClone; Cytiva) were seeded into a 96-well plate
(2×104 cells/well) pre-coated with Matrigel (Corning,
Inc.) at 37°C for 30 min. Following incubation for 8 h at 37°C with
5% CO2, the images of formed tubes were captured using a
light microscope (Leica Microsystems GmbH).
Detection of ferroptosis
To analyze ferroptosis, the levels of lipid reactive
oxygen species (ROS), total and ferrous iron (Fe2+),
malondialdehyde (MDA) and glutathione (GSH) in HTR-8/SVneo cells
were examined using a Fluorometric Intracellular ROS Kit (cat. no.
MAK143; MilliporeSigma), Iron Assay Kit (cat. no. ab83366; Abcam),
Lipid Peroxidation (MDA) Assay Kit (cat. no. MAK085;
MilliporeSigma), and GSH and Oxidized Glutathione Assay Kit (cat.
no. S0053; Beyotime Institute of Biotechnology), respectively,
according to the manufacturers' protocols.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells and tissues using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
and was purified and reverse transcribed into cDNA with the
PrimeScript™ RT reagent kit (cat. no. RR037A; Takara Biotechnology
Co., Ltd.) according to the manufacturer's protocol. The gene
expression was determined using a qPCR assay with a SYBR Green
mixture kit (Takara Bio, Inc.) according to the manufacturer's
instructions. The following thermocycling conditions were used:
94°C for 60 sec, and 40 cycles of 94°C for 5 sec, 60°C for 34 sec
and 72°C for 30 sec. mRNA expression levels were quantified using
the 2−∆∆Cq method and normalized to the internal
reference gene GAPDH (20). The
primer sequence used were: PEDF forward,
5′-CAGAAGAACCTCAAGAGTGCC-3′ and reverse,
5′-CTTCATCCAAGTAGAAATCC-3′; VEGF forward,
5′-CAACTTCTGGGCTCTTCTCG-3′ and reverse,
5′-CCTCTCCTCTTCCTTCTCTTCC-3′; and GAPDH forward,
5′-CAAGGTCATCCATGACAACTTTG-3′ and reverse,
5′-GTCCACCACCCTGTTGCTGTAG-3′.
Western blotting
Total protein was extracted from cells or tissues
using RIPA lysis buffer (Beyotime Institute of Biotechnology) and
quantified using a BCA kit. Protein (35 µg per lane) was separated
by 12% SDS-PAGE and transferred to PVDF membranes, which were
blocked in 5% non-fat milk for 1 h at room temperature. The
membranes were then incubated with primary antibodies against GPX4
(1:1,000; ab41787; Abcam), SLC7A11 (1:1,000; ab307601; Abcam), VEGF
(1:1,000; cat. no. AF-293-SP; R&D Systems, Inc.), Wnt (1:1,000;
ab15251; Abcam), β-catenin (1:1,000; ab32572; Abcam), E-cadherin
(1:1,000; ab231303; Abcam), vimentin (1:1,000; ab92547; Abcam), FGF
(1:1,000; ab131162; Abcam), PDGF (1:1,000; ab178409; Abcam),
proliferating cell nuclear antigen (PCNA; 1:1,000; ab29; Abcam) and
β-actin (1:1,000; ab8226; Abcam) for 12 h at 4°C. β-actin was used
as the loading control. Membranes were then incubated with goat
anti-mouse, goat anti-rabbit or rabbit anti-goat (1:3,000; cat.
nos. sc-2005, sc-2004 and sc-2768, respectively; Santa Cruz
Biotechnology, Inc.) IgG peroxidase-conjugated secondary antibodies
at 37°C for 1 h. Bands were visualized with ECL reagent (Merck
KGaA) and semi-quantified using ImageJ software (version 1.47;
National Institutes of Health).
Clinical sample detection
Placentas from pregnant women who delivered by
cesarean section were collected at Shengli Clinical Medical College
of Fujian Medical University (Fuzhou, China) between May 2017 and
July 2022, including 20 cases in the PAS group (age range, 26–39
years; mean age, 33 years old) and 20 pregnant woman giving birth
during a healthy term pregnancy in the healthy group (age range,
20–33 years; mean, 27 years old). The exclusion criteria were as
follows: i) Gestational hypertension, diabetes, kidney disease,
immune system diseases (including systemic red Lupus maculatus and
antiphospholipid syndrome), heart disease, tumor, epilepsy, acute
and chronic infectious diseases; ii) deformed or stillborn; and
iii) alcohol and addiction to drugs. The diagnostic criteria for
placental implantation were as follows: The villi of the placenta
invaded the myometrium in varying degrees. MRI examination showed
that the base of the lower segment of the uterus was thinning or
disappearing, and the boundary between the placenta and the
myometrium was unclear, and the placenta even penetrated the
myometrium. Intraoperatively, it was confirmed that placental villi
directly invaded the uterine myometrium, as observed on the
placental bed in the implanted placenta or the resected uterine
specimen.
The tissues were immediately frozen in liquid
nitrogen and stored at −80°C for subsequent experiments. The mRNA
expression levels of PEDF in these tissues were examined by
RT-qPCR. All experiments using clinical samples were approved by
the Ethics Committee of Shengli Clinical Medical College of Fujian
Medical University (approval no. K2020-090040; Fuzhou, China) in
July 2020. All patients provided written informed consent.
Statistical analysis
The data are presented as the mean ± standard
deviation and were analyzed by SPSS 20.0 software (IBM Corp.). All
experiments were repeated at least three times. Data with a normal
distribution were compared by Student's unpaired t-test. One-way
ANOVA followed by Tukey's post hoc test was used to assess the
differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
PEDF inhibits the proliferation,
migration and angiogenesis of EVTs in vitro
To determine the role of PEDF in PAS, its mRNA
expression levels in healthy placentas and placentas from patients
with PAS were analyzed. The results of RT-qPCR revealed
significantly decreased relative mRNA expression levels of PEDF in
the PAS group compared with that in the healthy group, suggesting
the potential regulatory effects of PEDF on PAS (Fig. S1). Next, the effects of PEDF on
the phenotypes of EVTs were determined using knockdown or
overexpression of PEDF. The transfection efficiencies of OE-PEDF,
OE-VEGF and siPEDF were confirmed by western blotting. OE-PEDF and
OE-VEGF significantly increased relative PEDF and VEGF expression
levels compared with the empty vector control. siPEDF significantly
decreased the relative expression levels of PEDF compared with
those in the siNC group (Fig.
