Introduction
Cell division cycle 45 (CDC45) is a component of the
CMG complex, which contains MCM2-7 and GINS and plays a role as an
essential helicase in DNA replication in eukaryotic cells (1). The CDC45 protein, recruiting
single-stranded DNA binding protein replication protein A (RPA)
(2), is required for restriction
of excess processing of the replication fork (3–5). In
yeast, DNA damage induces Rad53, of which the homologue is CHCK2 in
human cells and which causes phosphorylation of CDC45 to inhibit
both initiation and elongation processes of the DNA replication
(6). In addition, it has been
shown that the human DNA helicase B (HDHB/HELB), which plays a part
in DNA end resection (7),
associates with RPA (8,9) and CDC45 (10). At the process of inter-strand
crosslinks, FANCM associates with the CDC45-MCM complex while the
GINS are released (11).
Downregulation of GINS complex formation inhibits DNA replication,
arresting the cell cycle at G1 phase (12). On the other hand, overexpression of
the CDC45 causes replication stress, including S-phase arrest,
replication fork stalling and eventually apoptosis (13). These observations suggest that well
balanced expression of the CDC45, functioning together with HELB,
RPA and CHEK2, is essential for the regulation of DNA replication
initiation, which also affects the DNA repair system in eukaryotes.
However, it is not fully understood how the CDC45 gene
expression is controlled. To elucidate the gene expression
regulatory mechanism, 556-bp of the 5′-upstream region of the
CDC45 gene was cloned by PCR and it was ligated into a
luciferase (Luc) expression plasmid, which was used for
transfection and Luc reporter assay. The results showed that the
556-bp functions as a promoter with response to
trans-resveratrol (Rsv) in HeLa S3 cells. Analysis of the
sequence using the NCBI human genomic database revealed that the
556-bp is a bidirectional promoter, containing not only the
putative transcription start site (TSS) of the CDC45 gene,
but also the oppositely transcribed ubiquitin recognition factor in
the ER-associated degradation 1-encoding UFD1 gene (14). Notably, the UFD1 protein is
involved in the CMG helicase disassembly process (15). The 556-bp human CDC45 gene
promoter contains duplicated GGAA and GC-box elements (16,17),
which are the target of the ETS family (18) and Sp1/Sp3 (19) transcription factor (TF) proteins,
respectively. Previous studies showed that they cooperatively
regulate the transcription of the HELB and MCM4 genes
in response to Rsv in HeLa S3 cells (20,21).
In the present study, various deletion/point
mutations were made on the 556-bp region to show which elements are
essential for the promoter activity with the positive response to
natural compound Rsv. Additionally, reverse
transcription-quantitative (RT-q) PCR and western blotting were
performed to examine whether the transcripts and the translates of
the CDC45 gene accumulated following Rsv treatment of HeLa
S3 cells. Collectively, the present study aimed to reveal molecular
mechanisms that drive the CDC45 gene promoter in accordance
with induction of the HELB and MCM4 gene expression
in Rsv treated HeLa S3 cells.
Materials and methods
Materials
trans-Resveratrol (Rsv; cat. no. CAS501-36-0)
was purchased from Cayman Chemical Company (20–22).
Cells and cell culture
Human cervical carcinoma (HeLa S3) cells (Institute
of Medical Science, Tokyo University) (23) were grown in Dulbecco's modified
Eagle's medium (DMEM; Nacalai Tesque, Inc.) (20–22)
and supplemented with 10% fetal bovine serum (FBS; Biosera) and
penicillin-streptomycin at 37°C in a humidified atmosphere with 5%
CO2.
Construction of Luc reporter
plasmids
The Luc reporter plasmids, carrying 556-bp, which
contains a TSS of the human CDC45 gene, was constructed by
the slight modification of a previously described procedure
(20–22,24).
Briefly, PCR was performed with the hCDC45-9123/AhCDC45-9679 primer
pair (Table I) and genomic DNAs
that were extracted from HeLa S3 cells. The amplified DNA fragment
was treated with HindIII and then ligated into the
multi-cloning site of pGL4.10[luc2] (Promega Corporation).
The resultant plasmids, containing the 556-bp fragment in a correct
orientation, was named pGL4-CDC45-556. Similarly, other Luc
reporter plasmids were constructed by ligating a PCR-amplified DNA
fragment into the HindIII site of pGL4.10[luc2]. The
sense and anti-sense primers used for the amplification of the DNA
fragments are shown in Table II.
Nucleotide sequences were confirmed by a DNA sequencing service
(FASMAC, Greiner Japan Inc.) with Rv or GL primers (20–22,24).
The Luc reporter plasmids, pGL4-HDHB and pGL4-MCM4, were used as
Rsv-inducible positive control vectors (20,21).
 | Table I.Sequences of the primers for
amplifying 5′ upstream regions of the human CDC45 gene. |
Table I.
Sequences of the primers for
amplifying 5′ upstream regions of the human CDC45 gene.
| Primer | Sequence
(5′→3′) |
|---|
| hCDC45-9123 |
CCCAAGCTTAATGCAACGAAGAAACCCCGC |
| hCDC45-9184 |
TTTAAGCTTAAGCCGGTACGCCCCAGAGGCTCACC |
| hCDC45-9191 |
TTTAAGCTTACGCCCCAGAGGCTCACCGGAAGTGC |
| hCDC45-9191M |
TTTAAGCTTACGCCCCAGAGGCTCACCCCAAGTGC |
| hCDC45-9234 |
TTTAAGCTTAGGGGGGGTGCCCGGGACAAAGCGTC |
| hCDC45-9234M |
GCTAAGCTTAGGTGCGCTGCCCGGGACAAAGCGTC |
| hCDC45-9246 |
TTTAAGCTTCGGGACAAAGCGTCGGCTGCA |
| hCDC45-9285 |
GCCAAGCTTGGCTCTAAAACACCCTCAGTAGAAGC |
| hCDC45-9386 |
GCCAAGCTTCGTGTTGACAGTATTCCCCTCCAGAC |
| hCDC45-9386M1 |
GCCAAGCTTCGTGTTGACAGTATTGGCCTCCAGAC |
| hCDC45-9386M2 |
GCCAAGCTTCGTGTTGACAGCATTCCCCTCCAGAC |
| hCDC45-9477 |
TTCAAGCTTCAGCCATCGAGGACTCGGGCGGAACT |
| AhCDC45-9679 |
TTTAAGCTTGCGACGCTGGGCGGACATCTT |
| AhCDC45-9639 |
GATAAGCTTACTGCCTCCCACTGGGAACCCTCAGG |
| AhCDC45-9632 |
GCCAAGCTTCCCACTGGGAACCCTCAGGGAAAGTA |
| AhCDC45-9632MM |
GCCAAGCTTCCCACTGCCAACCCTCAGCCAAAGTA |
| AhCDC45-9584 |
GGCAAGCTTCTCAGTCACATACCCAATGGGGCAGC |
| AhCDC45-9515 |
GGTAAGCTTGTAGCTTAGTTCCGCCCGAGTCCTCG |
| AhCDC45-9493 |
GGTAAGCTTCCTCGATGGCTGAAGCAGAGGCAGTC |
| AhCDC45-9426 |
CCCAAGCTTGGCCCTACTAAATTCGTCTGG |
 | Table II.Primer pairs used for amplifying 5′
upstream regions of the human CDC45 gene. |
Table II.
