Introduction
Breast cancer is the most frequent cancer among
females. It is a heterogeneous disease, with distinct morphologies,
metastatic behaviour and therapeutic responses. Breast cancer is
now the most common cancer in developed and developing countries,
with 690,000 new cases estimated in each region. Incidence rates
are higher in the developed regions of the world than in the
majority of developing regions (1).
Despite advances in early detection, approximately 30% of patients
with early-stage breast cancer have recurrent disease. Although the
systemic treatment of patients with chemotherapy, hormonal therapy
and immunotherapy produces a high response rate initially,
progression invariably occurs following a variable time interval
(2). It remains the most frequent
cause of cancer mortality in females in developing and developed
countries. To improve the patient survival rate, elucidation of the
underlying molecular mechanisms of the tumorigenesis of breast
cancer is required. Previous studies have demonstrated that core
transcription factors, such as Oct3/4, Nanog and Sox2, involved in
the maintenance of pluripotency and self-renewal in embryonic stem
cells (ESCs), have been identified in tumors of various origins
(3).
Oct3/4, also known as OCT 3, OCT 4 and POU5F1, is
one of the earliest transcription factors expressed in the embryo
and is encoded by a homeobox-containing gene named Pou5f1 belonging
to the family of Pit Oct Unc (POU) genes and recognized as
fundamental in the maintenance of pluripotency and self-renewal in
ESCs and in primordial germ cells (4). Nanog is also a homeodomain
transcription factor thought to be a key factor in sustaining the
pluripotency of ESCs (5). Oct3/4
and Nanog expression have been identified in certain human tumors
(6). Studies of Oct3/4 and Nanog
have revealed that they are specific markers for seminoma/germinoma
and embryonal carcinoma in primary testicular and central nervous
system (CNS) tumors (7–10). The importance of Oct3/4 has also
been demonstrated in the diagnosis of metastatic seminoma and
embryonal carcinoma (11). In
addition, the ectopic expression of Oct3/4 and Nanog induced an
oncogenic potential in epithelial cells and NIH3T3 cells (12,13).
The genetic expression of Oct3/4 and Nanog and the protein
expression of Nanog have been identified in breast carcinoma and
the MCF7 breast carcinoma cell line (6,14).
Sox-2, a member of the SRY-related HMG-box family of transcription
factors, regulates ESC pluripotency and is also expressed in many
tumors (15–17). Previous studies of Sox-2 in breast
tumor samples and cell lines have suggested that Sox-2 has
oncogenic potential in breast carcinogenesis (18–20).
However, a systematic study of the expression of Oct3/4, Nanog and
Sox-2 in human breast cancer cell lines has not been reported.
The present study sought to detect the expression of
Oct3/4, Nanog and Sox-2 in human breast cancer cell lines. A
heterogeneous population of established human breast cancer cell
lines was examined to investigate the diverse expressions of
Oct3/4, Nanog and Sox-2 among them.
Materials and methods
Cell lines and culture conditions
The human breast cancer cell lines MCF7, T-47D and
MDA-MB-231 were purchased from the American Type Culture Collection
(Shanghai Institute of Cellular Biology, Chinese Academy of
Sciences, Shanghai, China). All cells were routinely cultured in
Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 medium (Gibco,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco)
in a humidified atmosphere of 5% CO2 in air at 37°C and
used when in the log phase of growth.
Semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR)
To assess the mRNA expression levels of Oct3/4,
Nanog and Sox-2, total RNA was extracted using an RNeasy Mini kit
(Qiagen Inc., Valencia, CA, USA). Reverse transcription was
performed using the SuperScript First-Strand Synthesis kit
(Invitrogen, Carlsbad, CA, USA). For semiquantitative PCR, 1
μl target cDNA conversion mixture was amplified using
Hotstar Taq DNA polymerase (Qiagen, Hilden, Germany) for 35
cycles at 94°C for 30 sec, at 55°C for 30 sec and at 72°C for 1
min. The PCR primers included Oct3/4A (forward,
5′-TGGAGAAGGAGAAGCTGGAGCAAAA-3′; reverse,
5′-GGCAGAGGTCGTTTGGCTGAATAGACC-3′, Genbank accession number:
NM_002701), Nanog (forward, 5′-TCCTCCTCTTCCTCTATACTAAC-3′; reverse,
5′-CCC ACAATCACAGGCATAG-3′, Genbank accession number: NM_024865)
and Sox-2 (forward, 5′-GGGAAATGGAGG GGTGCAAAAGAGG-3′; reverse,
5′-TTGCGTGAGTGT GGATGG GATTGGTG-3′, Genbank accession number:
NM_003106) and β-actin (forward, 5′GCGGGAAATCGT GCGTGACATT-3′;
reverse, 5′-GGCAGATGGTCGTTT GGCTGAATA-3′, Genbank accession number:
NM_001101). PCR products were electrophoresed on a 1.2% agarose gel
and stained with ethidium bromide.
