Introduction
Acute liver failure (ALF) is a complex syndrome
characterized by overactivation of innate immunity, and the
recruitment and differentiation of immune cells at the inflammatory
site, which plays important roles in inducing liver injury
(1). Our previous study suggested
that baseline serum interleukin (IL)-23 levels were highly
increased, and that upregulated baseline IL-23 levels were
associated with mortality in patients with liver failure (2). However, to the best of our knowledge,
few related mechanistic studies have been performed (2,3).
MicroRNAs (miRNAs/miRs) are noncoding RNAs composed
of 19–22 nucleotides that regulate gene expression at the
post-transcriptional level by inducing degradation of mRNA or
inhibiting its transcription (4).
A previous miRNA microarray analysis demonstrated that miR-21 is
increased in patients with sepsis (5); however, to the best of our knowledge,
the role of miR-21 in ALF has not been elucidated. Therefore, the
present study explored the role of miR-21 and its potential
mechanisms in the inflammatory responses of patients with ALF.
Krüppel-like-factor-6 (KLF6) is a widely expressed
nuclear transcriptional regulator, which has been considered a
tumor suppressor gene, and regulator of cell proliferation,
tumorigenesis, differentiation and signal transduction (6). The KLF family constitutes zinc finger
proteins that have a conserved zinc finger DNA-binding domain,
which can bind to GC-rich motifs on various target genes. KLF6 can
act as both a repressor and an activator of transcription (7). A previous study demonstrated that
KLF6 alleviates hepatic ischemia-reperfusion injury by inhibiting
autophagy (8). By contrast,
another study suggested that KLF6 is a transcriptional activator of
autophagy in acute liver injury (9). However, whether and how KLF6 plays a
role in ALF remain unknown.
Accumulating evidence has demonstrated that
autophagy serves a crucial role in ischemia-reperfusion injury of
the liver, lung, heart, kidney and brain (10). Numerous studies on
ischemia-reperfusion injury in the liver, heart and brain have
corroborated this perspective (9,11–15).
The regulation of autophagy is significantly influenced by the KLF
family. Notably, conserved KLFs/autophagy pathways have been
reported to regulate the lifespan of nematodes and mammalian
age-related vascular deterioration (16). In addition, the communication
between autophagy and KLF2 in acute liver injury determines the
endothelial phenotype and microvascular function (17). However, the effects and underlying
mechanism of KLF6 on autophagy in ALF are unknown. The present
study aimed to explore the role of miR-21 and its potential
mechanisms underlying inflammatory responses in ALF.
Materials and methods
Patients
From June 2015 to July 2017, a total of 66 patients
participated in the present study, including 40 patients with ALF
and 26 healthy controls (HCs). The median age of the study subjects
was 42 years (age range: 15–82 years) and 72.73% of patients were
male. Enrolled patients were treated in accordance with the
scheduled criteria (18), and the
inclusion criteria were as follows: An illness duration of <26
weeks in a patient without preexisting liver disease or cirrhosis
associated with any degree of mental status alteration
(encephalopathy) and coagulopathy (an international normalized
ratio ≥1.5). The exclusion criteria were as follows: i) Liver
transplant; ii) the presence of any concomitant illness; and iii)
the use of liver support devices. All enrolled participants were
hospitalized and followed-up at the Zhejiang Provincial People's
Hospital, People's Hospital of Hangzhou Medical College (Hangzhou,
China). Written informed consent was obtained from subjects prior
to participation. The study was performed according to the Ethical
Principles for Medical Research Involving Human Subjects outlined
in The Helsinki Declaration in 1975, and the research was approved
by the Ethics Committee of Zhejiang Provincial People's Hospital,
People's Hospital of Hangzhou Medical College (approval no.
ZRY-T123-59).
Construction of the animal model
A total of 21 C57BL/6 male mice (age, 6–8 weeks;
weight, 18–22 g), which were randomly divided into three groups
(n=7 mice/group), were obtained from the Experimental Animal Center
of the Chinese Academy of Sciences. All mice were raised at a room
temperature of 22±2°C and 55±5% relative humidity, with free access
to food and water. Mice were acclimated to the environment for 6–7
days under a 12-h dark/light cycle.
The combination of LPS/GalN (MilliporeSigma) was
dissolved and injected intraperitoneally (400 mg/kg GalN and 10
µg/kg LPS). To assess the role of miR-21 in LPS/GalN-induced ALF,
mice were challenged with a tail vein injection of 20 nmol miRNA
antagomir negative control (NC) or 20 nmol miR-21 antagomir (both
from Shanghai GenePharma Co., Ltd.). The mice were then challenged
intraperitoneally with 10 µg/kg LPS and 400 mg/kg GalN, or
phosphate-buffered saline (PBS; control), after 48 h. Mice were
sacrificed if they exhibited listlessness, respiratory
abnormalities, loss of appetite, weight loss (maximum 20%) and
disordered mobility. Otherwise, the mice were sacrificed 24 h after
LPS/GalN injection. Mice were euthanized by cervical dislocation
following anesthesia with an intraperitoneal injection of sodium
pentobarbital (35 mg/kg). All procedures were conducted in
accordance with the guide for the care and use of laboratory
animals (19) and the study was
approved by the Ethics Committee of Zhejiang Provincial People's
Hospital, People's Hospital of Hangzhou Medical College (approval
no. 20190010).
Cell culture
THP-1 cells were purchased from Pricella Life
Science & Technology Co., Ltd. The cells (3×105
cells/well) were cultured in 6-well plates in RPMI-1640 (cat. no.
11875119; Gibco; Thermo Fisher Scientific, Inc.) containing 10%
heat-inactivated fetal calf serum (cat. no. 12484028; Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Hyclone;
Cytiva) at 37°C in a 5% CO2 incubator. To obtain
THP-1-derived macrophages, THP-1 cells were then treated with 160
ng/ml phorbol 12-myristate 13-acetate (PMA; cat. no. P8139;
MilliporeSigma) followed by 24 h of incubation at 37°C in a 5%
CO2 incubator in RPMI-1640 to obtain M0 macrophages. The
macrophages were polarized to M1 macrophages by incubation with 10
pg/ml LPS (MilliporeSigma) and 20 ng/ml IFN-γ (R&D Systems,
Inc.) for 18 h at 37°C in a 5% CO2 incubator. Then, the
THP-1-derived macrophages were maintained in fresh RPMI-1640.
RNA extraction, and quantification of
mRNA and miRNA expression
Total miRNA was extracted and purified from 150 µl
mouse human plasma, which was obtained by centrifuging blood
samples at 2,000 × g at 4°C for 10 min using the miRNeasy
Serum/Plasma Kit (cat. no. 217184; Qiagen GmbH) according to the
manufacturer's instructions. The quality and concentration were
assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.) based on the ratio of absorbance at 260 nm. The
purified miRNA was immediately reverse-transcribed using the
miScript II Reverse Transcription Kit (Qiagen GmbH) according to
the manufacturer's instructions. The expression levels of serum
miR-21 were quantified using an Applied Biosystems 7500 real-time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
qPCR was performed using miScript SYBR Green PCR Kit with primers
specific for miR-21 (all from QIAGEN GmbH) according to the
manufacturer's instructions. The thermocycling protocol was: 15 min
at 95°C, followed by 40 cycles at 94°C for 15 sec, 55°C for 30 sec
and 70°C for 30 sec. The primer sequences are listed in Table I.
 | Table I.Primers for quantitative PCR
analysis. |
Table I.
Primers for quantitative PCR
analysis.
| Gene | Forward primer,
5′-3′ | Reverse primer,
5′-3′ |
|---|
| H-miR-21 |
TGCGGCTAGCTTATCAGACT | Universal primer:
GTGCAGGGTCCGAGGT |
| H-U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
| H-KLF6 |
CAAGGGAAATGGCGATGCCT |
CTTTTCTCCTGTGTGCGTCC |
| H-IL-23 |
GCTTCAAAATCCTTCGCAG |
TATCTGAGTGCCATCCTTGAG |
| GAPDH |
AGGCCGGATGTGTTCGC |
CAAATCCGTTGACTCCGACC |
| m-miR-21 |
TGCGGCTAGCTTATCAGACTGA | Universal primer:
GTGCAGGGTCCGAGGT |
| m-U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
Total RNA was extracted from THP-1-derived
macrophages and from mouse liver tissues, which were collected when
mice were sacrificed, using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). The quality and concentration of
RNA were assessed using a NanoDrop 2000 spectrophotometer based on
the ratio of absorbance at 260 nm. A PrimeScript ™ RT Reagent kit
(Takara Bio, Inc.) was used to reverse transcribe 2 µg RNA into
cDNA using the following protocol: 25°C for 5 min, 42°C for 30 min
and 85°C for 5 min, followed by maintenance at 4°C for 5 min.
Amplification of the cDNA was performed by qPCR using a SYBR Premix
Ex Taq™ II kit (Takara Bio, Inc.). The thermocycling protocol was:
95°C for 3 min, followed by 40 cycles of denaturation at 95°C for
30 sec, annealing at 60°C for 30 sec and extension at 72°C for 1
min, and a final extension step at 72°C for 7 min. The primer
sequences are listed in Table I.