Primer pairs used for amplifying 5′
upstream regions of the human CDC45 gene.
| Plasmid | Sense primer | Anti-sense
primer |
|---|
| pGL4-CDC45-556 | hCDC45-9123 | AhCDC45-9679 |
| pGL4-CDC45-D1 | hCDC45-9184 | AhCDC45-9679 |
| pGL4-CDC45-D2 | hCDC45-9234 | AhCDC45-9679 |
| pGL4-CDC45-D3 | hCDC45-9246 | AhCDC45-9679 |
| pGL4-CDC45-D4 | hCDC45-9285 | AhCDC45-9679 |
| pGL4-CDC45-D5 | hCDC45-9386 | AhCDC45-9679 |
| pGL4-CDC45-D6 | hCDC45-9477 | AhCDC45-9679 |
| pGL4-CDC45-D1 | hCDC45-9124 | AhCDC45-9639 |
| pGL4-CDC45-D2 | hCDC45-9125 | AhCDC45-9584 |
| pGL4-CDC45-D3 | hCDC45-9126 | AhCDC45-9515 |
| pGL4-CDC45-D4 | hCDC45-9127 | AhCDC45-9493 |
| pGL4-CDC45-D5 | hCDC45-9128 | AhCDC45-9426 |
| pGL4-CDC45-D11 | hCDC45-9184 | AhCDC45-9639 |
| pGL4-CDC45-D21 | hCDC45-9234 | AhCDC45-9639 |
| pGL4-CDC45-D31 | hCDC45-9246 | AhCDC45-9639 |
| pGL4-CDC45-D12 | hCDC45-9184 | AhCDC45-9584 |
| pGL4-CDC45-D22 | hCDC45-9234 | AhCDC45-9584 |
| pGL4-CDC45-D32 | hCDC45-9246 | AhCDC45-9584 |
| pGL4-CDC45-D33 | hCDC45-9246 | AhCDC45-9515 |
| pGL4-CDC45-D34 | hCDC45-9246 | AhCDC45-9493 |
| pGL4-CDC45-D42 | hCDC45-9285 | AhCDC45-9584 |
| pGL4-CDC45-D43 | hCDC45-9285 | AhCDC45-9515 |
| pGL4-CDC45-D44 | hCDC45-9285 | AhCDC45-9493 |
| pGL4-CDC45-D52 | hCDC45-9386 | AhCDC45-9584 |
| pGL4-CDC45-D53 | hCDC45-9386 | AhCDC45-9515 |
| pGL4-CDC45-D54 | hCDC45-9386 | AhCDC45-9493 |
| pGL4-CDC45-442 | hCDC45-9191 | AhCDC45-9632 |
|
pGL4-CDC45-442_M1 | hCDC45-9191M | AhCDC45-9632 |
|
pGL4-CDC45-442_1M | hCDC45-9191 | AhCDC45-9632MM |
|
pGL4-CDC45-442_M1M | hCDC45-9191M | AhCDC45-9632MM |
| pGL4-CDC45-399 | hCDC45-9234 | AhCDC45-9632 |
|
pGL4-CDC45-399_M2 | hCDC45-9234M | AhCDC45-9632 |
|
pGL4-CDC45-399_2M | hCDC45-9234 | AhCDC45-9632MM |
|
pGL4-CDC45-399_M2M | hCDC45-9234M | AhCDC45-9632MM |
|
pGL4-CDC45-D54_M3 | hCDC45-9386M1 | AhCDC45-9493 |
|
pGL4-CDC45-D54_M4 | hCDC45-9386M2 | AhCDC45-9493 |
Transcription factor binding sequence
analysis
The nucleotide sequence of the cloned 556-bp DNA
fragment was subjected to analysis of human transcription factor
binding elements by JASPAR 2020 (http://jaspar2020.genereg.net/).
Transient transfection and Luc
assay
Luc reporter plasmids were transfected into HeLa S3
cells by a DEAE-dextran method in 96-well plates as previously
described (25) and after 24 h of
transfection, the culture medium was changed to Rsv (20 µM)
containing DMEM with 10% FBS. After a further 24 h of incubation,
cells were collected and lysed with 100 µl of 1X cell culture lysis
reagent, containing 25 mM Tris-phosphate (pH 7.8), 2 mM DTT, 2 mM
1,2-diaminocyclohexane-N,N,N',N',-tetraacetic acid, 10% glycerol
and 1% Triton X-100 and then mixed and centrifuged at 12,000 × g
for 5 sec. The appropriate concentration and duration of treatment
with Rsv had been determined by MTS and Luc assay, respectively
(24). The supernatant was stored
at −80°C. The Luc assay was performed with a Luciferase assay
system (Promega Corporation) and relative Luc activities were
calculated as described previously (25).
Western blotting
Cells were collected after Rsv-treatment and lysed
in a RIPA buffer (20 mM Tris-HCl (pH 7.4), 0.1% SDS, 1% TritonX100,
and 1% sodium deoxychlate). Protein amount was analyzed with
Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad
Laboratories, Inc.) according to the manufactures protocol. After
SDS-PAGE (15% acrylamide) (15–25 µg proteins/lane) and blotting
onto a PVDF (Immobilon-P) membrane as previously described
(20–22), Blocking was carried out in a
Blocking solution, which is a TBS containing 1% Skim milk (Megmilk
Snow Brand), at 4°C for 15 h. Western blot analysis was carried out
with antibodies against CDC45 (cat. no. 11881, Cell Signaling,
Danvers, MA) (1:1,000), and β-actin (cat. no. A5441;
MilliporeSigma) (1:1,000) at 20°C for 1 h, followed by the
incubation with horseradish peroxidase-conjugated anti-rabbit (cat.
no. A0545) (1:10,000) or anti-mouse IgG (cat. no. A9917) secondary
antibodies (Sigma-Aldrich; Merck KGaA) (1:10,000) at 20°C for 1 h
in a TBS containing 1% TritonX100 and 2.5% Skim milk. Signal
intensities were detected with ImmunoStar LD (FUJIFILM Wako Pure
Chemical Corporation) and quantified with a ChemiDoc image analysis
system and ImageLab 6.0 software (Bio-Rad Laboratories, Inc.).
RT-qPCR
RNA extraction, cDNA synthesis, and qPCR were
performed according to the manufacturer's protocol. First-strand
cDNAs were synthesized with ReverTra Ace (Toyobo Life Science),
random primers (Takara Bio, Inc.) and total RNAs extracted from
HeLa S3 cells, which were cultured 1 to 5×106 cells/φ 10
cm dish. PCR analysis was carried out using a Mx3000P Real-Time
qPCR System (Stratagene; Agilent Technologies, Inc.) (20–22).
cDNAs were added to the Thunderbird Realtime PCR Master Mix (Toyobo
Life Science), containing 0.3 µM of each primer pair. The primer
pairs for amplifying the human CDC45 and GAPDH
transcripts were hCDC45-820: GACTGCACACGGATCTCCTT/AhCDC45-949:
TCTGTCCATGCACAGACCAC and hGAPDH556/hGAPDH642 (20–22),
respectively. Amplification was carried out initially for 1 min at
95°C, followed by 40 cycles at 95°C (15 sec) and 58°C (30 sec).