S2).
PEDF overexpression significantly decreased the
viability (Fig. 1A) and colony
formation of EVTs (Fig. 1C) and
decreased the number of EdU-positive cells (Fig. 1B) compared with the OE-NC group,
whereas PEDF knockdown using siPEDF significantly increased cell
proliferation (Fig. 1A) and colony
number (Fig. 1C) and increased the
number of EdU-positive cells compared with the siNC group.
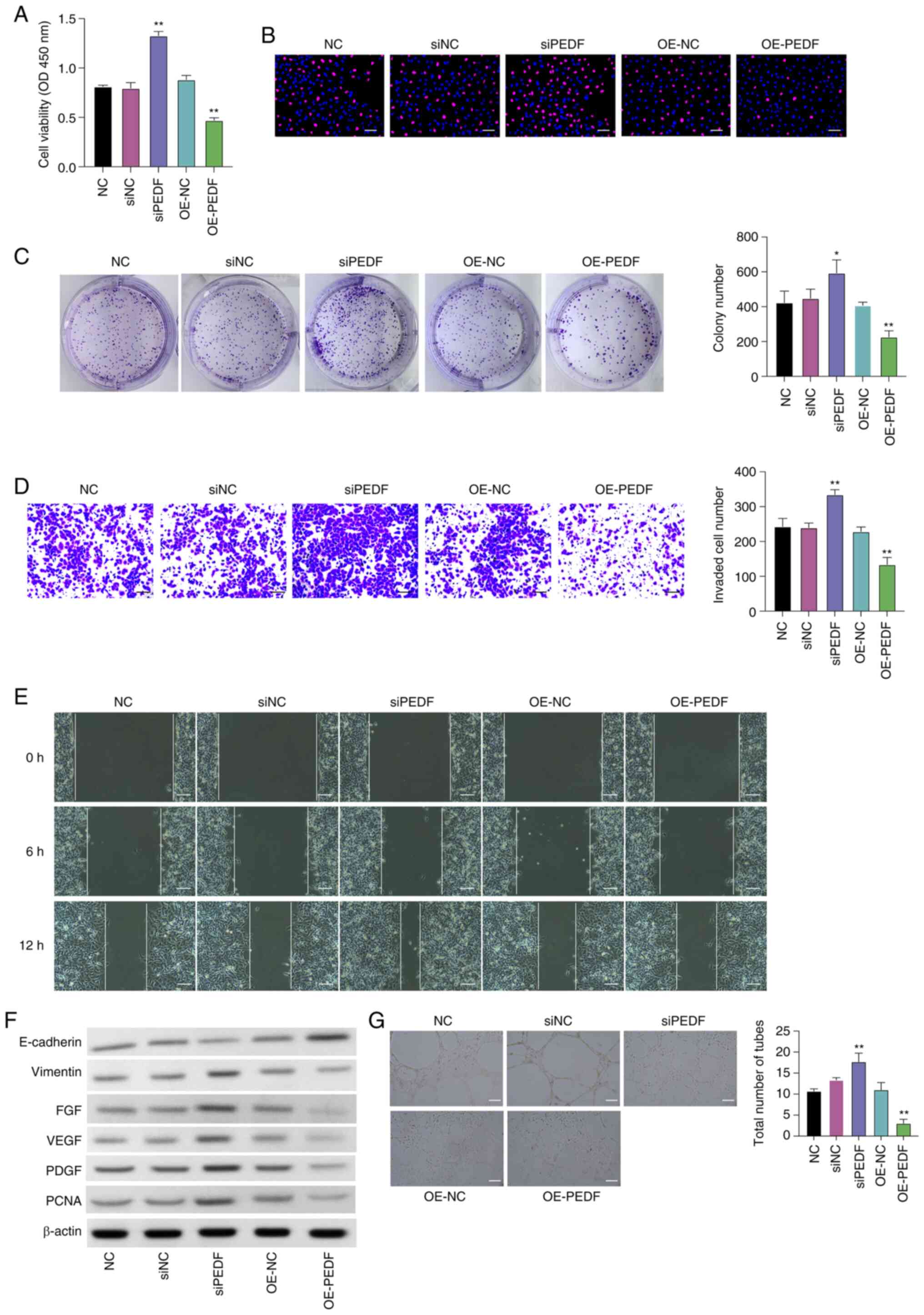 | Figure 1.PEDF inhibits the proliferation,
migration and angiogenesis of EVTs in vitro. EVTs were
transfected with OE-PEDF or siPEDF. Cell viability and
proliferation were assessed using. (A) Cell Counting Kit-8, (B)
5-ethynyl-2′-deoxyuridine (red, proliferative cells; blue, nuclei)
and (C) colony formation assays. (D) Cell invasion was assessed
using a Transwell assay. (E) Cell migration was detected using a
wound healing assay. (F) Protein expression levels of E-cadherin,
vimentin, FGF, VEGF, PDGF and PCNA were examined by western
blotting. (G) In vitro angiogenesis was examined using a
tube formation assay. Scale bar, 50 µm. Data are presented as the
mean ± SD, n=3. *P<0.05, **P<0.01 vs. NC or siPEDF. EVT,
extravillous trophoblast cell; FGF, fibroblast growth factor; NC,
negative control; OD, optical density; OE-NC, control empty vector
for overexpression; OE-PEDF, PEDF overexpression vector; PCNA,
proliferating cell nuclear antigen; PDGF, platelet-derived growth
factor; PEDF, pigment epithelium-derived factor; siNC, small
interfering RNA negative control; siPEDF, small interfering RNA
targeting PEDF; VEGF, vascular endothelial growth factor. |
The Transwell assay indicated a significantly
increased number of invasive cells following PEDF knockdown and a
significantly decreased number of invasive cells following PEDF
overexpression compared with the siNC group and OE-NC group,
respectively (Fig. 1D). The wound
healing ability of PEDF knockdown cells was higher than that of the
siNC group, whereas overexpression of PEDF reduced the wound
healing ability (Figs. 1E and
S3).