The relative expression levels of mRNA and miRNA were calculated
using the 2−ΔΔCq method (20). GAPDH was used as an endogenous
control for mRNA expression and U6 was used as an endogenous
control for miRNA expression. The specificity of the products was
verified using melting curve analysis.
Western blot analysis and
antibodies
Total protein was extracted from mouse liver tissue
and THP-1-derived macrophages using RIPA lysis buffer (Beyotime
Institute of Biotechnology) and proteins were quantified using a
bicinchoninic acid protein assay kit (cat. no. P0012S; Beyotime
Institute of Biotechnology). An equal amount of protein (50
µg/lane) was separated by SDS on 12% gels and was then transferred
to a nitrocellulose blotting membrane. The membranes were blocked
with 5% non-fat milk dissolved in TBS-0.1% Tween buffer for 2 h at
room temperature and were then probed with the following primary
antibodies: IL-23 (1:1,000; cat. no. ab190356; Abcam), KLF6
(1:1,000; cat. no. DF13114; Affinity Biosciences), LC3A/B (1:1,000;
cat.no. ab62721; Abcam), p62 (1:1,000; cat. no. ab155686; Abcam),
ATG7 (1:1,000; cat. no. ab133528; Abcam), Beclin1 (1:1,000; cat.
no. ab207612; Abcam) and GAPDH (1:2,500; cat. no. ab9485; Abcam).
The membranes were then incubated with HRP-conjugated anti-mouse or
anti-rabbit secondary antibodies (1:2,000; cat. nos. ab6789 and
ab6721; Abcam) for 2 h at room temperature. Signals were visualized
using enhanced chemiluminescence reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Densitometric
analysis was performed using ImageJ (Version 1.49; National
Institutes of Health).
Histological changes in mouse
livers
Liver sections collected from mice after sacrifice
were fixed with 10% neutral-buffered formalin for 36 h at room
temperature, embedded in paraffin and cut into 5-µm slices. After
deparaffinization and rehydration, the slices were stained with 1%
hematoxylin for 10 min and 0.5% eosin for 3 min at room
temperature. Histological changes were evaluated using a light
microscope by two experienced pathologists.
ELISA
Plasma was obtained by centrifuging mouse blood
samples at 2,000 × g for 10 min at 4°C and was then stored at
−80°C. Alanine transaminase (ALT; cat. no. C009-2; Nanjing
Jiancheng Bioengineering Institute), aspartate transaminase (AST;
cat. no. C010-2; Nanjing Jiancheng Bioengineering Institute) and
IL-23 (cat. no. BMS6017TEN; Invitrogen; Thermo Fisher Scientific,
Inc.) kits were used to assess the levels of ALT, AST and IL-23,
according to the manufacturers' protocols. The signal was measured
at 450 nm excitation using a SpectraMax® i3
multifunctional microplate reader (Molecular Devices, LLC). After
the standard curve was established, the plasma levels were
calculated based on the absorbance values of each sample.
Cell transfection
The miR-21 mimics (sense:
5′-UAGCUUAUCAGACUGAUGUUGA-3′; antisense:
5′-AACAUCAGUCUGAUAAGCUAUU-3′), miR-21 inhibitor
(5′-UCAACAUCAGUCUGAUAAGCUA-3′), miR-21 mimics negative control (NC)
(sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense:
5′-ACGUGACACGUUCGGAGAATT-3′) and miR-21 inhibitor NC
(5′-CAGUACUUUUGUGUAGUACAA-3′) were purchased from Guangzhou RiboBio
Co., Ltd. THP-1-derived macrophages (3×105 cells/well)
were inoculated in 6-well plates and the transfection experiment
was started when the cell confluence reached 70%. During
transfection, 12 µl Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) was added to 150 µl serum-free
opti-MEM (cat. no. 31985070; Gibco; Thermo Fisher Scientific, Inc.)
and mixed with 10 µl miR-21 mimics/inhibitors, then maintained at
room temperature for 20 min. The final concentration of miR-21
mimics and miR-21 mimics NC was 50 nM, and the final concentration
of the miR-21 inhibitor and miR-21 inhibitor NC was 100 nM. After
20 min at room temperature, the mixed solution was added to the
6-well plates to complete the transfection and incubated for 6 h at
37°C in a 5% CO2 incubator. Then, the medium was
replaced with RPMI-1640 containing 10% heat-inactivated fetal calf
serum and 1% penicillin-streptomycin. The cells were then incubated
for 48 h at 37°C in a 5% CO2 incubator.
In addition, THP-1-derived macrophages
(3×105 cells/well) were cultured with 5 µM rapamycin
(cat. no. 53123-88-9; MedChemExpress), which is an autophagy
inducer. After incubation for 1 h at 37°C in a 5% CO2
incubator, THP-1-derived macrophages were transfected with miR-21
mimics or miR-21 mimics NC using Lipofectamine 2000 for 6 h at 37°C
in a 5% CO2 incubator. Then, the medium was replaced
with RPMI-1640 containing 10% heat-inactivated fetal calf serum and
1% penicillin-streptomycin and incubated for 48 h at 37°C in a 5%
CO2 incubator.
Furthermore, 1 µg KLF6 overexpression plasmid (cat.
no. HG12365-ACG; Sino Biological, Inc.), with the pCMV3 backbone, 1
µg pCMV3-C-GFPSpark control vector (cat. no. CV026; Sino
Biological, Inc.), 50 nM miR-21 mimics NC and 50 nM miR-21 mimics
were transfected into THP-1-derived macrophages using Lipofectamine
2000 for 6 h at 37°C in a 5% CO2 incubator. Then, the
medium was replaced with RPMI-1640 containing 10% heat-inactivated
fetal calf serum and 1% penicillin-streptomycin and incubated for
48 h at 37°C in a 5% CO2 incubator.
Analysis of target genes of miRNA
using TargetScan
Target genes of miR-21 were evaluated using
TargetScan 8.0 software (https://www.targetscan.org/vert_80/).
Luciferase activity assay
For the luciferase assay, the KLF6-expression
construct was generated using a full-length of clone KLF6, which
was inserted into psiCHECK™-2 Vector (cat. no. C8021; Promega
Corporation). The miR-21-5p binding site found in the 3′-UTR of the
KLF6 mRNA using TargetScan 8.0 software, and its mutated version,
were cloned into a modified pGL3-control vector (cat. no. E1741;
Promega Corporation). Subsequently, 293T cells (cat. no. CL-0005;
Procell Life Science & Technology Co., Ltd.) were cotransfected
with reporter constructs containing wild-type (WT) or mutant (MUT)
KLF6 3′-UTR and miR-21-5p mimics (50 nM) or miR-21 mimics NC (50
nM) using Lipofectamine 2000. Then, 293T cells were harvested and
lysed 48 h after transfection. Luciferase reporter activities were
evaluated using the Dual-Glo Luciferase Assay System (Promega
Corporation). Firefly luciferase activity was standardized to
Renilla luciferase activity.
GFP-LC3 analyses
After cotransfection of THP-1-derived macrophages
with miR-21 mimics or miR-21 mimics NC and 1.5 µg pCMV-GFP-LC3B
(cat. no. D2815; Beyotime Institute of Biotechnology) using
Lipofectamine 2000 for 6 h at 37°C in a 5% CO2
incubator. Then, the medium was replaced with RPMI-1640 containing
10% heat-inactivated fetal calf serum and 1%
penicillin-streptomycin and incubated for 24 h at 37°C in a 5%
CO2 incubator. Nuclei were stained with DAPI for 20 min
at 4°C in the dark. Images of GFP-LC3 puncta were captured under a
confocal fluorescence microscope (Leica Microsystems, Inc.).
Statistical analysis
All data were assessed using GraphPad 5.0
(Dotmatics) and SPSS 19.0 (IBM Corp.). Results are presented as the
mean ± standard deviation. Comparisons between two groups were
carried out via paired Student's t-test or unpaired Student's
t-tests. Multiple comparisons were carried out via homogeneity test
of variance followed by one-way ANOVA and Tukey's post hoc test.
Two-tailed P≤0.05 was considered to indicate a statistically
significant difference. Each in vitro experiment was
repeated three times.