Quantitative PCR analysis for each sample was carried out in
triplicate. Relative gene expression values were obtained by
normalizing threshold cycle (CT) values of target genes
in comparison with CT values of the GAPDH gene
using the 2−ΔΔCq method (26).
Statistical analysis
Standard deviations (S.D.) for each data were
calculated and results are shown as means ± S.D. from three
independent experiments. Statistical analyses were performed with
the Student's t-test and asterisks indicate values of *P<0.05,
**P<0.01 and ***P<0.005. P<0.05 was considered to indicate
a statistically significant difference.
Results
Characterization of the human CDC45
promoter region
Duplicated GGAA motifs in the HELB and
MCM4 promoters respond to Rsv (20,21),
which can up-regulate the NAD+/NADPH ratio in HeLa S3
cells (27). The HELB is known to
interact with CDC45 that associates with MCM helicase to develop
the CDC45-MCM-GINS complex (1,12).
To examine whether the CDC45 promoter responds to Rsv
accompanied with the HELB and MCM4 promoter, the
present study amplified and isolated the 556-bp of the 5′-flanking
sequence of the CDC45 gene by PCR. Sequence analysis
revealed that the pGL4-CDC45-556 contained a nucleotide identical
to NCBI Sequence ID NC_000022.11 (nucleotide from
19419724-19479679) and that it covers the sequence of the most
upstream 5′ end of the cDNA (Sequence IDs: XM_011530416.1 and
XM_024452278.1, for CDC45 gene transcript variants X2 and
X3, respectively and XM_011530417.3 and XM_011530418.3, for
transcript variants X4 and X5, respectively; GENE ID, CDC45: 8318).
This 556-bp region also contains a 5′ upstream end of the
UFD1 mRNAs (sequence ID: NM_001035247.3, NM_005659.7 and
NM_001362910.2; GENE ID, UFD1: 7253) in a reverse orientation to
that of the CDC45 gene. The TSS was tentatively set as +1 at
the most upstream 5′ end of the CDC45 transcript variants X2
and X3. The JASPAR2020 database program (jaspar.genereg.net/)
indicated that the consensus recognition sequences of several known
transcription factors are present within the 556-bp (Fig. 1A). This DNA sequence has no obvious
TATA box or CCAAT motif but contains putative binding sites of
ZNF93 (−321 to −307), ETS family (−275 to −266), ZNF28 (−246 to
−237), SOX10 (−232 to −227, −55 to −50, −30 to −25), ZNF143 (−35 to
−20), SOX18 (−31 to −24), YY1 (+2 to +7), FOXC1 (+125 to +132) and
SPI1 (+166 to +171). To examine whether the 556-bp DNA fragment
functioned as a promoter, Luc reporter plasmids, pGL4-HDHB
(20), pGL4-MCM4 (21) and pGL4-CDC45-556, were transiently
transfected into HeLa S3 cells. All the relative Luc activities of
the plasmids-transfected cells increased after the addition of Rsv
to the cell culture (Fig. 1B).
Based on this observation, it was decided to examine the
CDC45 gene/protein expression and promoter activity.
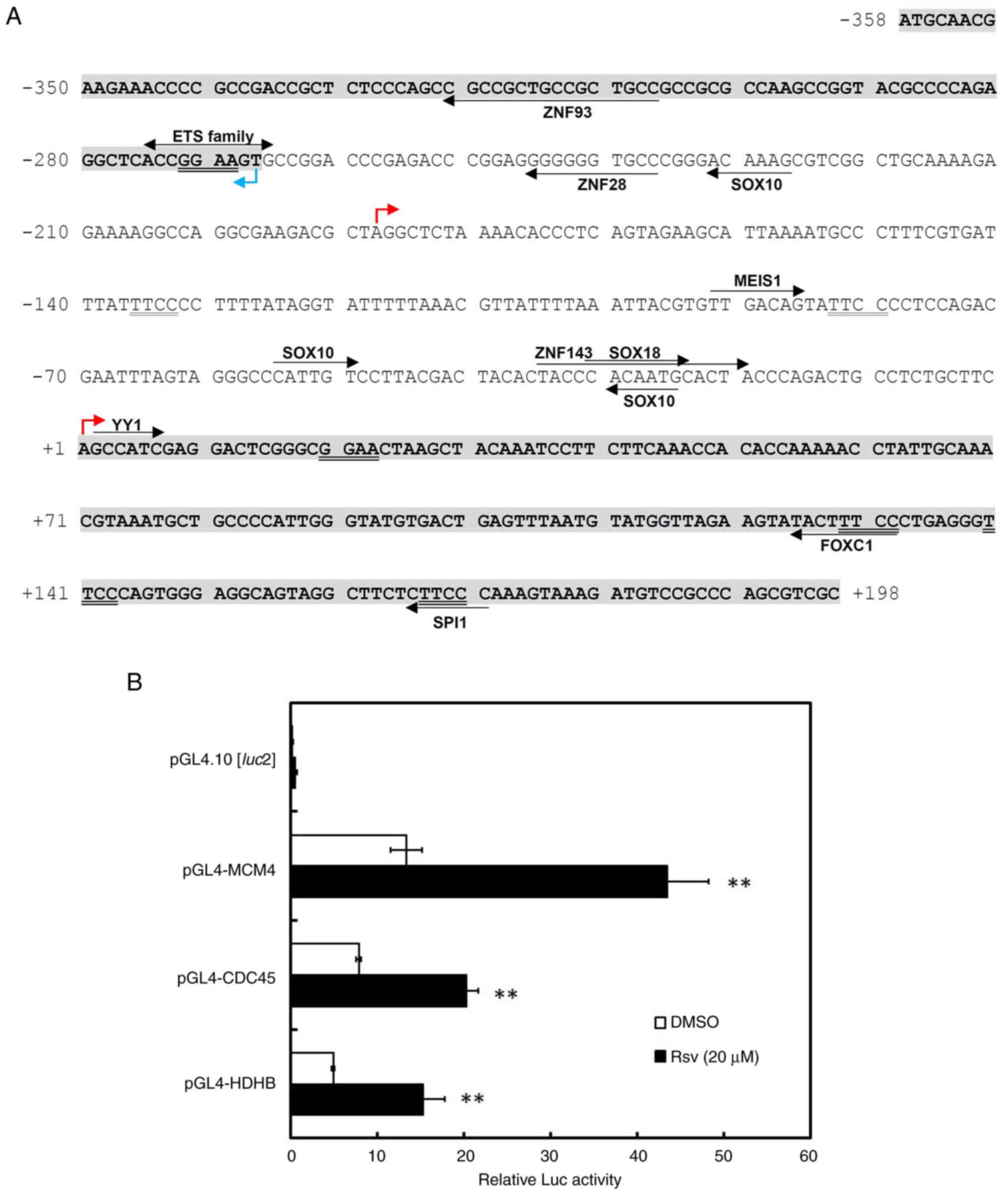 | Figure 1.Characterization of the human
CDC45/UFD1 bidirectional promoter region. (A) The nucleotide
sequence of the 556-bp fragment that was obtained from PCR is
shown. The most upstream 5′ end of the human CDC45
(XM_011530416.1/XM_024452278.1 and XM_011530417.3/XM_011530418.3)
and UFD1 (NM_001035247.3, NM_005659.7/NM_001362910.2zcDNAs
are designated by red and blue arrows, respectively. The most
upstream of the CDC45 gene transcript variants X2/X3 is
designated as nucleotide number +1 and that of the X4/X5 and the
UFD1 transcript are shown by arrows on −188 and −267,
respectively. Putative transcription factor-binding sites were
predicted by the JASPAR2020 database program and they (relative
score >98%) are indicated by arrows on or under the sequence.