Immunofluorescent staining
To identify the expression of Oct3/4, Nanog and
Sox-2 in breast cancer cell lines, cells were fixed in BD
Cytofix/Cytoperm solution (BD Biosciences, San Jose, CA, USA) for
20 min at 4°C. Following blocking for 20 min with donkey serum
(Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA) in
BD Perm/Wash Buffer (BD Biosciences), cells were incubated with
goat anti-Oct3/4 antibody (sc-8628; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and mouse anti-Sox-2 antibody (MAB-4343;
1:100; Millipore, Billerica, MA, USA) overnight at 4°C, followed by
Rhodamine red conjugated donkey anti-goat antibody (1:200; Jackson
Immunoresearch Laboratories, Inc.). For Nanog staining following
fixation as described above, cells were stained with fluorescein
isothiocyanate (FITC)-conjugated anti-human Nanog antibody
(eBioscience, Inc., San Diego, CA, USA). Negative control reactions
were included in each experiment and carried out by replacing
primary antibodies with PBS. Fluorescent cells were visualized and
digital images were captured using an Olympus microscope.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Statistically significant differences were determined using
SPSS version 16 software. P<0.05 was considered to indicate a
statistically significant result.
Results
Detection of Oct 3/4, Nanog and Sox-2 in
human breast cancer cell lines by RT-PCR
Oct3/4, Nanog and Sox-2 are essential transcription
factors that regulate and maintain the self-renewal and
pluripotency of ESCs (4). To
determine whether the genes for these proteins are expressed in
three breast cancer cell lines, semiquantitative RT-PCR analyses
were first performed. In addition, since the existence of 2 mRNA
and protein isoforms of Oct3/4 (Oct3/4A and Oct3/4B) has been
validated (21), we sought to
determine the gene expression of Oct3/4A specifically for the stem
cell-like properties. As shown in Fig.
1A, the 3 human breast cancer cell lines tested expressed
evident Oct3/4A, Nanog and Sox-2 mRNA at various expression levels.
The quantity of gene expression was normalized to β-actin to
determine quantitative differences among these 3 cell lines. As
shown in Fig. 1B, MDA-MB-231 and
MCF7 cells expressed significantly higher levels of Oct3/4A than
T-47D cells (P<0.05), whereas MCF7 and T-47D cells expressed
significantly higher levels of Nanog compared with MDA-MB-231 cells
(P<0.05; Fig. 1C). MCF7 and
T-47D cells expressed significantly higher levels of Sox-2 compared
with MDA-MB-231 cells and the highest expression of Sox-2 was
detected in MCF7 cells (P<0.05; Fig.
1D). The expression of various pluripotency marker genes was
noted within the three breast cancer cell lines, suggesting that
these pluripotency marker genes are reactivated during the process
of breast cancer development.