Furthermore, the protein expression levels of the
epithelial biomarker E-cadherin were decreased, and the levels of
mesenchymal biomarker vimentin, growth factors FGF, VEGF and PDGF,
and proliferative biomarker PCNA were elevated by PEDF knockdown,
whereas PEDF overexpression had the opposite effect (Fig. 1F).
The tube formation assay was conducted to examine
angiogenesis. siPEDF significantly increased the number of tubes,
while PEDF overexpression significantly decreased the number of
tubes compared with the siNC group or the OE-NC group, respectively
(Fig. 1G). These data demonstrated
that PEDF overexpression suppressed proliferation, migration and
angiogenesis of EVTs.
PEDF induces ferroptosis of EVTs
It was observed that PEDF overexpression
significantly increased the levels of lipid ROS (Fig. 2A), iron (Fig. 2B), Fe2+ (Fig. 2C) and MDA (Fig. 2D) compared with the control group,
which are key biomarkers for ferroptosis. The levels of GSH, the
antioxidant substrate of ferroptosis, were significantly decreased
by PEDF overexpression (Fig. 2E),
and the protein expression levels of GPX4 and SLC7A11, the
ferroptosis inhibitory factors, were decreased by PEDF
overexpression (Fig. 2F). These
data indicated that PEDF overexpression induced ferroptosis of
EVTs.
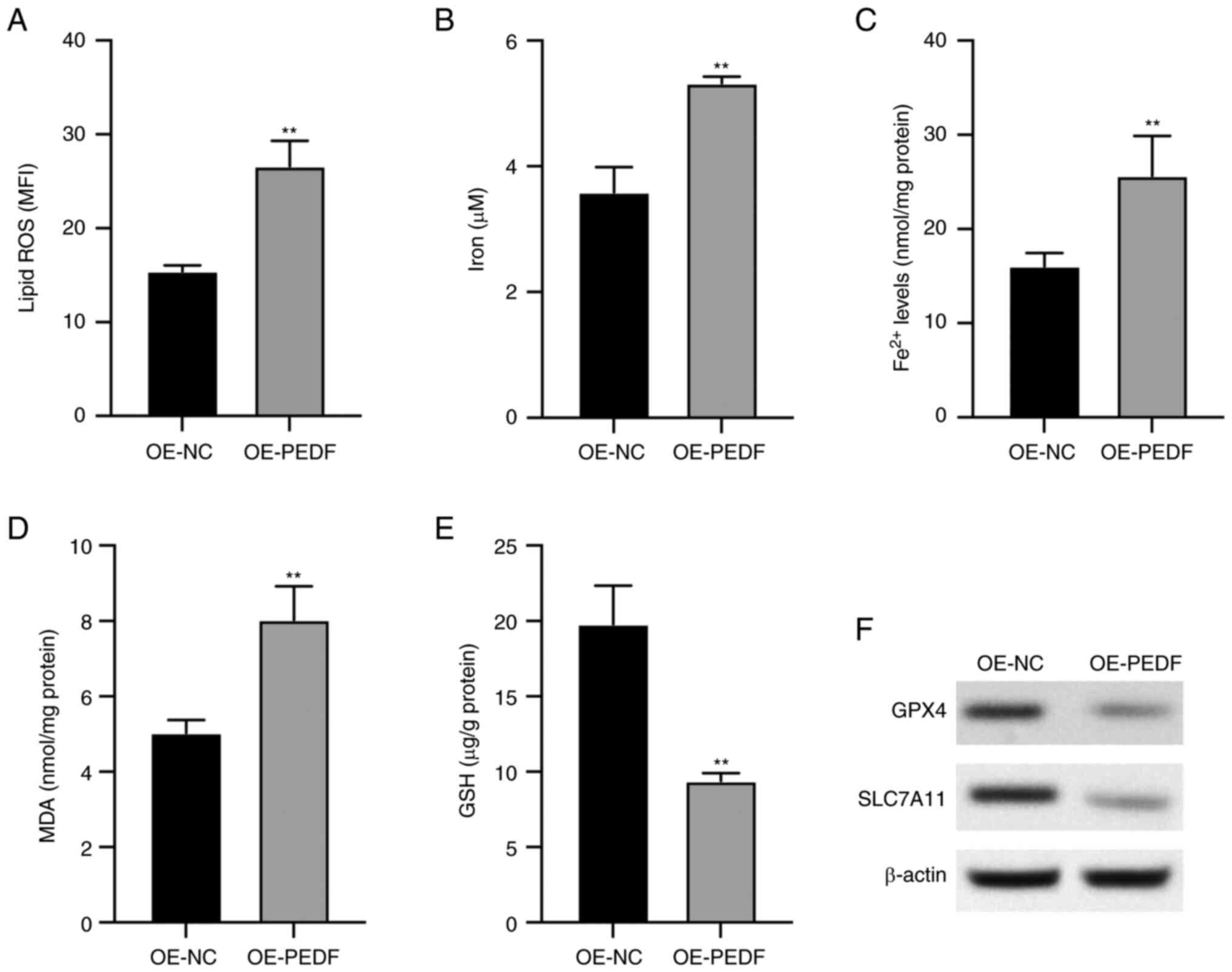 | Figure 2.PEDF overexpression induces
ferroptosis of extravillous trophoblast cells transfected with
OE-PEDF. Levels of (A) lipid ROS, (B) total iron, (C)
Fe2+, (D) MDA and (E) GSH were examined using the
respective kits. (F) Protein expression levels of GPX4 and SLC7A11
determined by western blotting. Data are presented as the mean ±
SD, n=3. **P<0.01 vs. OE-NC. GPX4, glutathione peroxidase 4;
GSH, reduced glutathione; MDA, malondialdehyde; MFI, mean
fluorescence intensity; OE-NC, control empty vector for
overexpression; OE-PEDF, PEDF overexpression vector; PEDF, pigment