The ‘ETS family’ includes ELK1, ELK3, ELK4, ERF, ERG, ETS1, ETS2,
ETV1, ETV3, ETV4, ETV5, ETV6, FEV, FLI1, GABPA and SPI1. (B) Luc
reporter plasmids, pGL4.10[luc2], pGL4-MCM4-309,
pGL4-CDC45-556 and pGL4-HDHB were transfected into HeLa S3 cells,
which were treated with or without Rsv (20 µM) for 24 h. Results
show relative Luc activities compared with that of
pGL4-PIF1-transfected and Rsv-non-treated cells. Results are shown
as means ± standard deviation from at least three independent
experiments. Statistical analysis for the results between
Rsv-treated and DMSO-treated control cells was performed with the
Student's t-test **P<0.01 vs. DMSO. Luc, luciferase; Rsv,
trans-resveratrol. |
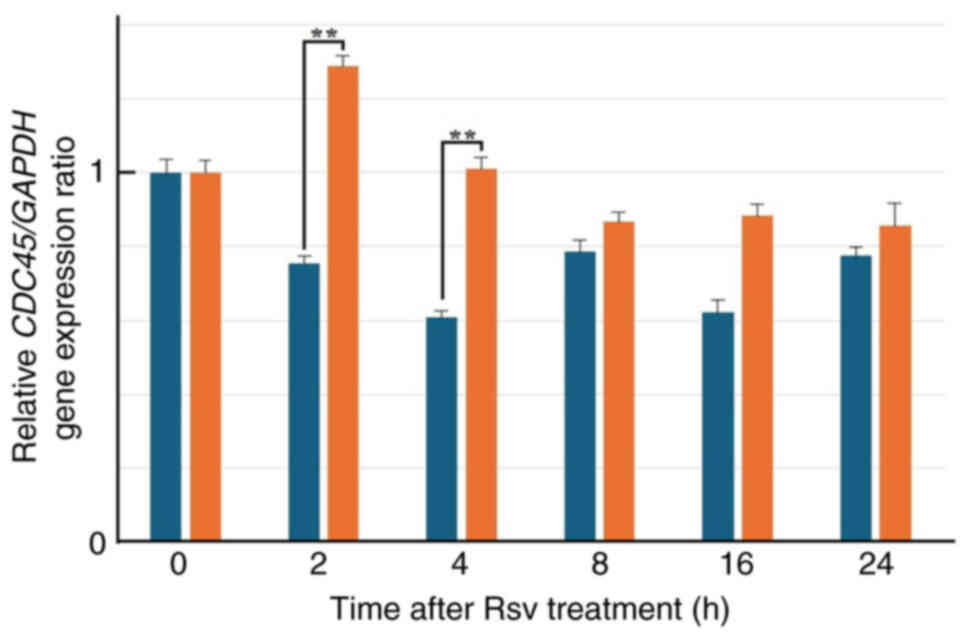
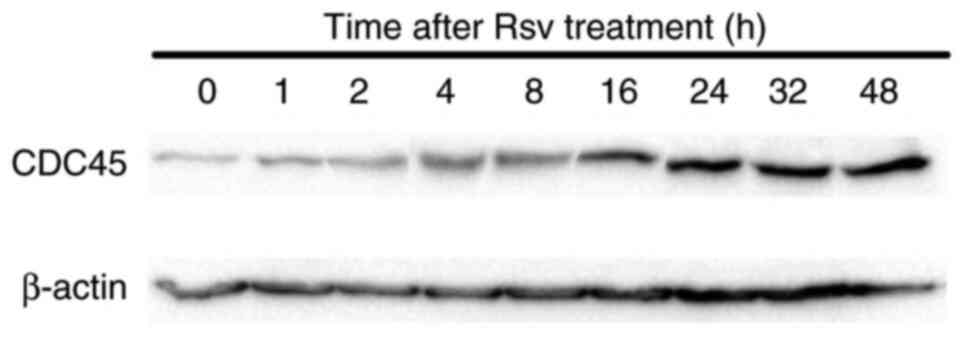
Effects of Rsv on CDC45 gene
expression and its protein amount in HeLa S3 cells
Next, total RNAs were extracted from cells after
adding Rsv to the culture medium and RT-qPCR was carried out
(Fig. 2). The relative gene
expression of CDC45 compared with that of the GAPDH
gene increased approximately two-fold at 2 to 4 h after Rsv
treatment. western blotting showed that the amount of CDC45 protein
accumulated from 16 to 48 h after the treatment (Fig. 3).
Determination of Rsv-response
element(s) in the CDC45 promoter
To examine the Rsv-responsive sequence, deletion
from the 5′ and 3′ ends of the 556-bp CDC45 promoter region
was introduced into the pGL4-CDC45-556 (Fig. 4A and B). The positive response to
Rsv was observed in the cells that were transfected with
pGL4-CDC45-D5. On the other hand, no apparent Luc activity was
detected in pGL4-CDC45-D6-transfected cells (Fig. 4A), indicating that the region from
−96 to −6 functions as a CDC45 gene promoter that positively
responds to Rsv. In addition, comparing the Luc activities of the
cells transfected with pGL4-CDC45-D4 and -D5, it was suggested that
the region from −55 to +12 is essentially required for CDC45
promoter activity and its positive response to Rsv (Fig. 4B). Because the deletions from −358
to −299 or −248 (Fig. 4A) and from
+198 to +159 (Fig. 4B) gave higher
Luc activities, these regions may have negative regulatory
element(s). The deletion from +158 to +104 from D11 and D12
constructs raised the basal promoter activity, suggesting that the
56 nucleotide contains negative elements) (Fig. 4C). Other deletions both from the
5′- and 3′- were examined in a similar transfection experiment
(Fig. 4D). However, apparent Luc
activities with positive response to Rsv were observed in cells
that were transfected by these plasmids, including pGL4-CDC45-D54,
which has the shortest 108-bp sequence. The obtained results
totally showed that the region from −96-+12 functions as a minimum
promoter that has Rsv response.