Results
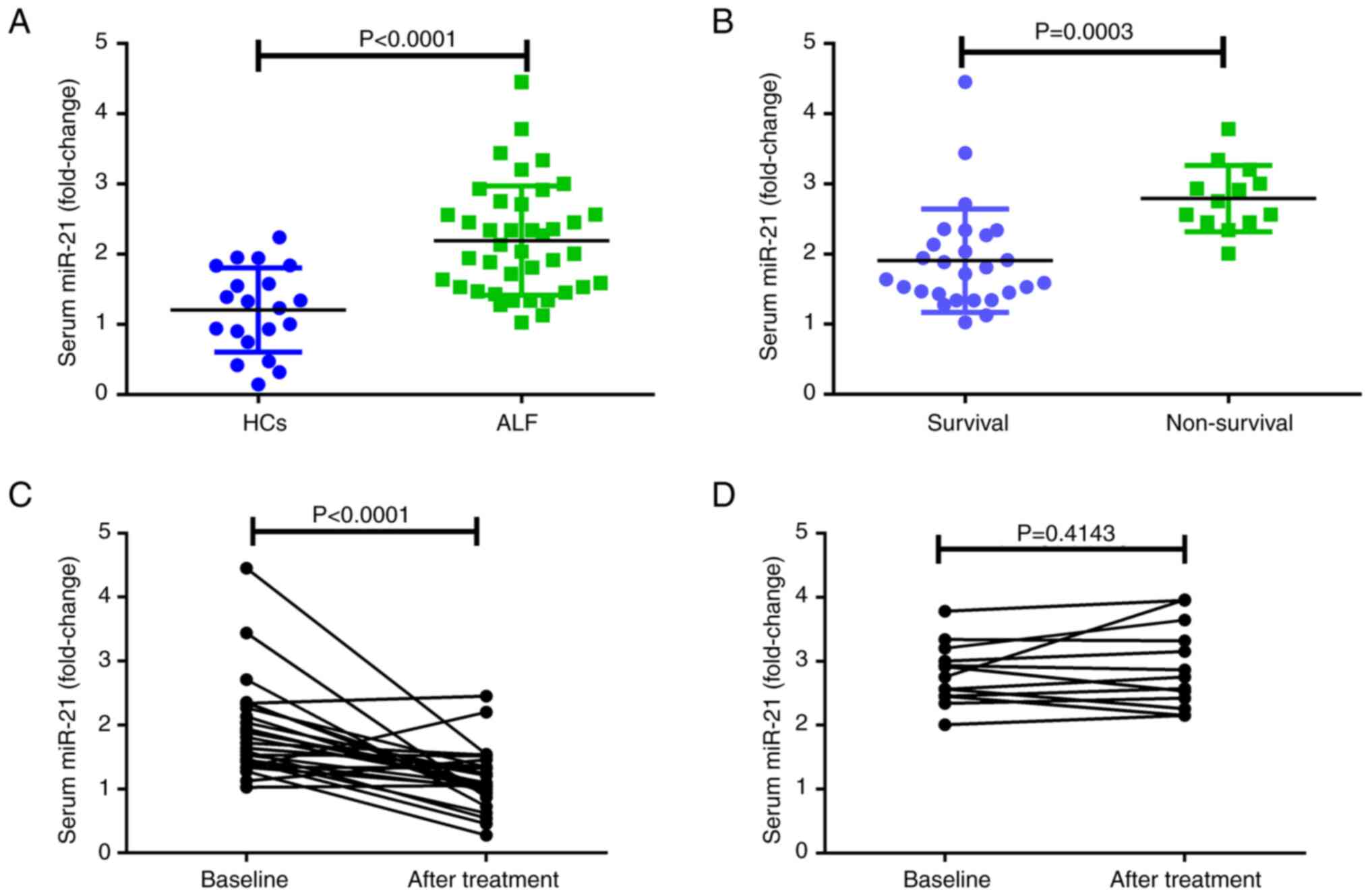
Serum miR-21 levels are significantly
increased in patients with ALF
Serum miR-21 levels, detected by qPCR, were compared
between the HC and ALF groups (Fig.
1A), and the results suggested that the serum levels of miR-21
were significantly higher in the ALF group than those in the HC
group. Moreover, serum miR-21 levels were highly upregulated in 13
non-surviving patients with ALF compared with those in the 27
surviving patients with ALF (Fig.
1B). To explore the role of miR-21 in patients with ALF, serum
miR-21 levels were evaluated before and after treatment in 27
surviving and 13 non-surviving patients with ALF before and after
treatment (Fig. 1C and D). The
results demonstrated that serum miR-21 levels were markedly
decreased in the surviving group of patients with ALF after
treatment. However, there was no statistically significant
difference in serum miR-21 levels before and after treatment in
non-surviving patients with ALF.
LPS/GalN-induced ALF is attenuated in
antagomir-21-treated mice
To further explore the role of miR-21 in the
pathogenesis of ALF, the effects of miR-21 antagomir were evaluated
in an LPS/GalN-induced male mouse model of ALF, which has a more
stable physical condition with fewer individual differences
compared with a female mouse model of ALF, similar to the liver
failure model we have previously constructed (3). Briefly, following treatments, serum
was isolated and liver tissues were collected. The expression
levels of miR-21 in the liver tissues were evaluated by qPCR
(Fig. 2A). The results
demonstrated that the expression levels of miR-21 were
significantly increased in the LPS/GalN-induced ALF mouse model
compared with in the PBS control group. However, the injection of
antagomir-21 blocked the upregulation of miR-21, demonstrating that
a mouse model of LPS/GalN-induced ALF with miR-21 inhibition was
successfully constructed. To explore the role of miR-21 in the
LPS/GalN-induced ALF mouse model, the present study further
explored liver function and pathological damage to the liver. As
shown in Fig. 2B, ALT and AST
levels in the LPS/GalN group were significantly increased, whereas
they were decreased in the antagomir-21 ALF mouse group. Similarly,
the degree of liver damage, including disordered structure of liver
lobules, increased infiltration of inflammatory cells (blue arrow),
hyperchromatic nuclei, hypochromatosis and appearance of necrosis
(red arrow), in the LPS/GalN-induced ALF mouse model was markedly
aggravated, whereas the degree of the aforementioned liver damage
was relieved in the antagomir-21 LPS/GalN mouse group (Fig. 2C). These results suggested that
LPS/GalN-induced ALF is attenuated by antagomir-21.
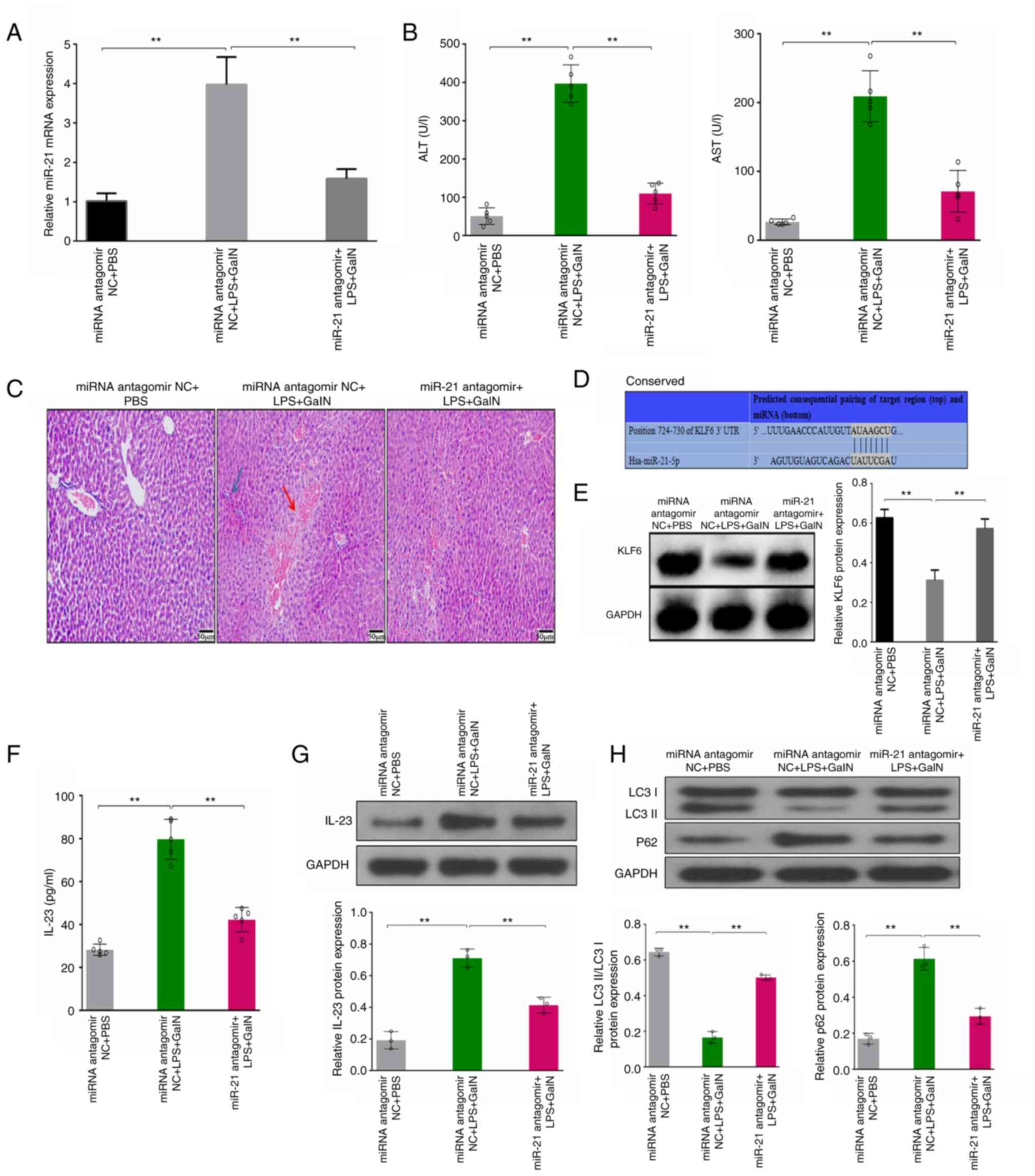 | Figure 2.LPS/GalN-induced ALF is attenuated in
antagomir-21-treated mice. (A) Relative expression levels of miR-21
in liver tissues from various groups were analyzed by quantitative
PCR. (B) Serum ALT and AST levels were measured by ELISA in each
group. (C) Representative photomicrographs of hematoxylin and
eosin-stained sections of liver tissue from each group
(magnification, ×50). (D) TargetScan database demonstrated that
KLF6 was a potential target of miR-21 with one putative miR-21-5p
seed match site. (E) Protein expression levels of KLF6 were
evaluated by western blotting of the liver tissues from each
groups. (F) Serum IL-23 levels from various groups were measured by
ELISA. Protein expression levels of (G) IL-23, and (H) LC3II/LC3I
and p62 were evaluated by western blotting in liver tissues from
various groups. **P<0.01. ALT, alanine transaminase; ALF, acute
liver failure; AST, aspartate transaminase; GalN, D-galactosamine;
IL, interleukin; LPS, lipopolysaccharide; miR, microRNA; NC,
negative control. |
To further evaluate the potential mechanisms of
miR-21, KLF6 (GenBank accession number: NM_001300.6) was considered
a target of miR-21, with one putative miR-21-5p seed match site
determined using the TargetScan database (Fig. 2D). Therefore, the expression levels
of KLF6 were assessed, and the results demonstrated that the
protein expression levels of KLF6 were markedly decreased in the
LPS/GalN group compared with those in the PBS control group. By
contrast, the decreased expression of KLF6 protein in the
LPS/GalN-induced ALF mouse model was abrogated in the antagomir-21
mouse group (Fig. 2E).