epithelium-derived factor; ROS, reactive oxygen species; SLC7A11,
solute carrier family 7 member 11. |
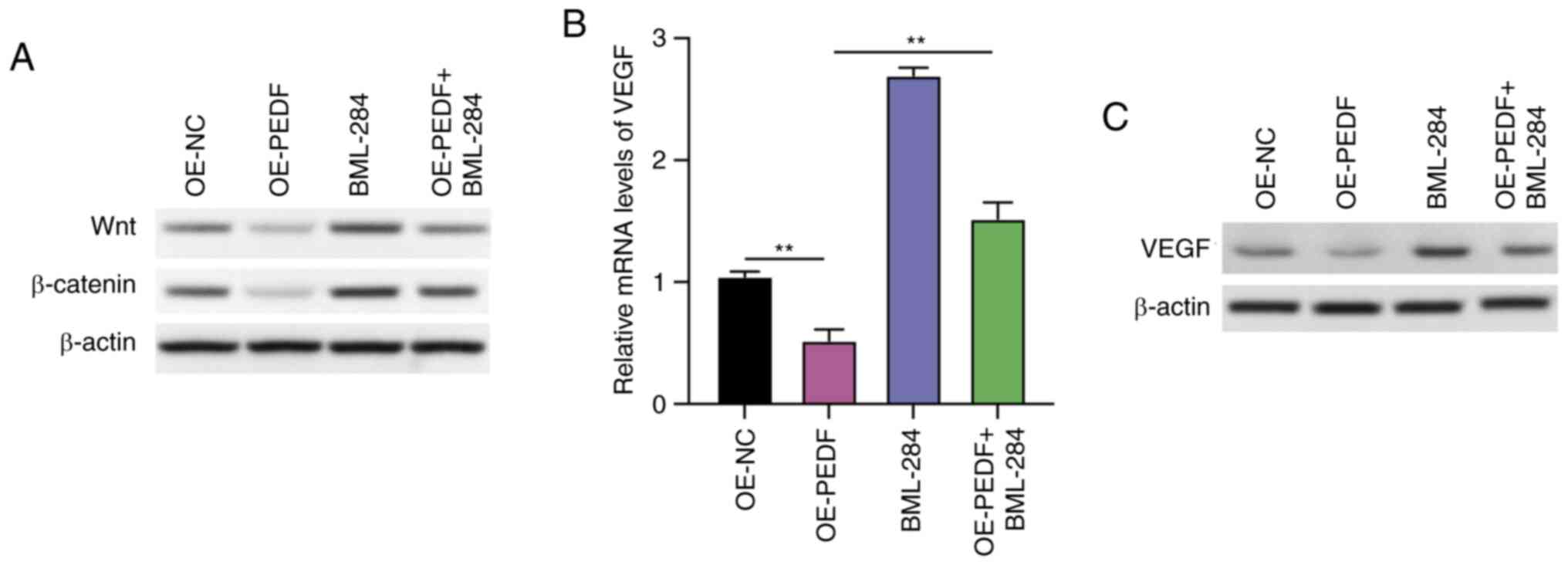
PEDF suppresses the Wnt-β-catenin/VEGF
signaling pathway in EVTs
Subsequently, the mechanisms that participate in the
PEDF-regulated angiogenesis of EVTs were explored using a Wnt
signaling activator. The overexpression of PEDF decreased the
protein expression levels of Wnt and β-catenin, which were
recovered by treatment with the Wnt signaling pathway agonist
BML-284, with BML-284 increasing the protein expression of Wnt and
β-catenin in EVTs treated with OE-PEDF (Fig. 3A). It was further observed that the
relative mRNA expression levels of VEGF were significantly
decreased by PEDF overexpression; however, this was reversed by
BML-284 treatment, which significantly increased the relative mRNA
expression levels of VEGF compared with the OE-PEDF group (Fig. 3B). The protein expression levels of
VEGF were decreased by PEDF overexpression, whereas treatment with
BML-284 reversed this decrease (Fig.
3C). These data indicated that PEDF suppressed Wnt signaling in
EVTs.
PEDF regulates the phenotypes of EVTs
through the Wnt-β-catenin/VEGF signaling pathway
To explore the potential mechanism of PEDF-regulated
phenotypes of EVTs, the roles of the Wnt-β-catenin/VEGF signaling
pathway and ferroptosis in PEDF-regulated phenotypes of EVTs were
assessed by treatment with the ferroptosis inhibitor Fer-1) and
BML-284, and overexpression of VEGF. Inhibition of ferroptosis by
Fer-1, activation of Wnt signaling by BML-284 and overexpression of
VEGF increased the cell viability that was suppressed by PEDF
overexpression (Fig. 4A),
Edu-positive cells (Fig. 4B) and
colony numbers (Fig. 4C), and
enhanced the invasion (Fig. 4D),
migration (Figs. 4E and S4) and angiogenesis (Fig. 4F) of EVTs.