Localization of Oct3/4, Nanog and Sox-2
in human breast cancer cell lines using immunofluorescence
staining
To determine whether these genes were expressed at
the protein level, the cells were examined using immunofluorescent
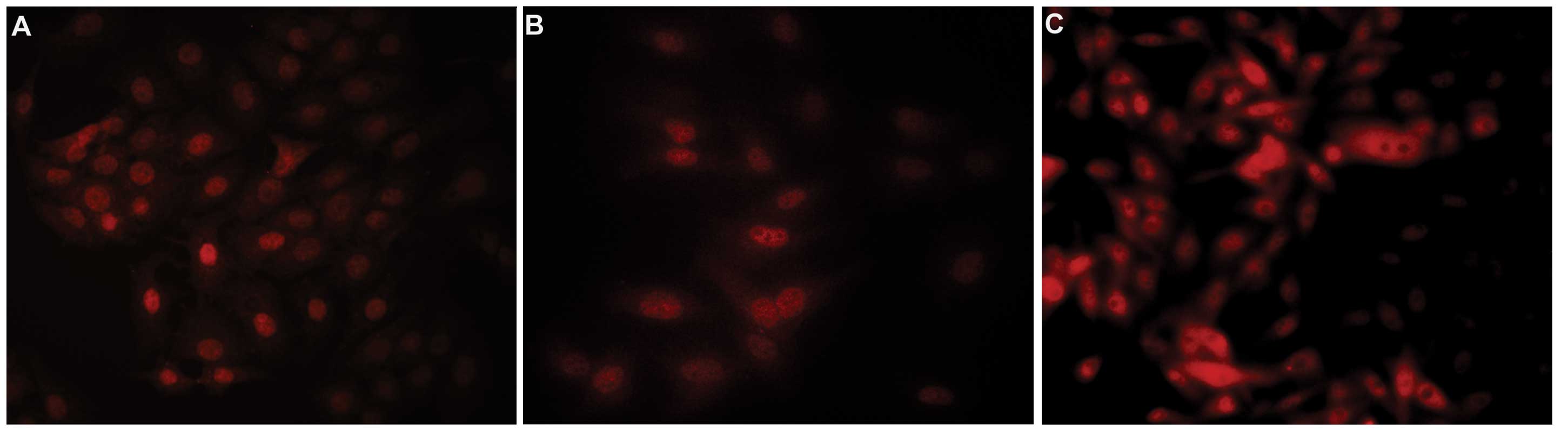
staining. As shown in Fig. 2, a
punctate nuclear staining of Oct3/4A was observed homogenously in
all three cell lines, which is consistent with a previous study
which demonstrated the same characteristic in type I breast stem
cells (22). As shown in Fig. 3, bright punctate nuclear staining of
Nanog was also displayed in a small population of all 3 breast
cancer cell lines tested. The Nanog-positive cells were scattered
in breast cancer cells. Fig. 4
confirms nuclear staining of Sox-2 in all three cell lines.
Discussion
In cancer biology, the correlation between
embryogenesis and oncogenesis has long been a prevailing theme.
Cancer cells and ESCs share numerous key biological properties. A
trait possessed by both cell types is extensive proliferative
potential for embryogenesis and tumor development. Pluripotency is
also fundamental to ESCs and gives rise to the myriad of
differentiated daughter cells present in the mature embryo
(23). It is important to study the
pluripotency-related genes associated with embryogenesis and
tumorigenesis. Transcription factors are critical molecular
switches regulating ESC fate, which may also operate in renewing
cancer cells. Three pluripotency-related transcription factors, Oct
3/4, Nanog and Sox-2, form a core regulatory network that
coordinates to determine the self-renewal and differentiation of
ESCs. These ESC self-renewal molecules may also contribute to
tumorigenesis (24). The
dysregulated expression of Oct 3/4, Nanog and Sox-2 has been shown
in numerous types of tumors and it is possible that this may
contribute to the neoplastic process and play a role in cancer
development.
The purpose of the current study was to corroborate
the presence of pluripotency-associated markers in human breast
cancer cell lines and further investigate whether the diverse
expressions of these markers relate to the inherent tumorigenicity
among various breast cancer cell lines. Oct3/4 is an embryonic
transcription factor highly expressed in ESCs, carcinoma cells and
oocytes. Oct3/4 belongs to the POU transcription factor family and
plays a critical role in maintaining the pluripotent and
self-renewing state of stem cells (25). In humans, two isoforms are encoded,
Oct3/4A and Oct3/4B, which share a common C-transactivation domain
but have different N-transactivation domains. The existence of 2
isoforms is likely to be functionally significant but only Oct3/4A
is responsible for the stem cell-like properties of cancer cells
(21,26). It has been previously proposed that
Oct3/4 acts as a multifunctional factor in cancer biology based on
reports that Oct3/4 increases the malignant potential of ESCs in a
dose-dependent manner (12,27). The expression of Oct3/4 has also
been shown in some tumors and it is considered to play a critical
role in tumorigenesis (6,28–32).
However, Oct3/4 expression studies on tumors are generally carried
out without considering the two isoforms. The current study sought
to examine Oct3/4 A at the mRNA and protein levels in three human
breast cancer cell lines. The results revealed that Oct3/4A was
expressed in all three human breast cancer cell lines, although
various expression patterns were noted. Oct3/4A was detected in the
nuclei of breast cancer cells, suggesting a subset of cells with
stem cell attributes were present in the breast cancer cell lines.
In stem cells, Oct3/4A functions as a master switch during
differentiation by regulating the pluripotent potential (33,34).