Furthermore, the levels of IL-23 were detected in
the antagomir-21 mouse model. The results demonstrated that serum
IL-23 was significantly suppressed in the antagomir-21 ALF mouse
group compared with that in the miRNA antagomir NC ALF mouse group,
as detected by ELISA (Fig. 2F). As
shown in Fig. 2G, the protein
expression levels of IL-23 in the liver tissues exhibited the same
trend, as determined by western blotting. These results indicated
that miR-21 may promote the expression of IL-23.
Moreover, the levels of autophagy markers were
explored in the antagomir-21 mouse model. The results demonstrated
that the LC3II/I ratio was upregulated and the protein expression
levels of p62 were reduced in the antagomir-21 ALF mouse group
compared with those in the miRNA NC ALF mouse group (Fig. 2H). These results demonstrated that
miR-21 could inhibit autophagy.
miR-21 promotes the expression of
IL-23 and inhibits the expression of KLF6
To explore the underlying mechanisms of miR-21, the
miR-21 inhibitor, miRNA inhibitor NC, miR-21 mimics and miR-21
mimics NC were used to interfere with the expression levels of
miR-21 in THP-1-derived macrophages, which was confirmed by qPCR.
As shown in Fig. 3A, the
transfection efficiency of the miR-21 inhibitor and miR-21 mimics
were confirmed by qPCR. The relative expression levels of miR-21
were significantly upregulated in the miR-21 mimics group compared
with those in the miR-21 mimics NC group, and the relative
expression levels of miR-21 were significantly decreased in the
miR-21 inhibitor group compared with those in the miRNA inhibitor
NC group. Therefore, the miR-21 mimics and miR-21 inhibitor were
used for subsequent overexpression and knockdown experiments.
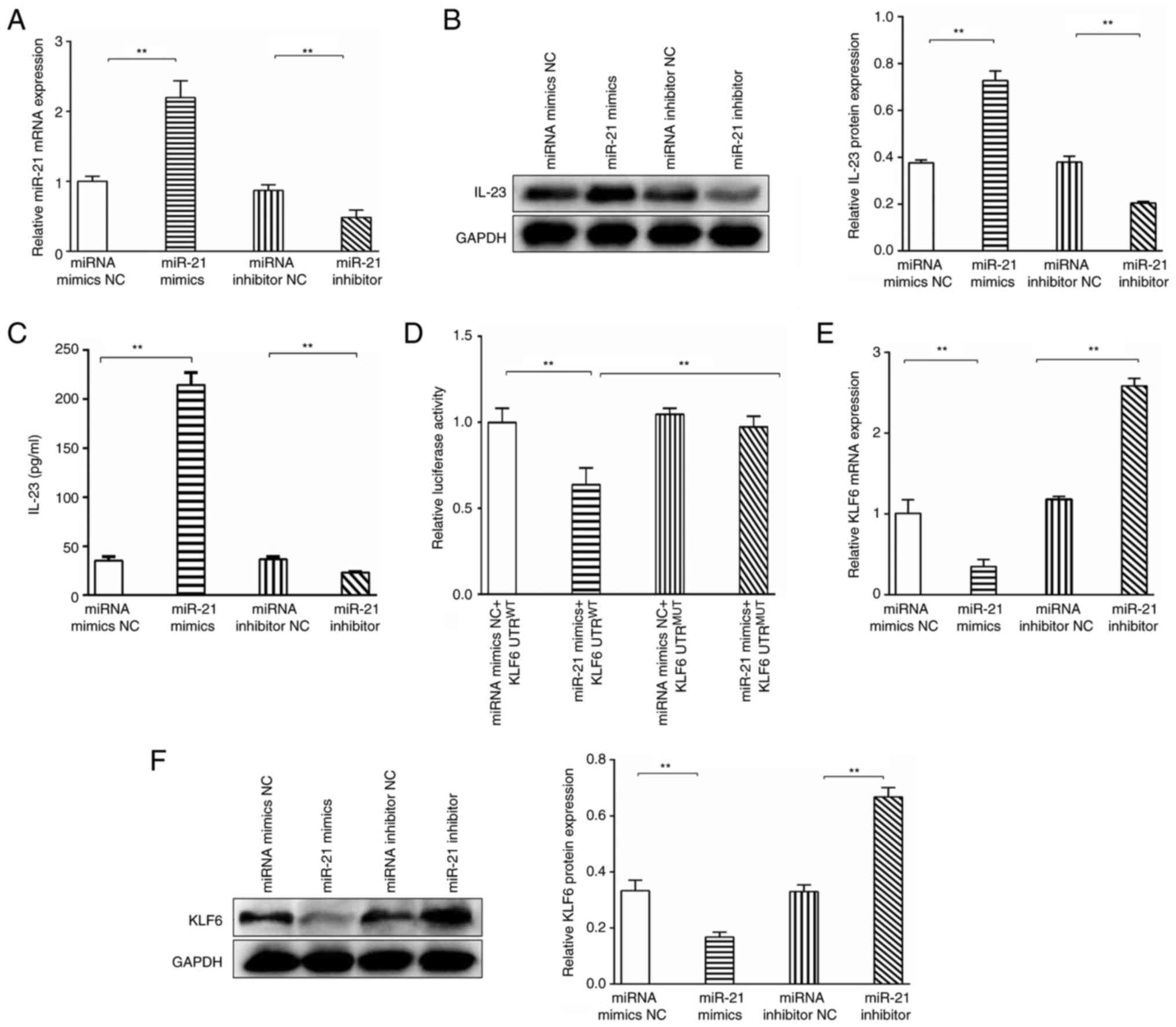 | Figure 3.miR-21 promotes the expression of
IL-23 and inhibits the expression of KLF6. (A) miR-21 mimics,
miR-21 mimics NC, miR-21 inhibitor and miRNA inhibitor NC were
constructed and transfected into THP-1-derived macrophages; the
expression levels of miR-21 were confirmed by qPCR. (B) IL-23
protein expression levels were analyzed in THP-1-derived
macrophages post-transfection by western blotting. (C) Levels of
IL-23 in the cell supernatants were evaluated by ELISA. (D)
Reporter constructs (pGL3-KLF6) with putative WT or MUT 3′-UTRs
downstream of the firefly luciferase were cloned and cotransfected
into cells with miR-21 mimics NC or miR-21 mimics, and relative
luciferase activity was evaluated in the various groups. The
relative expression levels of KLF6 were evaluated by (E) qPCR and
(F) western blotting in various groups. **P<0.01. IL,
interleukin; KLF6, Krüppel-like-factor-6; miR, microRNA; NC,
negative control; MUT, mutant; ns, not significant; qPCR,
quantitative PCR; WT, wild-type. |
Our previous study suggested that IL-23 was highly
increased in patients with ALF (3). To explore whether a relationship
exists between miR-21 and IL-23 in THP-1-derived macrophages, the
expression levels of IL-23 were explored in the miR-21
overexpression and knockdown groups. The results demonstrated that
IL-23 expression was significantly increased in the miR-21 mimics
group compared with that in the miR-21 mimics NC group, whereas
IL-23 expression was significantly suppressed in the miR-21
inhibitor group compared with that in the miRNA inhibitor NC group,
as detected by western blotting (Fig.
3B). As shown in Fig. 3C, the
levels of IL-23 in the cell supernatants exhibited the same trend,
as determined by ELISA. These results indicated that miR-21
promoted the expression of IL-23.