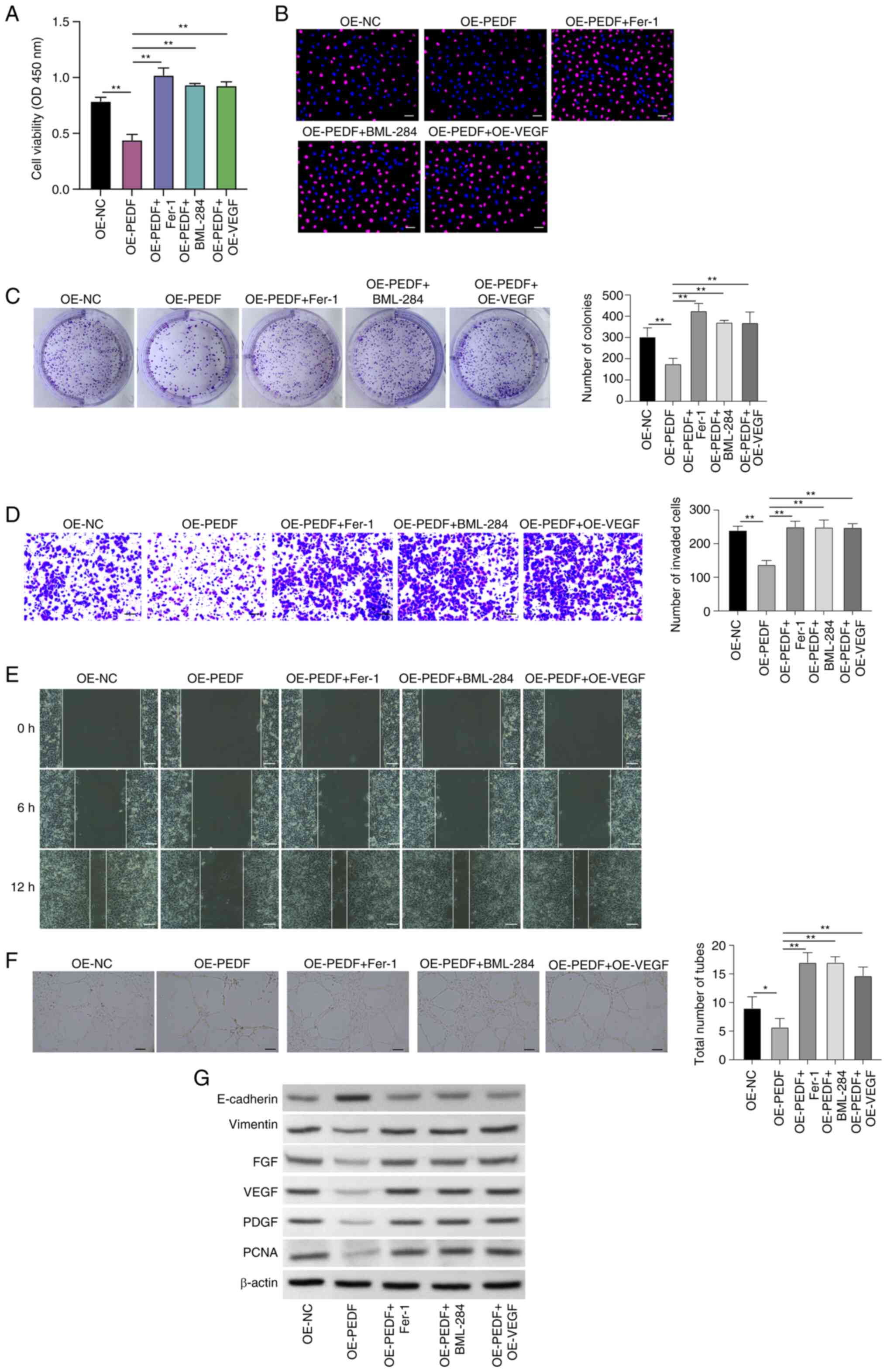 | Figure 4.PEDF regulates phenotypes of EVTs via
the Wnt-β-catenin/VEGF signaling pathway. EVTs were transfected
with OE-PEDF and treated with Fer-1 or BML-284 or transfected with
OE-VEGF. Cell viability and proliferation were examined using (A)
Cell Counting Kit-8, (B) 5-ethynyl-2′-deoxyuridine (red,
proliferative cells; blue, nuclei) and (C) colony formation assays.
Cell (D) invasion and (E) migration were assessed by Transwell and
wound healing assays. (F) Angiogenesis was analyzed using a tube
formation assay. (G) Expression levels of E-cadherin, vimentin,
FGF, VEGF, PDGF and PCNA were examined by western blotting. Scale
bar, 50 µm. Data are presented as the mean ± SD, n=3. *P<0.05
and **P<0.01 vs. OE-NC or OE-PEDF. EVT, extravillous trophoblast
cell; Fer-1, ferrostatin-1; FGF, fibroblast growth factor; OD,
optical density; OE-NC, control empty vector for overexpression;
OE-PEDF, PEDF overexpression vector; OE-VEGF, VEGF overexpression
vector; PCNA, proliferating cell nuclear antigen; PDGF,
platelet-derived growth factor; PEDF, pigment epithelium-derived
factor; VEGF, vascular endothelial growth factor. |
The protein expression levels of vimentin, FGF,
VEGF, PDGF and PCNA were increased, and the expression levels of
E-cadherin were decreased in PEDF-overexpressing EVTs treated with
Fer-1 or BML-284 or transfected with OE-VEGF compared with the
OE-PEDF group (Fig. 4G). These
data showed that activation of the Wnt signaling pathway could
reverse the suppressive effects of PEDF overexpression on the
proliferation, migration and angiogenesis of EVTs, indicating that
Wnt signaling and VEGF may be a mediator of PEDF-regulated EVT
phenotype.
PEDF induces ferroptosis of EVTs by
suppressing the Wnt-β-catenin/VEGF signaling pathway
The role of the Wnt-β-catenin/VEGF signaling pathway
in PEDF-regulated ferroptosis was verified in EVTs. In
PEDF-overexpressing cells, treatment with Fer-1 and BML-284 and
VEGF overexpression significantly decreased the levels of lipid ROS
(Fig. 5A), iron (Fig. 5B), Fe2+ (Fig. 5C) and MDA (Fig. 5D) compared with those in cells
overexpressing PEDF. Furthermore, in PEDF-overexpressing cells,
treatment with Fer-1 and BML-284, and VEGF overexpression
significantly increased the GSH levels compared with those in cells
overexpressing PEDF (Fig. 5E).