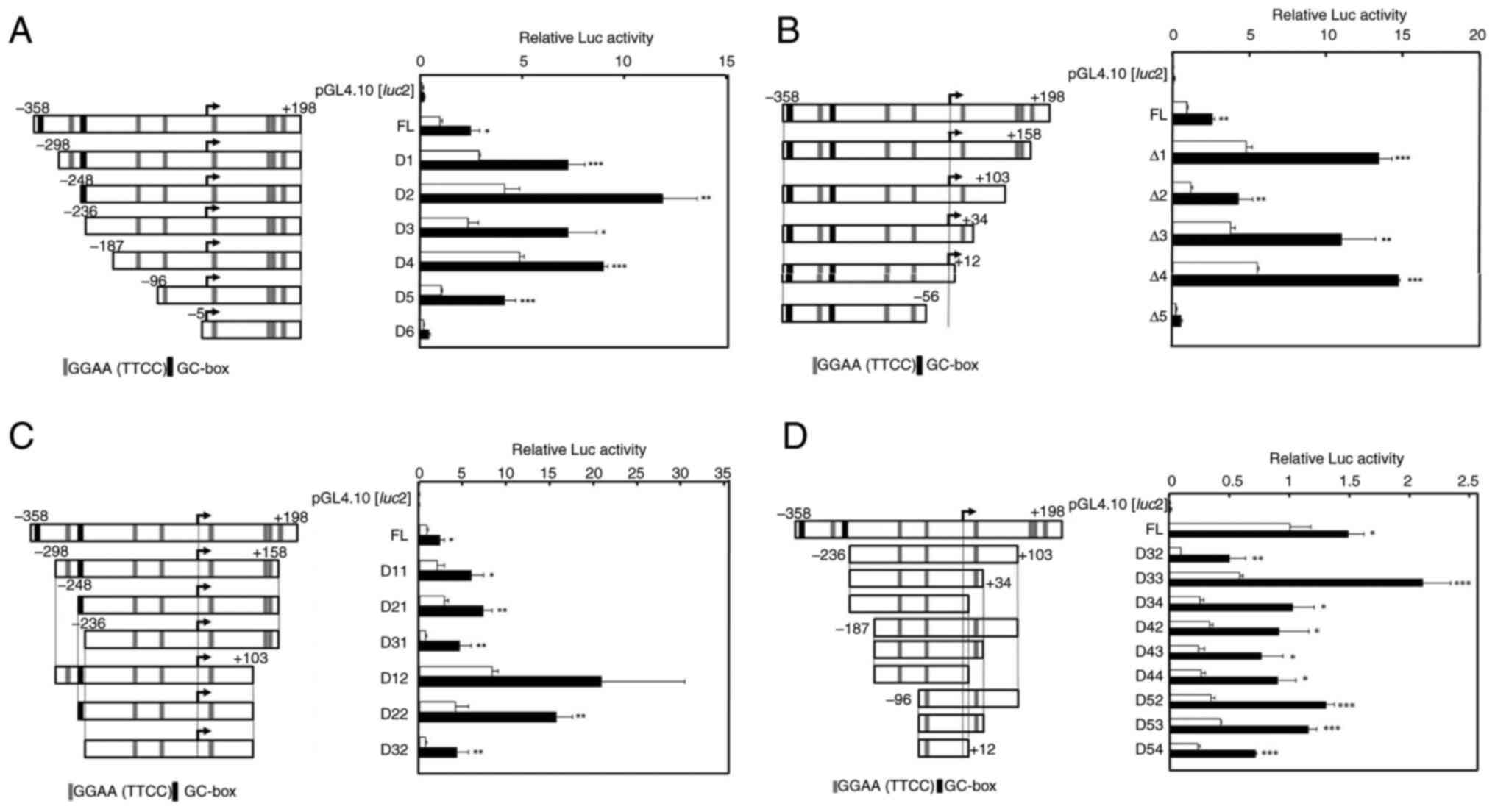 | Figure 4.Effect of Rsv on human CDC45
promoter activity. (Left panels) The 5′ upstream end of the human
CDC45 gene, which has been ligated upstream of the
Luciferase gene of the pGL4.10[luc2], is shown. The
5′-end of the cDNA of the X2 and X3 transcript variants is
designated +1. The GGAA (TTCC) and GC-box elements are
schematically shown. (Right panels) Luc reporter plasmids were
transiently transfected into HeLa S3 cells and treated with (closed
bars) or without (open bars) Rsv (20 µM) for 24 h. (A) Transfection
was performed with (A) pGL4-CDC45-556, pGL4-CDC45-D1, -D2, -D3,
-D4, -D5 and -D6, (B) pGL4-CDC45-556, pGL4-CDC45-D1, -D2, -D3, -D4,
-D5 and -D6, (C) pGL4-CDC45-556, pGL4-CDC45-D11, -D21, -D31, -D12,
-D11 and -D32, (D) GL4-CDC45-556, pGL4-CDC45-D32, -D33, -D34, -D42,
-D43, -D44, -D52, -D53 and -D54. Luc activities were normalized to
that of the pGL4-PIF1-transfected cells. Histograms show relative
Luc activities of deletion-introduced plasmid-transfected cells
compared with that of the pGL4-CDC45-556-transfected cells without
Rsv treatment. Results are shown as means ± standard deviation from
three independent experiments. Statistical analysis for the results
between Rsv-treated and non-treated cells was performed with the
Student's t-test. *P<0.05, **P<0.01 and ***P<0.005. Rsv,
trans-resveratrol; Luc, luciferase. |
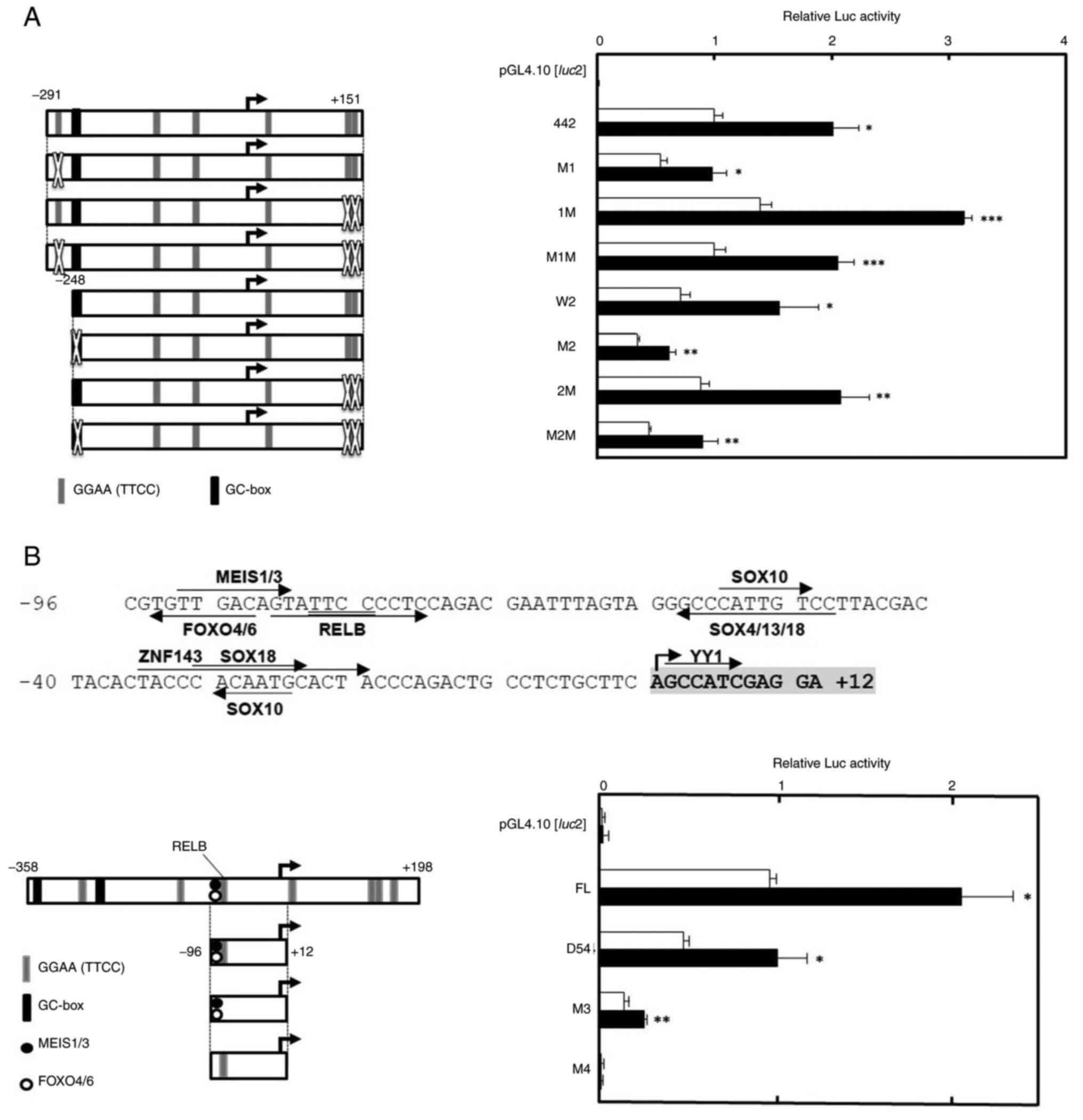
Introduction of point mutations on the
CDC45 promoter
To indicate the Rsv-responsive sequence more
precisely, point mutations were introduced to the CDC45
promoter (Fig. 5). To examine the
contribution of the GGAA (TTCC) motifs (ACC GGAAGT; −275 to −267 and
ATACTTTCCCTGAGGGTTCCCAGTG; +124 to +148) and
the GC-box (GGAGGGGGGTGCC; −249 to −237) to the drug response,
point mutations were introduced on pGL4-CDC45-442 and −399
(Fig. 5A). Transfection and Luc
assay showed that most mutation-introduced plasmids had a positive
response to Rsv. However, the response was much reduced in
pGL4-CDC45-399-M2 and -M2M-transfected cells (Fig. 5A), suggesting that the GC-box,
which was predicted as ZNF28 recognition sequence by JASPAR2020
program, plays a part in the regulation of CDC45
transcription.
Next, the minimum core Rsv-responding 108-bp from
−96 to +12 was examined to see whether it contained a Rsv response
element(s) (Fig. 5B). Previous
studies on the human HELB, MCM4 and TP53 gene
promoters showed that a GGAA (TTCC) motif plays a primarily
important role in the response to Rsv (20–22).
Therefore, it was hypothesized that the GGAA (TTCC) core motif also
regulates the CDC45 promoter activity. However, no
TF-binding sites with GGAA (TTCC) were indicated by the JASPAR2020
program when the threshold was set at 98% (Fig. 1A). Therefore, the present study
examined setting the threshold at 95%. This time, the putative RELB
binding sequence, which is located just 3′-downstream of the MEIS1
binding motif was indicated (Fig.
5B). Compared with the wild type pGL4-CDC45-D54, mutation in
the pGL4-CDC45-D54_M3 reduced the Luc activity, but a positive