KLF6 was identified as a target gene of miR-21, with
one putative miR-21-5p seed match site (KLF6 3′-UTR 724–730), using
the TargetScan database. To explore whether miR-21-5p directly
targets the KLF6 mRNA 3′-UTR (724–730 bp), reporter constructs
(pGL3-KLF6) with putative WT or MUT 3′-UTR downstream of the
firefly luciferase were cloned. 293T cells were cotransfected with
pGL3-KLF6 and miR-21-5p mimics or miR-21 mimics NC. The results
demonstrated that transfection with the miR-21-5p mimics resulted
in a significant downregulation of the luciferase activity of the
reporter plasmid containing the WT 3′-UTR; however, the
downregulation of luciferase activity was fully abolished after
point mutation in the miR-21-5p binding site in the 3′-UTR of KLF6
(3′-UTR MUT) (Fig. 3D). These
results indicated a direct interaction between miR-21-5p and the
3′-UTR of KLF6 mRNA.
To further confirm the bioinformatics-based
predictions, THP-1-derived macrophages were transfected with miR-21
inhibitor or miR-21 mimics, and the mRNA and protein expression
levels of KLF6 were evaluated by qPCR and western blotting,
respectively. The relative expression levels of KLF6 were
downregulated in the miR-21 mimics group compared with those in the
miR-21 mimics NC group. However, introduction of the miR-21-5p
inhibitor resulted in upregulation of the mRNA expression levels of
KLF6 compared with those in the miRNA inhibitor NC group (Fig. 3E). As shown in Fig. 3F, KLF6 protein expression exhibited
the same trend, as determined by western blotting. These results
indicated that miR-21 inhibited the expression of KLF6.
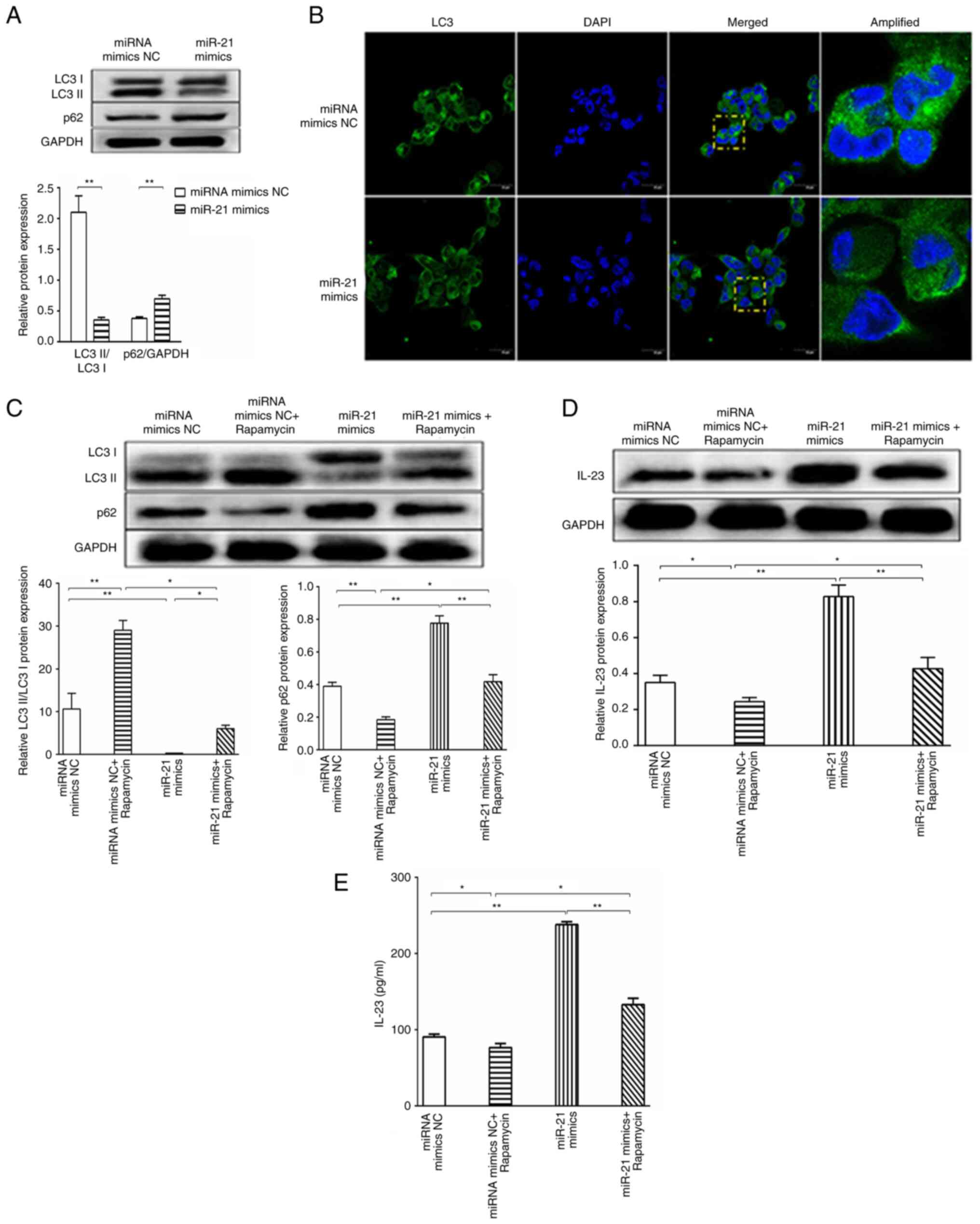
miR-21 promotes the expression of
IL-23 via inhibiting autophagy
It has been reported that 5-methoxytryptophan
effectively alleviates liver cirrhosis by regulating
FOXO3a/miR-21/ATG5 signaling pathway-mediated autophagy (21); therefore, it was hypothesized that
miR-21 promoted the expression of IL-23 by impairing autophagy in
patients with ALF. To experimentally verify this hypothesis,
THP-1-derived macrophages were transfected with miR-21 mimics or
miR-21 mimics NC. As expected, LC3I to LC3II conversion was highly
upregulated and p62 protein expression was decreased in the miR-21
mimics NC group compared with those in the miR-21 mimics group, as
determined by western blotting (Fig.
4A), which demonstrated that miR-21 inhibited autophagy.
Consistently, overexpression of miR-21 highly suppressed GFP-LC3
puncta formation (Fig. 4B).
Moreover, THP-1-derived macrophages were cultured with 5 µM
rapamycin, which is an autophagy inducer. After 1 h, THP-1-derived
macrophages were transfected with the miR-21 mimics or miR-21
mimics NC. The results demonstrated that the miRNA mimics NC +
rapamycin group exhibited increased LC3I to LC3II conversion,
decreased p62 protein expression (Fig.
4C) and decreased IL-23 protein expression (Fig. 4D) compared with in the miRNA mimics
NC group. In the miR-21 mimics group, LC3I to LC3II conversion was
decreased, p62 protein expression was increased (Fig. 4C) and IL-23 protein expression was
increased (Fig. 4D) compared with
in the miRNA mimics NC group. Compared with in the miR-21 mimics
group, the miR-21 mimics + rapamycin group exhibited increased LC3I
to LC3II conversion, decreased p62 protein expression (Fig. 4C) and decreased IL-23 protein
expression (Fig. 4D). Compared
with in the miRNA mimics NC + rapamycin group, the miR-21 mimics +
rapamycin group exhibited decreased LC3I to LC3II conversion,
increased p62 protein expression (Fig.
4C) and increased IL-23 protein expression (Fig. 4D). Similarly, the cell supernatant
levels of IL-23 exhibited the same trend, as determined by ELISA
(Fig. 4E). In conclusion, these
results demonstrated that miR-21 promoted the expression of IL-23
via inhibiting autophagy.
KLF6 promotes autophagy, and KLF6 blocks
miR-21-induced increase in IL-23 levels. To explore the underlying
mechanisms of KLF6, a KLF6 overexpression plasmid was used to
induce the overexpression of KLF6, which was confirmed by western
blotting. The KLF6 overexpression plasmid significantly increased
the expression levels of KLF6 in THP-1-derived macrophages compared
with the vector group (Fig. 5A),
indicating that the KLF6 overexpression plasmid was successfully
constructed. To explore whether the KLF6 overexpression plasmid
inhibited the downregulation of KLF6 induced by miR-21,
THP-1-derived macrophages were transfected with 50 nM miR-21 mimics
or 50 nM miR-21 mimics NC, and 1 µg KLF6 overexpression plasmid or
1 µg KLF6 control plasmid. The results demonstrated that miR-21
mimics could significantly inhibit KLF6 protein expression compared
with miRNA mimics NC group, and KLF6 overexpression could
significantly inhibit the downregulation of KLF6 expression induced
by miR-21 mimics compared with the miR-21 mimics group (Fig. 5B).