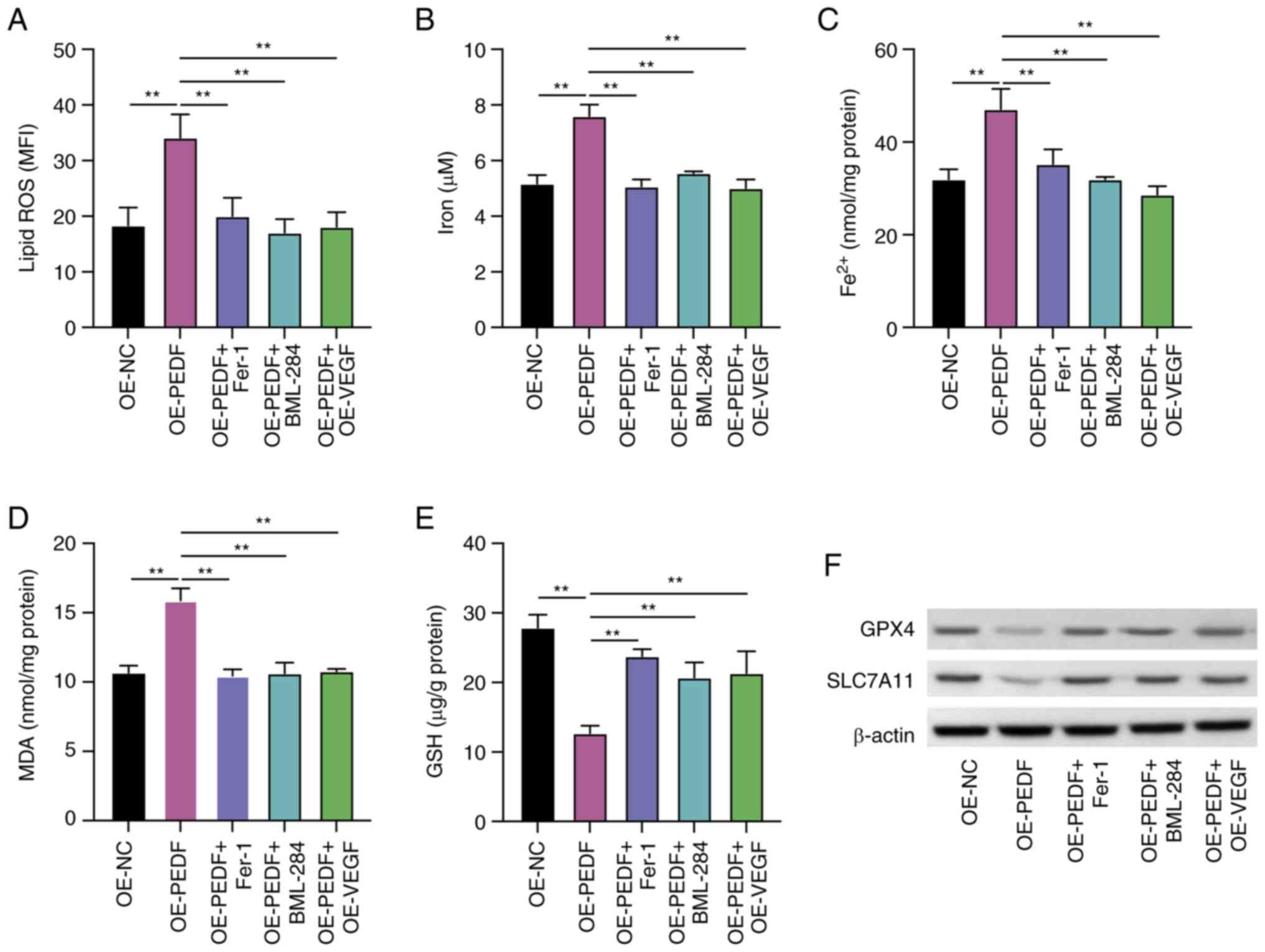 | Figure 5.PEDF induces ferroptosis of EVTs by
suppressing the Wnt-β-catenin/VEGF pathway. EVTs were transfected
with OE-PEDF and OE-VEGF or transfected with OE-PEDF and treated
with Fer-1 or BML-284. The levels of (A) lipid ROS, (B) total iron,
(C) Fe2+, (D) MDA and (E) GSH were examined using the
respective kits. (F) Protein expression levels of GPX4 and SLC7A11
were determined by western blotting. Data are presented as the mean
± SD, n=3. **P<0.01 vs. OE-NC or OE-PEDF. EVT, extravillous
trophoblast cell; Fer-1, ferrostatin-1; GPX4, glutathione
peroxidase 4; GSH, reduced glutathione; MDA, malondialdehyde; MFI,
mean fluorescence intensity; OE-NC, control empty vector for
overexpression; OE-PEDF, PEDF overexpression vector; OE-VEGF, VEGF
overexpression vector; PEDF, pigment epithelium-derived factor;
ROS, reactive oxygen species; SLC7A11, solute carrier family 7
member 11; VEGF, vascular endothelial growth factor. |
The PEDF-reduced levels of GPX4 and SLC7A11 were
recovered by Fer-1, BML-288 and overexpression of VEGF compared
with the OE-PEDF group (Fig. 5F).
These data demonstrated that the Wnt-β-catenin/VEGF signaling
pathway potentially mediates PEDF-regulated ferroptosis.
Discussion
PAS is characterized by excessive trophoblast
invasion (21). According to the
depth of placental invasion into the uterine muscle layer, it can
be categorized into placenta accreta, placenta increta and placenta
percreta, which are dangerous complications in obstetrics (21). During normal pregnancy, after the
placenta implants, mononuclear trophoblast cells begin to
proliferate and form columnar cells. Some of these nourishing cells
lose their proliferative ability and differentiate into cells with
high invasive capabilities, similar to tumor cells, known as EVTs
(2,8). EVTs exhibit a high level of
invasiveness, which is a normal and controlled physiological
behavior strictly regulated by the maternal body. However, various
molecules, such as VEGF and Notch, can influence the invasive and
angiogenic abilities of trophoblast cells, leading to early
placental abnormalities, and in turn, resulting in
pregnancy-related diseases associated with the placenta (22,23).
PAS represents a typical example of these conditions (24,25).
In patients with PAS, a significant number of EVTs are observed at
the maternal-fetal interface. EVT invasion leads to changes in
vascular remodeling and affects the formation of new maternal-fetal
blood vessels in the uterus (24,25).
Compared with a healthy placenta, patients with PAS exhibit complex
and irregular blood vessel proliferation within the placenta, with
tortuous vessel paths and varying diameters, indicating rich and
abnormal blood flow (26). All the
aforementioned findings suggest the involvement of transitional
invasion by EVTs and neovascularization in PAS; however, the
specific mechanisms are still not fully understood (27).