response to Rsv was still observed (Fig. 5B). On the other hand, the response
was completely abolished in the pGL4-CDC45-D54_M4. These results
suggested that the sequence between putative MEIS1 and RELB
recognition sites is of primary importance for the CDC45
promoter activity and the positive response to Rsv.
Discussion
The present study showed that treatment with Rsv (20
µM) induced CDC45 gene and protein expression in HeLa S3
cells. Deletion and mutation analyses revealed that the putative
RELB binding sequence was required for the CDC45 promoter
activity to positively respond to Rsv.
The CDC45-UFD1 bidirectional promoter
has been isolated and characterized to suggest a putative TATA-box
within the region (28). Although
the TATA-like sequence, TTTTATAGG (from −129 to −122) is contained
in the pGL4-CDC45-D5 construct, it does not have promoter activity
(Figs. 4 and 5B), indicating that the sequence is not
essential for CDC45 promoter in HeLa S3 cells. The 556-bp,
containing TSSs of both genes, possesses a duplicated GGAA (TTCC)
motif, which is contained in the promoter regions of many genes
that encode DNA repair and genome maintenance factors (16,29).
The duplicated GGAA motif in the human TP53 promoter has
been shown to have an essential role to respond to Rsv in HeLa S3
cells (22). However, the
duplicated GGAA (TTCC) (from +124 to +148) in the 556-bp of the
human CDC45 promoter seems to be non-essential (Fig. 5A). The GC-box, which is a
Sp1-recognitionsequence that is commonly contained in the
HELB and MCM4 promoter regions, serves a part in the
response to Rsv (20,21). However, the Rsv-responding sequence
from −96 to +12 (Fig. 5B) does not
contain any GC-boxes or GC-box like motifs. In the present study,
the functional transcription promoter with the response to Rsv was
shown to be present in the sequence 5′-CAGTATTCCCCTCC-3′ (−88 to −75),
which overlaps with the MEIS1 and RELB recognition motifs (Fig. 5B). MEIS1 gene expression is
prominently suppressed when THP-1 cells are induced to
differentiate into monocyte-macrophage-like cells by
12-O-tetradecanoylphorbol-13-acetate (TPA) (30). In murine myeloid cells, MEIS1 acts
as a suppressor of granulocyte colony stimulation factor-induced
differentiation (31). The amount
of RELB protein increases in granulocyte macrophage colony
stimulation factor-induced macrophage cells only following
stimulation by lipopolysaccharide (32). In addition, RELB is required for
TNF-induced differentiation of bone marrow cells into M1
macrophages in mice (33). Our
previous studies indicated that the duplicated GGAA-motif is
responsible for the activation of the E2F4 and ZNFX1
genes during TPA-induced macrophage-like differentiation of HL-60
cells (34,35). In HeLa S3 cells, the Sp1/PU.1 ratio
is notably increased following the Rsv treatment (21). It should be determined whether the
RELB/MEIS1 ratio in HeLa S3 cells is increased in response to Rsv
and if they are competing to bind to the Rsv response element in
the CDC45 promoter.
The natural compound Rsv has been shown to
upregulate expression of the HELB and MCM4 genes and
its encoded proteins in HeLa S3 cells (20,21).