Thus, the over-expression of Oct3/4A in human breast cancer cells
implies a link between Oct3/4A and tumorigenesis via the activation
of its downstream target genes (35). Furthermore, Oct3/4A mRNA expression
was significantly upregulated in the MCF7 and MDA-MB-231 cells
compared with the T-47D cells. This suggests that there is higher
OCT3/4A expression in the adenocarcinoma cells since MCF7 and
MDA-MB-231 cells are derived from an adenocarcinoma. Lower Oct3/4A
expression may be seen in the ductal carcinoma cells since T-47D
cells are derived from a ductal carcinoma. However, further
research using additional cell lines and primary tumors is required
to confirm this result.
Nanog is a recently identified transcription factor
and is typically expressed in ESCs. The constitutive expression of
Nanog maintains the stem cell phenotype allowing for self-renewal
and propagation of the cell line, even in the presence of agents
that promote differentiation. The overexpression of Nanog in human
ECSs promotes pluripotency and is associated with increased
self-renewal capacity, whereas the knockdown of Nanog induces
differentiation into mature cell types (36,37).
Previous studies of Nanog in tumors have suggested its tumorigenic
potential and regulation of tumor development (14,38,39).
The current study demonstrated that Nanog was detected at the mRNA
and protein levels in the three human breast cancer cell lines
tested. The scattered Nanog-positive cells were observed in breast
cancer cells by immunostaining, suggesting that Nanog is expressed
only in a small subset of cancer cells. A small population of cells
capable of proliferating extensively and initiating tumors are
termed cancer stem cells (40).
Higher expression of Nanog has also been shown in the cancer stem
cells in human osteosarcoma (41).
Jeter et al revealed that the downregulation of Nanog
inhibited MCF7 breast cancer cell clonal expansion and tumor
development (14). Therefore, the
expression of Nanog in breast cancer cells may correlate with the
tumorigenesis of breast cancer.
Sox-2, a transcription factor located on 3q26.3-q27,
is one of the transcription factors expressed by stem cells. There
is growing evidence to suggest that this gene is essential for the
maintenance of stem cell proliferation and differentiation
capabilities (42).
A number of links have been identified between Sox-2
transcription factors and human cancers (43). Sox-2 expression has been observed in
embryonal carcinoma, teratoma, lung, pancreactic and gastric
adenocarcinoma (44). The present
study demonstrated that Sox-2 was expressed in all 3 of the human
breast cancer cell lines tested. MCF7 and T-47D cells expressed
significantly higher levels of Sox-2 compared with MDA-MB-231
cells. These results are consistent with a previous study by Chen
et al (20) that identified
higher Sox-2 protein expression in MCF7 and T-47D cells than in
MDA-MB-231 cells. Chen et al further demonstrated that the
downregulation of Sox-2 using si-RNA in MCF7 cells has been shown
to inhibit cell proliferation and tumor growth (20). In breast cancer, Sox-2 has also been
shown to be a possible driver of the basal-like phenotype and play
an early role in breast carcinogenesis (18,19).
In conclusion, the present study confirms the
expression of the pluripotency-associated markers Oct3/4, Nanog and
Sox-2 in 3 separate breast cancer cell lines, as demonstrated by
RT-PCR and immnocytohistochemistry. Further experiments are
required to explore the complex role of Oct3/4, Nanog and Sox2 in
human breast cancer.
References
|
1.
|
J FerlayHR ShinF BrayD FormanC MathersDM
ParkinEstimates of worldwide burden of cancer in 2008: GLOBOCAN
2008Int J Cancer12728932917201010.1002/ijc.2551621351269
|
|
2.
|
AM Gonzalez-AnguloF Morales-VasquezGN
HortobagyiOverview of resistance to systemic therapy in patients
with breast cancerAdv Exp Med
Biol608122200710.1007/978-0-387-74039-3_117993229
|
|
3.
|
I Ben-PorathMW ThomsonVJ CareyAn embryonic
stem cell-like gene expression signature in poorly differentiated
aggressive human tumorsNat
Genet40499507200810.1038/ng.12718443585
|
|
4.
|
M PesceHR SchölerOct-4: gatekeeper in the
beginnings of mammalian developmentStem
Cells19271278200110.1634/stemcells.19-4-27111463946
|
|
5.
|
DJ RoddaJL ChewLH LimTranscriptional
regulation of nanog by OCT4 and SOX2J Biol
Chem2802473124737200510.1074/jbc.M50257320015860457
|
|
6.