In the present study, it was demonstrated that
overexpression of PEDF suppressed the proliferation, invasion and
tube formation of EVTs, whereas knockdown of PEDF had the opposite
effect. A decrease in the protein expression levels of PCNA, FGF,
VEGF, PDGF and vimentin was observed following overexpression of
PEDF, while an increase was observed following small interfering
RNA knockdown of PEDF. The PCNA, FGF, VEGF and PDGF were critical
regulators participate in the cell proliferation, angiogenesis and
invasion; decrease of these factors following overexpression of
PEDF suggested that PEDF modulated the growth and angiogenesis of
EVTs.
The mechanisms underlying the altered proliferation,
invasion and angiogenesis of EVTs following PEDF regulation were
subsequently determined. Ferroptosis has been widely studied in
recent years and is involved in various diseases (28,29).
Several studies have demonstrated the participation of ferroptosis
in EVT phenotypes, and that excessive iron levels can be
detrimental to pregnancy (30) and
is associated with reproductive disorders such as endometriosis
(31). In the present study,
overexpression of PEDF led to decreased proliferation of
HTR-8/SVneo cells, along with the induction of ferroptosis, which
was detected by the accumulation of lipid ROS, increases in total
iron, Fe2+ and MDA levels, and the decreased levels of
GSH, as well as decreased protein expression levels of GPX4 and
SLC7A11, which are inhibitors of ferroptosis. The detection of
total iron and Fe2+ levels was a specific indication of
ferroptosis induction and should be sufficient to rule out the
possibility of the results arising from oxidative damage,
pyroptosis or other mechanisms (32). These data indicated that PEDF may
contribute to ferroptosis of EVTs.
In a previous study, the relative expression levels
of PEDF and VEGF in placenta from healthy and PAS clinical samples
were analyzed (33). It was
identified that in the group with abnormal placental positions
(placenta accreta and placenta previa), there was a low level of
PEDF in the placenta. This low expression was negatively associated
with the VEGF level and microvessel density, which suggests that
the reduced expression of PEDF in the placental tissue of the group
with abnormal placental positions may contribute to insufficient
negative regulation of VEGF and the pathological vascular formation
in the placenta (34). However,
the detailed molecular mechanisms by which PEDF regulates PAS are
unclear.
Furthermore, in the present study, it was
demonstrated that the protein expression levels of Wnt, β-catenin
and VEGF were decreased following overexpression of PEDF, which is
consistent with a previous study demonstrating that PEDF can
suppress Wnt signaling (35). The
PEDF-suppressed proliferation and angiogenesis of EVTs were
recovered by treatment with the Wnt agonist BML-284, suggesting
that PEDF regulated VEGF expression and EVT phenotypes via the
Wnt/β-catenin signaling pathway.
Furthermore, the Wnt signaling pathway serves a
critical role in cell differentiation, and stemness maintenance
during the development of tissues and organs (36,37).
When the pathway is activated, the Wnt receptors prevent the
degradation of β-catenin, which consequently stimulates the
function of transcription factors T-cell factor/lymphoid enhancer
factor and the expression of targeted genes, including VEGF
(38). Wnt is necessary for the
expansion of trophoblast progenitors and stem cells (7). It has been reported that Wnt-3a
activation induces matrix metalloproteinase-2 secretion and
trophoblast migration (5). In the
present study, it was demonstrated that the VEGF protein expression
and tube formation of EVTs were suppressed by PEDF overexpression
and were recovered by Wnt pathway activation by treatment with
BML-284. Therefore, it was predicted that PEDF modulated Wnt
signaling to suppress VEGF expression and angiogenesis.
To summarize, it was determined that PEDF
overexpression inhibited the proliferation, invasion and
angiogenesis of EVTs, and induced ferroptosis by modulating Wnt
signaling to suppress VEGF expression. The present study revealed
the importance of ferroptosis in PEDF-regulated EVT functions and
provided novel evidence for the pathological role of PEDF in
PAS.
Supplementary Material
Supporting Data
Acknowledgements
This abstract was presented at the Chinese Medical
Association 17th National Conference on Perinatal
Medicine (November 17–19, 2023; Guangzhou, China), and was
published as Abstract no. PO-295.
Funding
This study was supported by Fujian Province Health and Health
Science and Technology Program Projects (grant no. 2020GGB006).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LZ designed the study. RL and LZ wrote the
manuscript. RL, XW, XH and JW performed the experiments. RL and XW
performed statistical analysis of the data. LZ, RL, XW, XH and JW
confirm the authenticity of all raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experiments concerning clinical samples were
approved by the Ethics Committee of Shengli Clinical Medical
College of Fujian Medical University (approval no. K2020-090040;
Fuzhou, China). All patients provided written informed consent for
participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moser G and Huppertz B: Implantation and
extravillous trophoblast invasion: From rare archival specimens to
modern biobanking. Placenta. 56:19–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei XW, Zhang YC, Wu F, Tian FJ and Lin Y:
The role of extravillous trophoblasts and uterine NK cells in
vascular remodeling during pregnancy. Front Immunol. 13:9514822022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Illsley NP, DaSilva-Arnold SC, Zamudio S,
Alvarez M and Al-Khan A: Trophoblast invasion: Lessons from
abnormally invasive placenta (placenta accreta). Placenta.
102:61–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horgan R and Abuhamad A: Placenta accreta
spectrum: Prenatal diagnosis and management. Obstet Gynecol Clin
North Am. 49:423–438. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sonderegger S, Haslinger P, Sabri A,
Leisser C, Otten JV, Fiala C and Knöfler M: Wingless (Wnt)-3A
induces trophoblast migration and matrix metalloproteinase-2
secretion through canonical Wnt signaling and protein kinase B/AKT
activation. Endocrinology. 151:211–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knöfler M and Pollheimer J: Human
placental trophoblast invasion and differentiation: A particular
focus on Wnt signaling. Front Genet. 4:1902013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dietrich B, Haider S, Meinhardt G,
Pollheimer J and Knöfler M: WNT and NOTCH signaling in human
trophoblast development and differentiation. Cell Mol Life Sci.