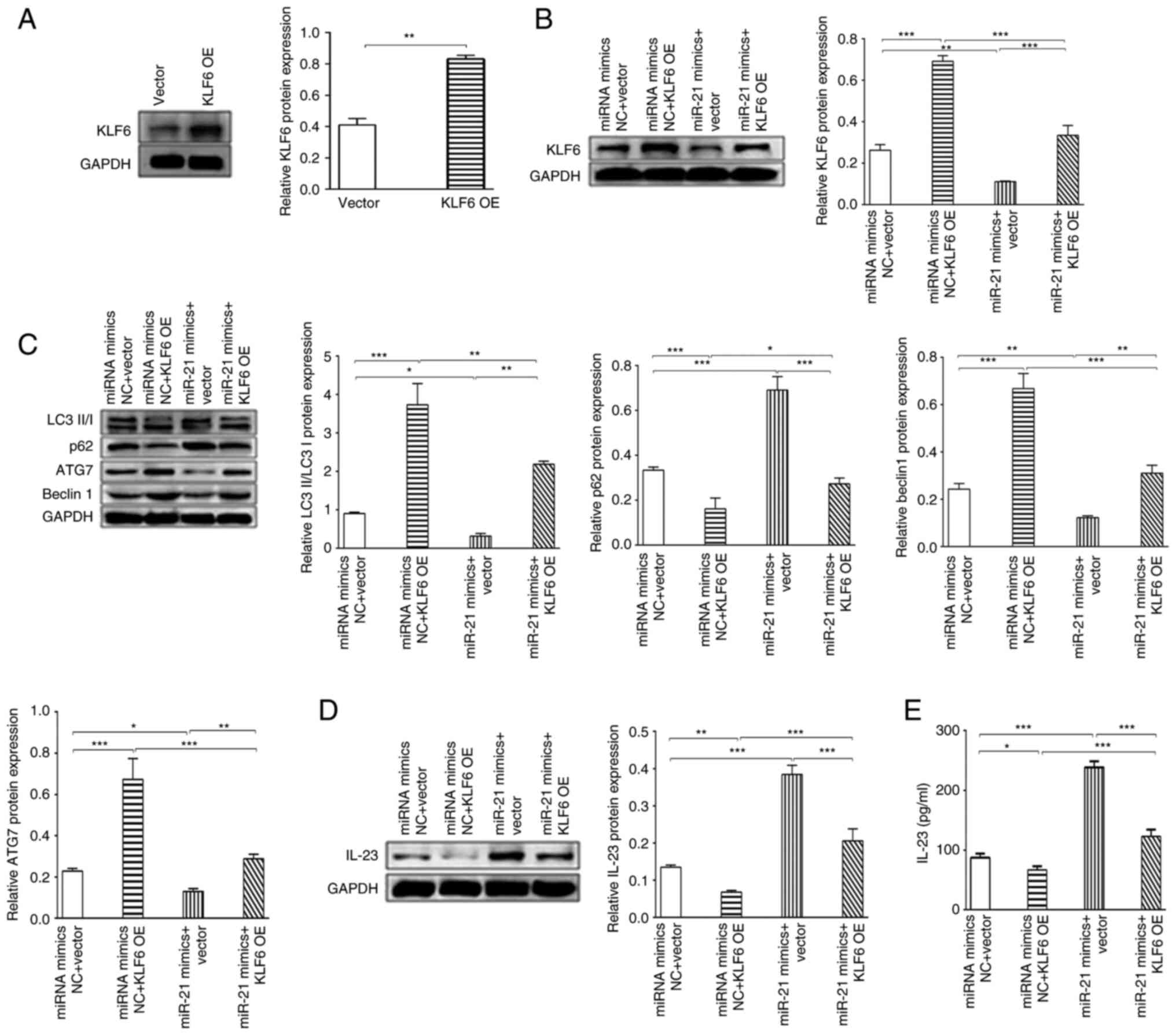 | Figure 5.KLF6 promotes autophagy, and KLF6
blocks the miR-21-induced increase in IL-23 levels. (A) KLF6 OE
plasmid and NC plasmid were transfected into THP-1-derived
macrophages, and the expression levels of KLF6 were confirmed by
western blotting. (B) Relative protein expression levels of KLF6
were evaluated by western blotting in THP-1-derived macrophages
post-transfection. (C) Western blot analysis of LC3II/I, p62, ATG7
and Beclin 1 expression in macrophages post-transfection. (D) IL-23
expression was evaluated in THP-1-derived macrophages
post-transfection. (E) ELISA evaluated the expression of IL-23 in
the supernatant post-transfection. *P<0.05, **P<0.01,
***P<0.001. IL, interleukin; KLF6, Krüppel-like-factor-6; miR,
microRNA; NC, negative control; OE, overexpression. |
The present study also evaluated whether KLF6
blocked the miR-21-induced decrease in autophagy levels. Compared
with in the miRNA mimics NC group, overexpression of KLF6 could
significantly upregulate the LC3II/I ratio, and the protein
expression levels of Beclin1 and ATG7, and reduce the protein
expression levels of p62 (Fig.
5C). Further study suggested that overexpression of KLF6 could
upregulate miR-21-induced LC3II/I ratio, and the protein expression
levels of Beclin1 and ATG7, and downregulate the protein expression
levels of p62, which suggested that overexpression of KLF6 could
block miR-21-induced downregulation of autophagy (Fig. 5C).
Moreover, the present study explored whether KLF6
blocked the miR-21-induced increase in IL-23 expression. The
results suggested that overexpression of KLF6 could significantly
reduce miR-21 mimics-induced IL-23 expression in THP-1-derived
macrophages compared with miR-21 mimics group (Fig. 5D). The levels of IL-23 exhibited
the same trend in the supernatant, as determined by ELISA (Fig. 5E). These results indicated that
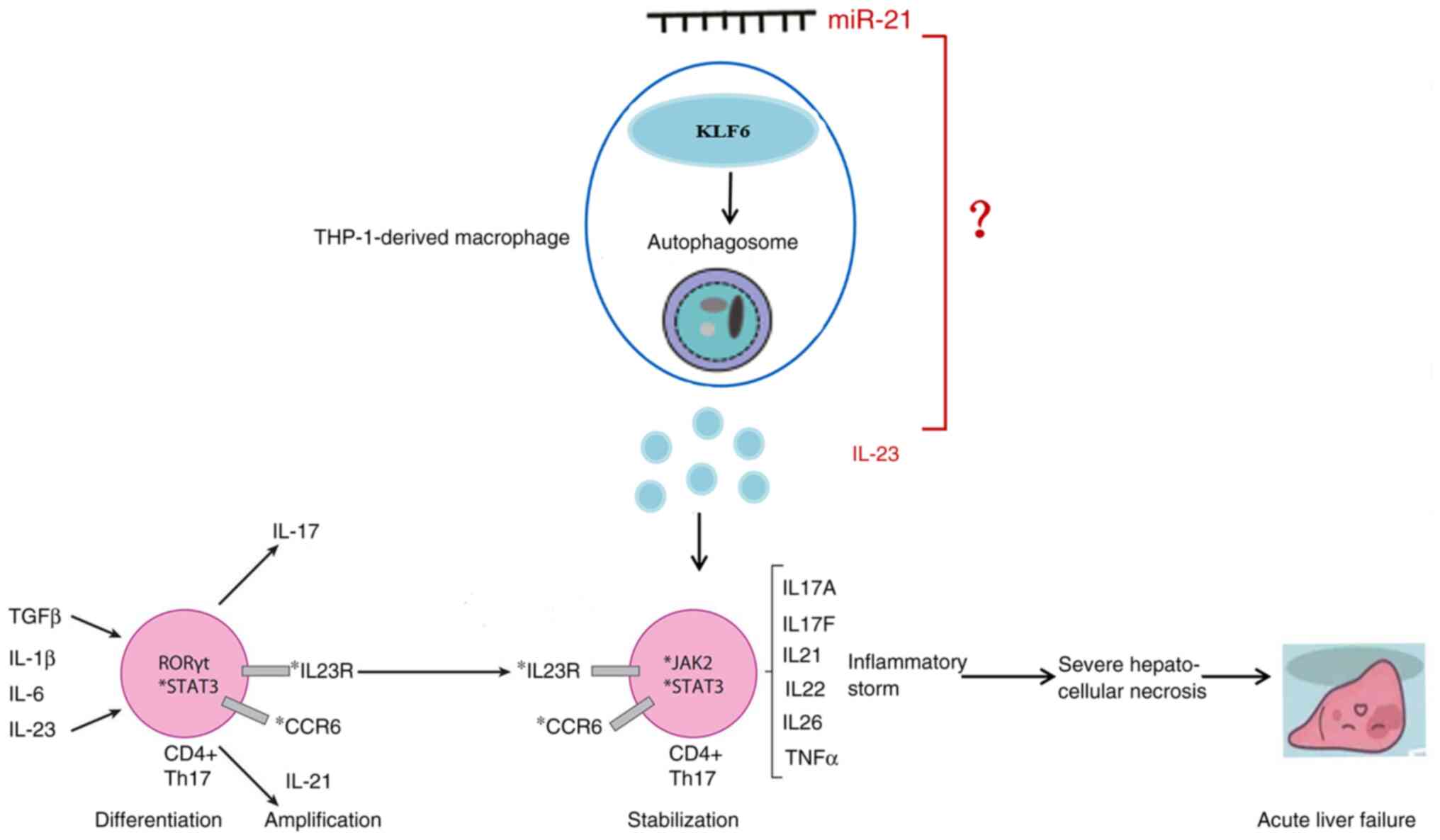
KLF6 blocked miR-21 induced-increases in IL-23 expression. In
conclusion, miR-21 promoted the expression of IL-23 by inhibiting
KLF6, which regulates autophagy, thus promoting inflammatory
storms, and subsequent severe hepatocellular necrosis and ALF
(Fig. 6).