|
UI EzehPJ TurekRA ReijoAT ClarkHuman
embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are
expressed in both seminoma and breast
carcinomaCancer10422552265200510.1002/cncr.2143216228988
|
|
7.
|
AH HartL HartleyK ParkerThe pluripotency
homeobox gene NANOG is expressed in human germ cell
tumorsCancer10420922098200510.1002/cncr.2143516206293
|
|
8.
|
CE Hoei-HansenK AlmstrupJE NielsenStem
cell pluripotency factor NANOG is expressed in human fetal
gonocytes, testicular carcinoma in situ and germ cell
tumoursHistopathology474856200510.1111/j.1365-2559.2005.02182.x15982323
|
|
9.
|
S SantagataJL HornickKL LigonComparative
analysis of germ cell transcription factors in CNS germinoma
reveals diagnostic utility of NANOGAm J Surg
Pathol3016131618200610.1097/01.pas.0000213320.04919.1a17122519
|
|
10.
|
TD JonesTM UlbrightJN EbleLA BaldridgeL
ChengOCT4 staining in testicular tumors: a sensitive and specific
marker for seminoma and embryonal carcinomaAm J Surg
Pathol28935940200410.1097/00000478-200407000-0001415223965
|
|
11.
|
L ChengEstablishing a germ cell origin for
metastatic tumors using OCT4
immunohistochemistryCancer10120062010200410.1002/cncr.2056615386301
|
|
12.
|
K HochedlingerY YamadaC BeardR
JaenischEctopic expression of Oct-4 blocks progenitor-cell
differentiation and causes dysplasia in epithelial
tissuesCell121465477200510.1016/j.cell.2005.02.018
|
|
13.
|
J ZhangX WangB ChenExpression of Nanog
gene promotes NIH3T3 cell proliferationBiochem Biophys Res
Commun33810981102200510.1016/j.bbrc.2005.10.07116259959
|
|
14.
|
CR JeterM BadeauxG ChoyFunctional evidence
that the self-renewal gene NANOG regulates human tumor
developmentStem Cells279931005200910.1002/stem.2919415763
|
|
15.
|
Y SanadaK YoshidaM OharaM OedaK KonishiY
TsutaniHistopathologic evaluation of stepwise progression of
pancreatic carcinoma with immunohistochemical analysis of gastric
epithelial transcription factor SOX2: comparison of expression
patterns between invasive components and cancerous or nonneoplastic
intraductal componentsPancreas321641702006
|
|
16.
|
XL LiY EishiYQ BaiExpression of the
SRY-related HMG box protein SOX2 in human gastric carcinomaInt J
Oncol24257263200414719100
|
|
17.
|
AJ BassH WatanabeCH MermelSOX2 is an
amplified lineage-survival oncogene in lung and esophageal squamous
cell carcinomasNat Genet4112381242200910.1038/ng.46519801978
|
|
18.
|
C LengerkeT FehmR KurthExpression of the
embryonic stem cell marker SOX2 in early-stage breast carcinomaBMC
Cancer1142201110.1186/1471-2407-11-4221276239
|
|
19.
|
SM Rodriguez-PinillaD SarrioG
Moreno-BuenoSox2: a possible driver of the basal-like phenotype in
sporadic breast cancerMod
Pathol20474481200710.1038/modpathol.380076017334350
|
|
20.
|
Y ChenL ShiL ZhangThe molecular mechanism
governing the oncogenic potential of SOX2 in breast cancerJ Biol
Chem2831796917978200810.1074/jbc.M80291720018456656
|
|
21.
|
J LeeHK KimJY RhoYM HanJ KimThe human
OCT-4 isoforms differ in their ability to confer self-renewalJ Biol
Chem2813355433565200610.1074/jbc.M60393720016951404
|
|
22.
|
MH TaiCC ChangM KiupelJD WebsterLK OlsonJE
TroskoOct4 expression in adult human stem cells: evidence in
support of the stem cell theory of
carcinogenesisCarcinogenesis26495502200515513931
|
|
23.
|
JE VisvaderGJ LindemanCancer stem cells in
solid tumours: accumulating evidence and unresolved questionsNat
Rev Cancer8755768200810.1038/nrc249918784658
|
|
24.
|
CC ChangGS ShiehP WuCC LinAL ShiauCL
WuOct-3/4 expression reflects tumor progression and regulates
motility of bladder cancer cellsCancer
Res6862816291200810.1158/0008-5472.CAN-08-009418676852
|
|
25.