79:2922022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pollheimer J, Vondra S, Baltayeva J,
Beristain AG and Knöfler M: Regulation of placental extravillous
trophoblasts by the maternal uterine environment. Front Immunol.
9:25972018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abbas Y, Turco MY, Burton GJ and Moffett
A: Investigation of human trophoblast invasion in vitro. Hum Reprod
Update. 26:501–513. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shang Z, Li C, Liu X, Xu M, Zhang X, Li X,
Barnstable CJ, Zhao S and Tombran-Tink J: PEDF gene deletion
disrupts corneal innervation and ocular surface function. Invest
Ophthalmol Vis Sci. 62:182021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma B, Zhou Y, Liu R, Zhang K, Yang T, Hu
C, Gao Y, Lan Q, Liu Y, Yang X and Qi H: Pigment epithelium-derived
factor (PEDF) plays anti-inflammatory roles in the pathogenesis of
dry eye disease. Ocul Surf. 20:70–85. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ansari D, Althini C, Ohlsson H, Bauden M
and Andersson R: The role of PEDF in pancreatic cancer. Anticancer
Res. 39:3311–3315. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao X, Zeng J, Huang H, Ma C, Wang L, Wang
F, Liao X and Song X: Cancer-targeted PEDF-DNA therapy for
metastatic colorectal cancer. Int J Pharm. 576:1189992020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Loegl J, Nussbaumer E, Hiden U,
Majali-Martinez A, Ghaffari-Tabrizi-Wizy N, Cvitic S, Lang I,
Desoye G and Huppertz B: Pigment epithelium-derived factor (PEDF):
A novel trophoblast-derived factor limiting feto-placental
angiogenesis in late pregnancy. Angiogenesis. 19:373–388. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei X, Yi X, Zhu XH and Jiang DS:
Posttranslational modifications in ferroptosis. Oxid Med Cell
Longev. 2020:88320432020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu X, Li Y, Zhang S and Zhou X:
Ferroptosis as a novel therapeutic target for cardiovascular
disease. Theranostics. 11:3052–3059. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Kang R, Kroemer G and Tang D:
Ferroptosis in infection, inflammation immunity. J Exp Med.
218:e202105182021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hirschhorn T and Stockwell BR: The
development of the concept of ferroptosis. Free Radic Biol Med.
133:130–143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bloomfield V, Rogers S and Leyland N:
Placenta accreta spectrum. CMAJ. 192:E9802020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Q, Yu S, Huang X, Tan Y, Zhu C, Wang
YL and Wang H, Lin HY, Fu J and Wang H: New insights into the
function of Cullin 3 in trophoblast invasion and migration.
Reproduction. 150:139–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scalise ML, Amaral MM, Reppetti J, Damiano
AE, Ibarra C and Sacerdoti F: Cytotoxic effects of Shiga toxin-2 on
human extravillous trophoblast cell lines. Reproduction.
157:297–304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jauniaux E, Jurkovic D, Hussein AM and
Burton GJ: New insights into the etiopathology of placenta accreta
spectrum. Am J Obstet Gynecol. 227:384–391. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma Y, Hu Y and Ma J: Animal models of the
placenta accreta spectrum: Current status and further perspectives.
Front Endocrinol (Lausanne). 14:11181682023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia H, Ke SC, Qian RR, Lin JG, Li Y and
Zhang X: Comparison between abdominal ultrasound and nuclear
magnetic resonance imaging detection of placenta accreta in the
second and third trimester of pregnancy. Medicine (Baltimore).
99:e179082020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burton GJ and Jauniaux E: Pathophysiology
of placental-derived fetal growth restriction. Am J Obstet Gynecol.
218:S745–S761. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y,
Fang J, Xu S, Gao Y, Chen X, Sui X and Li G: The emerging role of
ferroptosis in inflammation. Biomed Pharmacother. 127:1101082020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan Q, Luo Y, Xia Q and He K: Ferroptosis
and liver fibrosis. Int J Med Sci. 18:3361–3366. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Georgieff MK, Krebs NF and Cusick SE: The
benefits and risks of iron supplementation in pregnancy and
childhood. Annu Rev Nutr. 39:121–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Zeng X, Lu D, Yin M, Shan M and Gao
Y: Erastin induces ferroptosis via ferroportin-mediated iron
accumulation in endometriosis. Hum Reprod. 36:951–964. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ortega MA, Saez MÁ, Asúnsolo Á, Romero B,
Bravo C, Coca S, Sainz F, Álvarez-Mon M, Buján J and
García-Honduvilla N: Upregulation of VEGF and PEDF in placentas of
women with lower extremity venous insufficiency during pregnancy
and its implication in villous calcification. Biomed Res Int.
2019:53209022019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duzyj CM, Buhimschi IA, Laky CA, Cozzini
G, Zhao G, Wehrum M and Buhimschi CS: Extravillous trophoblast
invasion in placenta accreta is associated with differential local
expression of angiogenic and growth factors: A cross-sectional
study. BJOG. 125:1441–1448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Protiva P, Gong J, Sreekumar B, Torres R,
Zhang X, Belinsky GS, Cornwell M, Crawford SE, Iwakiri Y and Chung
C: Pigment epithelium-derived factor (PEDF) inhibits Wnt/β-catenin
signaling in the liver. Cell Mol Gastroenterol Hepatol.
1:535–549.e14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Baccouche B, Olayinka O, Serikbaeva
A and Kazlauskas A: The role of the Wnt pathway in
VEGF/anti-VEGF-dependent control of the endothelial cell barrier.
Invest Ophthalmol Vis Sci. 62:172021. View Article : Google Scholar
|
|
37
|
Jiang L, Yin M, Wei X, Liu J, Wang X, Niu
C, Kang X, Xu J, Zhou Z, Sun S, et al: Bach1 represses
Wnt/β-catenin signaling and angiogenesis. Circ Res. 117:364–375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kovács B, Vajda E and Nagy EE: Regulatory
effects and interactions of the Wnt and OPG-RANKL-RANK signaling at
the bone-cartilage interface in osteoarthritis. Int J Mol Sci.
20:46532019. View Article : Google Scholar : PubMed/NCBI
|