The human HELB (HDHB) gene encodes a DNA
replication-associated helicase (36), which binds to DNA double-strand
breaks to inhibit DNA end resection (7). In addition, the HELB protein is known
to interact with the CDC45 at initiation of DNA replication
(10,37). Notably, the MCM complex, whose
structure was revealed by cryo-electron microscopy (38), associates with CDC45 and GINS
(1,39). The timing of CMG complex formation
at the origin of replication should be limited to initiate DNA
replication at a suitable time (40). The duplicated GGAA (TTCC) motifs
are not only present in the 5′ upstream regions of the CDKN1A,
RB1, TP53 and MCM4 genes (21,22),
but also in that of the GINS1, GINS3 and GINS4 genes
(41). Expression of genes
encoding proteins that regulate entering the S-phase could be
accurately regulated by GGAA-recognizing TFs.
The present study showed that human CDC45
gene promoter can be activated by Rsv in HeLa S3 cells. CDC45 is a
component of CMG helicase which is essential for responses to
replicative stress and stability of mammalian genomes (37,42).
If, in some cancer cells, the CMG helicase play an important role
as a tumor suppressor, maintaining required expression level would
stop aberrant proliferation. By contrast, in other types of cancer,
hyper expression of the CDC45 gene may be harmful,
functioning as an oncogenic factor (43). In this case, the CDC45 might be one
of the targets, inhibition of which can lead to apoptosis or
programmed cell death. Rsv can prolong the life span of organisms
(44,45) and has been revealed to have
beneficial effects for health (46). Further investigations are required
to elucidate the mechanisms by which Rsv-induced signals regulate
DNA replication/repair-associated gene expression. The present
study not only examine biological effect of the Rsv, but also
suggested a molecular mechanism how Rsv affects transcription of
CDC45 gene. Evaluation of the efficacies of other candidate
drugs, gene expression vectors and nucleotides, including miRNAs,
the multiple transfection assay would be useful to indicate which
gene promoters are activated. The limitation of the present study
is that it cannot be directly applied to diagnosis with tissue
samples obtained by biopsy. However, it could be applied on iPS
cells or patient-derived cell lines. Another concern is that the
experimental system to analyze multiple gene promoter activities is
only applicable with the use of/verification in only one cell line
at the same time. Although it needs to be improved, this multiple
promoter analysis, an easy, reproducible and cost-effective method,
which does not always need transfection-efficiency estimation, can
suggest which drugs or gene expression vectors should be used for
the treatment.
Acknowledgements
The authors would like to thank Mr. Hiroyasu Nakao
(Tokyo University of Science, Noda, Chiba, JAPAN) and Ms. Marie
Nose (Tokyo University of Science, Noda, Chiba, JAPAN) for their
excellent technical assistance. The present study was performed
under the permission of recombinant DNA experimental committee
admission nos. 1825 and 1917 of Tokyo University of Science.
Funding
The present study was supported in part by JSPS KAKENHI grant
no. 24510270 and a Research Fellowship from the Research Center for
RNA Science, RIST, Tokyo University of Science.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JA and FU confirm the authenticity of all the raw
data. JA and HK constructed the Luc reporter plasmid and performed
experiments and analyzed the data (transfection assay, RT-qPCR and
western blotting). FU interpreted the data and wrote the
manuscript. ST collected and analyzed/interpreted the data. TM, YO
and MA interpreted the data and edited the manuscript. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
threshold cycle
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
Luc
|
luciferase
|
|
MCM
|
minichromosome maintenance
|
|
Rsv
|
trans-resveratrol
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
TF
|
transcription factor
|
|
TSS
|
transcription start site
|
References
|
1
|
Simon AC, Sannino V, Costanzo V and
Pellegrini L: Structure of human Cdc45 and implications for CMG
helicase function. Nat Commun. 7:116382016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Szambowska A, Tessmer I, Prus P, Schlott
B, Pospiech H and Grosse F: Cdc45-induced loading of human RPA onto
single-stranded DNA. Nucleic Acids Res. 45:3217–3230.
2017.PubMed/NCBI
|
|
3
|
Krastanova I, Sannino V, Amenitsch H,
Gileadi O, Pisani FM and Onesti S: Structural and functional
insights into the DNA replication factor Cdc45 reveal an
evolutionary relationship to the DHH family of phosphoesterases. J
Biol Chem. 287:4121–4128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szambowska A, Tessmer I, Kursula P,
Usskilat C, Prus P, Pospiech H and Grosse F: DNA binding properties
of human Cdc45 suggest a function as molecular wedge for DNA
unwinding. Nucleic Acids Res. 42:2308–2319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruck I and Kaplan DL: Cdc45
protein-single-stranded DNA interaction is important for stalling
the helicase during replication stress. J Biol Chem. 288:7550–7563.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Can G, Kauerhof AC, Macak D and Zegerman
P: Helicase subunit Cdc45 targets the checkpoint kinase Rad53 to
both replication initiation and elongation complexes after fork
stalling. Mol Cell. 73:562–573.e3. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tkáč J, Xu G, Adhikary H, Young JTF, Gallo
D, Escribano-Díaz C, Krietsch J, Orthwein A, Munro M, Sol W, et al:
HELB is a feedback inhibitor of DNA end resection. Mol Cell.
61:405–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guler GD, Liu H, Vaithiyalingam S, Arnett
DR, Kremmer E, Chazin WJ and Fanning E: Human DNA helicase B (HDHB)
binds to replication protein A and facilitates cellular recovery
from replication stress. J Biol Chem. 287:6469–6481. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu H, Yan P and Fanning E: Human DNA
helicase B functions in cellular homologous recombination and
stimulates Rad51-mediated 5′-3′ heteroduplex extension in vitro.
PLoS One. 10:e01168522015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gerhardt J, Guler GD and Fanning E: Human
DNA helicase B interacts with the replication initiation protein
Cdc45 and facilitates Cdc45 binding onto chromatin. Exp Cell Res.
334:283–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang J, Zhang J, Bellani MA, Pokharel D,
Gichimu J, James RC, Gali H, Ling C, Yan Z, Xu D, et al: Remodeling
of interstrand crosslink proximal replisomes is dependent on ATR,
FANCM, and FANCD2. Cell Rep. 27:1794–1808.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aparicio T, Guillou E, Coloma J, Montoya G
and Méndez J: The human GINS complex associates with Cdc45 and MCM
and is essential for DNA replication. Nucleic Acids Res.
37:2087–2095. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Köhler C, Koalick D, Fabricius A, Parplys
AC, Borgmann K, Pospiech H and Grosse F: Cdc45 is limiting for
replication initiation in humans. Cell Cycle. 15:974–985. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen M, Gutierrez GJ and Ronai ZA:
Ubiquitin-recognition protein Ufd1 couples the endoplasmic
reticulum (ER) stress response to cell cycle control. Proc Natl
Acad Sci USA. 108:9119–9124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maric M, Mukherjee P, Tatham MH, Hay R and
Labib K: Ufd-Npl4 recruit Cdc48 for disassembly of ubiquitylated
CMG helicase at the end of chromosome replication. Cell Rep.