Discussion
Although multiple miRNAs, including miR-21, have
been found to be key regulators of liver regeneration (22,23),
the potential mechanism in ALF is still incompletely understood.
The present study demonstrated that serum miR-21 levels were
significantly upregulated in patients with ALF. To explore the role
of miR-21, a mouse model of ALF was constructed using LPS/GalN. The
results suggested that the antagomir-21 treated mice alleviated
LPS/GalN-induced ALF, as demonstrated by changes in liver function
and liver pathology. Moreover, miR-21 promoted the expression of
IL-23 via inhibiting KLF6, and KLF6 regulated autophagy. These
results demonstrated that miR-21 may have a significant role in the
pathogenesis of LPS/GalN-induced ALF and that blocking the
expression of miR-21 could alleviate the degree of ALF.
miRNAs regulate gene expression at the
post-transcriptional level by inhibiting transcription or inducing
degradation of mRNA (24).
However, the role of miR-21 in ALF has not been completely
elucidated. Previous research has demonstrated that miR-21
contributes to ALF in septic mice by inhibiting PPARα expression
(25). The overexpression of
miR-21 has been shown to promote hepatocyte proliferation and liver
regeneration by targeting PTEN, thus suggesting that increased
miR-21 may be a treatment strategy to promote liver regeneration
(26). miR-21 might also be
considered a potential biomarker for the development and
progression of sepsis, which could predict the prognosis of
patients with sepsis (27).
Another study has demonstrated that dysregulation of miR-21 may
explain the abnormal inflammation and persistent M1 macrophage
polarization seen in diabetic wounds (28). The present study indicated that
serum miR-21 levels were increased in patients with ALF, and this
result was further verified in LPS/GalN-induced ALF mouse model.
The injection of antagomir-21, which negatively regulates miR-21
expression, alleviated LPS/GalN-induced ALF, as demonstrated by
pathological changes in the liver.
Autophagy is a ubiquitous homeostatic mechanism in
eukaryotic cells that serves an important role in maintaining cell
differentiation, development, homeostasis and survival (29). Autophagy is maintained at a low
level to regulate cellular homeostasis, such as protein or
organelle turnover, under normal physiological conditions (30). When the external environment
changes, such as in response to a lack of nutrients or stress,
autophagy can help cells survive (31). Studies have shown that certain
cytokines, such as IL-6 and IL-8, can regulate the production of
autophagy, and autophagy can also regulate the secretion of
cytokines, such as IL-1β and IL-10 (32–34).
The present study demonstrated that autophagy was significantly
downregulated in LPS/GalN-induced liver injury.
The diverse roles of the KLF family in regulating
autophagy have previously been reported. KLF15 transcriptionally
activates ATG14 to promote autophagy and attenuate damage of
oxidized low density lipoprotein-induced human aortic endothelial
cells (35). KLF4 strengthens the
sensitivity of colon cancer cells to 5-fluorouracil by targeting
RAB26 to restrain autophagy (36).
KLF2 critically regulates the neurogenesis of dental pulp-derived
stem cells by inducing mitophagy and autophagy (37). The present study demonstrated that
KLF6 promoted autophagy, and KLF6 blocks miR-21-induced increases
in IL-23 expression.
The mobilization and differentiation of immune cells
in the inflammatory site is caused and enhanced by inflammatory
cytokines, such as IL-6, IL-17 and IL-23, which serve key roles in
causing ALF (18).
Antigen-presenting cells, including macrophages, are important
modulators of immunity, and are important in adjusting innate and
adaptive immune responses (38). A
previous study suggested that KLF6 can promote inflammatory and
hypoxic responses in macrophages (39). Azilsartan, which is a drug
currently used to treat hypertension, attenuates oscillatory shear
stress-induced endothelial dysfunction and inflammatory responses
by upregulating KLF6 (40).
However, another study demonstrated that inhibiting KLF6 expression
in keratinocytes increases inflammatory responses during aging and
accelerates cellular senescence (41). Consequently, different disease
models, cell types or stimulation conditions may have a role in the
dual effect of KLF6 on inflammatory responses. Nevertheless, few
related studies have reported on the role of KLF6 in inflammatory
responses. The present study demonstrated that the inflammatory
cytokine IL-23 was significantly upregulated in serum samples from
patients with ALF, whereas overexpression of KLF6 could reduce the
expression of IL-23.
A previous study demonstrated that higher hepatic
KLF6 expression is associated with a better prognosis in primary
sclerosing cholangitis, which is a chronic liver disease, and KLF6
regulates FXR signaling by binding to FXREs (42). KLF6 can also affect lipid
metabolism and activate the mTOR signaling pathway, thereby
promoting the progression of renal clear cell carcinoma (43). Nevertheless, the potential
mechanisms are insufficiently understood. The present study
demonstrated that LPS/GalN-induced ALF was alleviated in
antagomir-21 mice. Further study demonstrated that miR-21 promoted
the expression of IL-23 by inhibiting KLF6, and KLF6 regulated
autophagy. Upregulated IL-23 is associated with inflammatory
storms, which have been suggested to be involved in multiple stages
of Th17 lineage fate, including in differentiation, expansion and
stabilization of Th17 cells (44).
TGF-β and IL-6 can induce the expression of retinoic acid-related
orphan receptor γt via STAT3 (45), which promote the differentiation of
Th17 cells. Upon engagement of IL-23 to its receptor complex,
sequential activation of JAK2 and STAT3 occurs (46). Subsequently, cytokines secreted by
Th17 cells lead to a combination of inflammatory storms, and
subsequent severe hepatocellular necrosis and ALF.
Since the mechanism was determined in vitro
and may not reflect the actual conditions in vivo, further
research is needed to expand these findings in mouse models. If
reproduced, miR-21 may represent a promising therapeutic strategy
for ALF. In conclusion, miR-21 promoted the expression of IL-23 by
targeting KLF6, which regulates autophagy, thus promoting
inflammatory storms, and subsequent severe hepatocellular necrosis
and ALF.
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhejiang Provincial Natural
Science Foundation of China (grant no. LQ20H030013), the Zhejiang
Province Medical and Health Science and Technology Program (grant
no. 2020361785), the National Natural Science Foundation of China
(grant no. 82100625), and the Fundamental Research Program Funding
of Ninth People's Hospital Affiliated to Shanghai Jiao Tong
University School of Medicine (grant no. JYZZ127).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SB, WZ and JX contributed to the research design,
experimental operation and statistical analysis. RY contributed to
the experimental operation and analysis. SB contributed to the
manuscript writing and funding. JX and WZ contributed to the
critical revision, and helped with the administration and technical
support. SB and WZ confirm the authenticity of all the raw data.
All authors contributed to the data acquisition and read and
approved the final manuscript.
Ethics approval and consent to
participate
The research was approved by the Ethics Committee of
Zhejiang Provincial People's Hospital, People's Hospital of
Hangzhou Medical College (approval no. for human studies:
ZRY-T123-59; approval no. for mice studies: 20190010; Hangzhou,
China) Written informed consent was obtained from all
participants.
Patient consent for publication
Patients provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tujios S, Stravitz RT and Lee WM:
Management of acute liver failure: Update 2022. Semin Liver Dis.
42:362–378. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bao S, Zheng J, Li N, Huang C, Chen M,
Cheng Q, Li Q, Lu Q, Zhu M, Ling Q, et al: Role of interleukin-23
in monocyte-derived dendritic cells of HBV-related acute-on-chronic
liver failure and its correlation with the severity of liver
damage. Clin Res Hepatol Gastroenterol. 41:147–155. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao S, Zhao Q, Zheng J, Li N, Huang C,
Chen M, Cheng Q, Zhu M, Yu K, Liu C and Shi G: Interleukin-23
mediates the pathogenesis of LPS/GalN-induced liver injury in mice.
Int Immunopharmacol. 46:97–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Portius D, Sobolewski C and Foti M:
MicroRNAs-dependent regulation of PPARs in metabolic diseases and
cancers. PPAR Res. 2017:70584242017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xue Z, Xi Q, Liu H, Guo X, Zhang J, Zhang
Z, Li Y, Yang G, Zhou D, Yang H, et al: miR-21 promotes NLRP3
inflammasome activation to mediate pyroptosis and endotoxic shock.