|
MM MatinJR WalshPJ GokhaleSpecific
knockdown of Oct4 and beta2-microglobulin expression by RNA
interference in human embryonic stem cells and embryonic carcinoma
cellsStem Cells22659668200410.1634/stemcells.22-5-65915342930
|
|
26.
|
G CauffmanI LiebaersA Van SteirteghemH Van
de VeldePOU5F1 isoforms show different expression patterns in human
embryonic stem cells and preimplantation embryosStem
Cells2426852691200610.1634/stemcells.2005-061116916925
|
|
27.
|
S GidekelG PizovY BergmanE PikarskyOct-3/4
is a dose-dependent oncogenic fate determinantCancer
Cell4361370200310.1016/S1535-6108(03)00270-814667503
|
|
28.
|
LH LooijengaH StoopHP de LeeuwPOU5F1
(OCT3/4) identifies cells with pluripotent potential in human germ
cell tumorsCancer Res6322442250200312727846
|
|
29.
|
Y AtlasiSJ MowlaSA ZiaeeAR BahramiOCT-4,
an embryonic stem cell marker, is highly expressed in bladder
cancerInt J Cancer12015981602200710.1002/ijc.2250817205510
|
|
30.
|
GM SeigelAS HackamA GangulyLM MandellF
Gonzalez-FernandezHuman embryonic and neuronal stem cell markers in
retinoblastomaMol Vis13823832200717615543
|
|
31.
|
SH ChiouCC YuCY HuangPositive correlations
of Oct-4 and Nanog in oral cancer stem-like cells and high-grade
oral squamous cell carcinomaClin Cancer
Res1440854095200810.1158/1078-0432.CCR-07-440418593985
|
|
32.
|
YC ChenHS HsuYW ChenOct-4 expression
maintained cancer stem-like properties in lung cancer-derived
CD133-positive cellsPLoS
One3e2637200810.1371/journal.pone.000263718612434
|
|
33.
|
K OkitaT IchisakaS YamanakaGeneration of
germline-competent induced pluripotent stem
cellsNature448313317200710.1038/nature0593417554338
|
|
34.
|
IH ParkR ZhaoJA WestReprogramming of human
somatic cells to pluripotency with defined
factorsNature451141146200810.1038/nature0653418157115
|
|
35.
|
GJ PanZY ChangHR SchölerD PeiStem cell
pluripotency and transcription factor Oct4Cell
Res12321329200210.1038/sj.cr.729013412528890
|
|
36.
|
H DarrY MaysharN BenvenistyOverexpression
of NANOG in human ES cells enables feeder-free growth while
inducing primitive ectoderm
featuresDevelopment13311931201200610.1242/dev.0228616501172
|
|
37.
|
H ZaehresMW LenschL DaheronSA StewartJ
Itskovitz-EldorGQ DaleyHigh-efficiency RNA interference in human
embryonic stem cellsStem
Cells23299305200510.1634/stemcells.2004-025215749924
|
|
38.
|
L WangP ParkCY LinCharacterization of stem
cell attributes in human osteosarcoma cell linesCancer Biol
Ther8543552200910.4161/cbt.8.6.769519242128
|
|
39.
|
MK SiuES WongHY ChanHY NganKY ChanAN
CheungOverexpression of NANOG in gestational trophoblastic
diseases: effect on apoptosis, cell invasion, and clinical
outcomeAm J
Pathol17311651172200810.2353/ajpath.2008.08028818772339
|
|
40.
|
CT JordanML GuzmanM NobleCancer stem
cellsN Engl J Med35512531261200610.1056/NEJMra06180816990388
|
|
41.
|
L WangP ParkH ZhangF La MarcaCY
LinProspective identification of tumorigenic osteosarcoma cancer
stem cells in OS99-1 cells based on high aldehyde dehydrogenase
activityInt J Cancer128294303201110.1002/ijc.2533120309879
|
|
42.
|
T WilbertzP WagnerK PetersenSOX2 gene
amplification and protein overexpression are associated with better
outcome in squamous cell lung cancerMod
Pathol24944953201110.1038/modpathol.2011.4921460799
|
|
43.
|
C DongD WilhelmP KoopmanSox genes and
cancerCytogenet Genome Res105442447200410.1159/000078217
|
|
44.
|
S SantagataKL LigonJL HornickEmbryonic
stem cell transcription factor signatures in the diagnosis of
primary and metastatic germ cell tumorsAm J Surg
Pathol31836845200710.1097/PAS.0b013e31802e708a17527070
|