18:3033–3042. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uchiumi F, Miyazaki S and Tanuma S: The
possible functions of duplicated ets (GGAA) motifs located near
transcription start sites of various human genes. Cell Mol Life
Sci. 68:2039–2051. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
FitzGerald PC, Shlyakhtenko A, Mir AA and
Vinson C: Clustering of DNA sequences in human promoters. Genome
Res. 14:1562–1574. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei GH, Badis G, Berger MF, Kivioja T,
Palin K, Enge M, Bonke M, Jolma A, Varjosalo M, Gehrke AR, et al:
Genome-wide analysis of ETS-family DNA-binding in vitro and in
vivo. EMBO J. 29:2147–2160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng D, Zhao Y, Wang S, Jia W, Kang J and
Zhu J: Human telomerase reverse transcriptase (hTERT) transcription
requires Sp1/Sp3 binding to the promoter and a permissive chromatin
environment. J Biol Chem. 290:30193–30203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uchiumi F, Arakawa J, Iwakoshi S,
Ishibashi S and Tanuma S: Characterization of the 5′-flanking
region of the human DNA helicase B (HELB) gene and its response to
trans-resveratrol. Sci Rep. 6:245102016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uchiumi F, Katsuda C, Akui M, Kusaka M,
Tanaka M, Asai M and Tanuma SI: Effect of the natural compound
trans-resveratrol on human MCM4 gene transcription. Oncol Rep.
44:283–292. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uchiumi F, Shoji K, Sasaki Y, Sasaki M,
Sasaki Y, Oyama K, Sugisawa S and Tanuma S: Characterization of the
5′-flanking region of the human TP53 gene and its response to the
natural compound, resveratrol. J Biochem. 159:437–447. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanuma S and Kanai Y:
Poly(ADP-ribosyl)ation of chromosomal proteins in the HeLa S3 cell
cycle. J Biol Chem. 257:6565–6570. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uchiumi F, Watanabe T, Hasegawa S, Hoshi
T, Higami Y and Tanuma S: The effect of resveratrol on the werner
syndrome RecQ helicase gene and telomerase activity. Curr Aging
Sci. 4:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uchiumi F, Larsen S and Tanuma S:
Application of DEAE-dextran to an efficient gene transfer system.
Dextran: Chemical Structure, Application and Potential Side
Effects. Figgs GP: Nova Science Publishers; Hauppauge, NY: pp.
143–156. 2014
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takihara Y, Sudo D, Arakawa J, Takahashi
M, Sato A, Tanuma S and Uchiumi F: Nicotinamide adenine
dinucleotide (NAD+) and cell aging. New Research on Cell
Aging and Death. Strakoš R and Lorens B: Nova Science Publishers;
Hauppauge, NY: pp. 131–158. 2018
|
|
28
|
Igaki H, Nakagawa K, Aoki Y, Ohtomo K,
Kukimoto I and Kanda T: Characterization of the bi-directional
transcriptional control region between the human UFD1L and CDC45
genes. Biochem Biophys Res Commun. 283:569–576. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uchiumi F, Larsen S and Tanuma S:
Biological systems that control transcription of DNA repair and
telomere maintenance-associated genes. New Research in Directions
in DNA Repair. Chen C: InTech Open; London: pp. 309–325. 2013
|
|
30
|
Martino V, Bianchera A, Reia L, Bussolati
O, Fazzina R, Marino F, Montemurro L, Tonelli R, Pession A, Gazzola
GC and Sala R: Down regulation of HOXA4, HOXA7, HOXA10, HOXA11 and
MEIS1 during monocyte-macrophage differentiation in THP-1 cells.
Mol Med Rep. 2:241–244. 2009.PubMed/NCBI
|
|
31
|
Calvo KR, Knoepfler PS, Sykes DB, Pasillas
MP and Kamps MP: Meis1a suppresses differentiation by G-CSF and
promotes proliferation by SCF: Potential mechanisms of
cooperativity with Hox9 in myeloid leukemia. Proc Natl Acad Sci
USA. 98:13120–13125. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li T, Morgan MJ, Choksi S, Zhang Y, Kim YS
and Liu ZG: MicroRNAs modulate the noncanonical transcription
factor NF-kappaB pathway by regulating expression of the kinase
IKKalpha during macrophage differentiation. Nat Immunol.
11:799–805. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao Z, Hou X, Yin X, Li Y, Duan R, Boyce
BF and Yao Z: TNF induction of NF-kB RelB enhances RANKL-induced
osteoclastogenesis by promoting inflammatory macrophage
differentiation but also limits it through suppression of NFATc1
expression. PLoS One. 10:e01357282015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hamada H, Goto Y, Arakawa J, Murayama E,
Ogawa Y, Konno M, Oyama T, Asai M, Sato A, Tanuma S and Uchiumi F:
Characterization of the human E2F4 promoter region and its
response to 12-O-tetradecanoylphorbol-13-acetate. J Biochem.
166:363–373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hamada H, Yamamura M, Ohi H, Kobayashi Y,
Niwa K, Oyama T, Mano Y, Asai M, Tanuma SI and Uchiumi F:
Characterization of the human zinc finger nfx-1-type containing 1
encoding ZNFX1 gene and its response to
12-O-tetradecanoyl-13-acetate in HL-60 cells. Int J Oncol.
55:869–904. 2019.
|
|
36
|
Taneja P, Gu J, Peng R, Carrick R, Uchiumi
F, Ott RD, Gustafson E, Podust VN and Fanning E: A
dominant-negative mutant of human DNA helicase B blocks the onset
of chromosomal DNA replication. J Biol Chem. 277:40853–40861. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hazeslip L, Zafar MK, Chauhan MZ and Byrd
AK: Genome maintenance by DNA helicase B. Genes (Basel).
11:5782020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miller TCR, Locke J, Greiwe JF, Diffley
JFX and Costa A: Mechanism of head-to-head MCM double-hexamer
formation revealed by cryo-EM. Nature. 575:704–710. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Douglas ME, Ali FA, Costa A and Diffley
JFX: The mechanism of eukaryotic CMG helicase activation. Nature.
555:265–268. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Araki H: Molecular mechanisms of DNA
replication. DNA Replication, Recombination, and Repair. Hanaoka F
and Sugasawa K: Springer; Japan, Tokyo: pp. 3–22. 2016, View Article : Google Scholar
|
|
41
|
Uchiumi F: Transcriptional regulatory
systems of the human helicases. Advances in Medicine and Biology.
Berhardt LV: Nova Science Publishers; Hauppauge, NY: pp. 115–153.
2020
|
|
42
|
Xiang S, Reed DR and Alexandrow MG: The
CMG helicase and cancer: A tumor ‘engine’ and weakness with missing
mutations. Oncogene. 42:473–490. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu Y, Chen X, Liu F, Yu H, Zhang Y, Du K,
Nan Y and Huang Q: Systematic pan-cancer analysis identifies CDC45
as having an oncogenic role in human cancers. Oncol Rep.
48:1852022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stefani M, Markus MA, Lin RC, Pinese M,
Dawes IW and Morris BJ: The effect of resveratrol on a cell model
of human aging. Ann N Y Acad Sci. 1114:407–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kaeberlein M: Resveratrol and rapamycin:
Are they anti-aging drugs? Bioessays. 32:96–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Uchiumi F, Arakawa J, Takihara Y, Akui M,
Ishibashi S and Tanuma S: The effect of trans-resveratrol on
the expression of the human DNA-repair associated genes. Int Mol
Med. 3:783–792. 2016.
|