Cell Death Dis. 10:4612019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu K, Zheng QK, Ma RJ, Ma C, Sun ZG and
Zhang N: Kruppel-like factor 6 splice variant 1: An oncogenic
transcription factor involved in the progression of multiple
malignant tumors. Front Cell Dev Biol. 9:6617312021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mallipattu SK, Horne SJ, D'Agati V, Narla
G, Liu R, Frohman MA, Dickman K, Chen EY, Ma'ayan A, Bialkowska AB,
et al: Krüppel-like factor 6 regulates mitochondrial function in
the kidney. J Clin Invest. 125:1347–1361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Yu D, He C, Yu Q, Huo Z, Zhang Y and
Zhang S: KLF6 alleviates hepatic ischemia-reperfusion injury by
inhibiting autophagy. Cell Death Dis. 14:3932023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sydor S, Manka P, Best J, Jafoui S, Sowa
JP, Zoubek ME, Hernandez-Gea V, Cubero FJ, Kälsch J, Vetter D, et
al: Kruppel-like factor 6 is a transcriptional activator of
autophagy in acute liver injury. Sci Rep. 7:81192017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu C, Zhao L, Zhang F and Li L: Regulation
of autophagy protects against liver injury in liver surgery-induced
ischaemia/reperfusion. J Cell Mol Med. 25:9905–9917. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang T, Guo J, Gu J, Chen K, Li H and
Wang J: Protective Role of mTOR in liver ischemia/reperfusion
injury: Involvement of inflammation and autophagy. Oxid Med Cell
Longev. 2019:78612902019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng JJ, Shi HQ, Ren FF, Zhao XS, Chen QY,
Wang DJ, Wu LP, Chu MP, Lai TF and Li L: Notoginsenoside R1
protects against myocardial ischemia/reperfusion injury in mice via
suppressing TAK1-JNK/p38 signaling. Acta Pharmacol Sin.
44:1366–1379. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang J, Ni L, Zhang X, Wang H, Liu L, Wei
M, Li G and Bei Y: Platelet membrane-fused circulating
extracellular vesicles protect the heart from ischemia/reperfusion
injury. Adv Healthc Mater. 12:e23000522023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Wu J, Zhu J, Yang G, Tian J, Zhao
Y and Wang Y: Artesunate provides neuroprotection against cerebral
ischemia-reperfusion injury via the TLR-4/NF-κB pathway in rats.
Biol Pharm Bull. 44:350–356. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Niu H, Li L, Han J, Liu Z, Chu M,
Sha X and Zhao J: Anti-CHAC1 exosomes for nose-to-brain delivery of
miR-760-3p in cerebral ischemia/reperfusion injury mice inhibiting
neuron ferroptosis. J Nanobiotechnology. 21:1092023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsieh PN, Zhou G, Yuan Y, Zhang R,
Prosdocimo DA, Sangwung P, Borton AH, Boriushkin E, Hamik A,
Fujioka H, et al: A conserved KLF-autophagy pathway modulates
nematode lifespan and mammalian age-associated vascular
dysfunction. Nat Commun. 8:9142017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guixé-Muntet S, de Mesquita FC, Vila S,
Hernández-Gea V, Peralta C, García-Pagán JC, Bosch J and
Gracia-Sancho J: Cross-talk between autophagy and KLF2 determines
endothelial cell phenotype and microvascular function in acute
liver injury. J Hepatol. 66:86–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Squires JE, McKiernan P and Squires RH:
Acute liver failure: An update. Clin Liver Dis. 22:773–805. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the care and use of
laboratory animals. Washington (DC): National Academies Press (US);
1996
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong M, Zheng Q, Liu M, Chen L, Lin YH,
Tang SG and Zhu YM: 5-methoxytryptophan alleviates liver fibrosis
by modulating FOXO3a/miR-21/ATG5 signaling pathway mediated
autophagy. Cell Cycle. 20:676–688. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parrish A, Srivastava A, Juskeviciute E,
Hoek JB and Vadigepalli R: Dysregulation of miR-21-associated miRNA
regulatory networks by chronic ethanol consumption impairs liver
regeneration. Physiol Genomics. 53:546–555. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rowe MM and Kaestner KH: The role of
non-coding RNAs in liver disease, injury, and regeneration. Cells.
12:3592023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu TX, Munitz A and Rothenberg ME:
MicroRNA-21 is up-regulated in allergic airway inflammation and
regulates IL-12p35 expression. J Immunol. 182:4994–5002. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du X, Wu M, Tian D, Zhou J, Wang L and
Zhan L: MicroRNA-21 contributes to acute liver injury in
LPS-induced sepsis mice by inhibiting PPAR α expression. PPAR Res.
2020:66330222020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Song M, Chen W,
Dimitrova-Shumkovska J, Zhao Y, Cao Y, Song Y, Yang W, Wang F,
Xiang Y and Yang C: MicroRNA-21 contributes to liver regeneration
by targeting PTEN. Med Sci Monit. 22:83–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Na L, Ding H, Xing E, Zhang Y, Gao J, Liu
B, Yu J and Zhao Y: The predictive value of microRNA-21 for sepsis
risk and its correlation with disease severity, systemic
inflammation, and 28-day mortality in sepsis patients. J Clin Lab
Anal. 34:e231032020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liechty C, Hu J, Zhang L, Liechty KW and
Xu J: Role of microRNA-21 and its underlying mechanisms in
inflammatory responses in diabetic wounds. Int J Mol Sci.
21:33282020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Klionsky DJ, Petroni G, Amaravadi RK,
Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cadwell K,
Cecconi F, Choi AMK, et al: Autophagy in major human diseases. EMBO
J. 40:e1088632021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klionsky DJ, Abdel-Aziz AK, Abdelfatah S,
Abdellatif M, Abdoli A, Abel S, Abeliovich H, Abildgaard MH, Abudu
YP, Acevedo-Arozena A, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy (4th
edition)1. Autophagy. 17:1–382. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vargas JNS, Hamasaki M, Kawabata T, Youle
RJ and Yoshimori T: The mechanisms and roles of selective autophagy
in mammals. Nat Rev Mol Cell Biol. 24:167–185. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mishra J, Vishwakarma J, Malik R, Gupta K,
Pandey R, Maurya SK, Garg A, Shukla M, Chattopadhyay N and
Bandyopadhyay S: Hypothyroidism induces interleukin-1-dependent
autophagy mechanism as a key mediator of hippocampal neuronal
apoptosis and cognitive decline in postnatal rats. Mol Neurobiol.
58:1196–1211. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He WS, Zou MX, Yan YG, Yao NZ, Chen WK, Li
Z, Wang WJ and Ouyang ZH: Interleukin-17A promotes human disc
degeneration by inhibiting autophagy through the activation of the
phosphatidylinositol 3-kinase/Akt/Bcl2 signaling pathway. World
Neurosurg. 143:e215–e223. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maneechotesuwan K, Kasetsinsombat K,
Wongkajornsilp A and Barnes PJ: Role of autophagy in regulating
interleukin-10 and the responses to corticosteroids and statins in
asthma. Clin Exp Allergy. 51:1553–1565. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han D, Huang M, Chang Z and Sun W: KLF15
transcriptionally activates ATG14 to promote autophagy and
attenuate damage of ox-LDL-induced HAECs. Mol Biotechnol.
66:112–122. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng Y, Wu J, Chen H, Lin D, Chen H,
Zheng J, Xia H, Huang L and Zeng C: KLF4 targets RAB26 and
decreases 5-FU resistance through inhibiting autophagy in colon
cancer. Cancer Biol Ther. 24:22263532023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Prateeksha P, Naidu P, Das M, Barthels D
and Das H: KLF2 regulates neural differentiation of dental
pulp-derived stem cells by modulating autophagy and mitophagy. Stem
Cell Rev Rep. 19:2886–2900. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Santamaria J, Darrigues J, van Meerwijk
JPM and Romagnoli P: Antigen-presenting cells and T-lymphocytes
homing to the thymus shape T cell development. Immunol Lett.
204:9–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim GD, Ng HP, Chan ER and Mahabeleshwar
GH: Kruppel-like factor 6 promotes macrophage inflammatory and
hypoxia response. FASEB J. 34:3209–3223. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei G, Zhu D, Sun Y, Zhang L, Liu X, Li M
and Gu J: The protective effects of azilsartan against oscillatory
shear stress-induced endothelial dysfunction and inflammation are
mediated by KLF6. J Biochem Mol Toxicol. 35:1–8. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zou Z, Long X, Zhao Q, Zheng Y, Song M, Ma
S, Jing Y, Wang S, He Y, Esteban CR, et al: A single-cell
transcriptomic atlas of human skin aging. Dev Cell. 56:383–397.e8.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sydor S, Manka P, van Buren L, Theurer S,
Schwertheim S, Best J, Heegsma J, Saeed A, Vetter D, Schlattjan M,
et al: Hepatocyte KLF6 expression affects FXR signalling and the
clinical course of primary sclerosing cholangitis. Liver Int.
40:2172–2181. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Syafruddin SE, Rodrigues P, Vojtasova E,
Patel SA, Zaini MN, Burge J, Warren AY, Stewart GD, Eisen T, Bihary
D, et al: A KLF6-driven transcriptional network links lipid
homeostasis and tumour growth in renal carcinoma. Nat Commun.
10:11522019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu B and Wan Y: Molecular control of
pathogenic Th17 cells in autoimmune diseases. Int Immunopharmacol.
80:1061872020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang D, Xing Q, Su Y, Zhao X, Xu W, Wang
X and Dong C: The conserved non-coding sequences CNS6 and CNS9
control cytokine-induced Rorc transcription during T helper 17 cell
differentiation. Immunity. 53:614–626.e4. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Abraham C and Cho JH: IL-23 and
autoimmunity: New insights into the pathogenesis of inflammatory
bowel disease. Annu Rev Med. 60:97–110. 2009. View Article : Google Scholar : PubMed/NCBI
|