Introduction
Globally, gastric cancer (GC) is the fourth most
common malignancy among all types of cancers, and mortality due to
GC is second only to lung cancer (1). Of all GC cases, >70% occur in
developing countries and half the world total occurs in Eastern
Asia (mainly China) (2). Despite
improvements in surgical techniques and the development of new
chemotherapeutic regimens, the results are often disappointing. The
overall five-year survival rate for patients who undergo curative
surgical resections for GC ranges between 47.0 and 60.4% (3). Although a number of studies have
investigated the pathogenesis of the disease, the true mechanisms
of GC carcinogenesis remain obscure (3).
In the past few years, it has been hypothesized that
tumors are most likely initiated by a minority of cells, known as
cancer stem cells (CSCs) (4).
According to the American Association for Cancer Research (AACR),
CSCs are defined as subpopulations of cells within a tumor that
possess the capacity for self-renewal and cause the heterogeneous
lineage of cancer cells that constitute the tumor (5). The difficulty in eradicating tumors
may be due to the fact that conventional treatments target the bulk
of the tumor cells, leaving the CSCs, which are involved in tumor
maintenance, therapy resistance, tumor progression, recurrence and
distant metastasis, unaffected. According to this hypothesis,
identifying and eradicating CSCs may be an effective treatment
modality (6).
The question of how to isolate and identify CSCs is
key to the research into them. Three distinct methodologies based
on the properties of CSCs have been used successfully for the
isolation of these cells from solid tumors (5,7,8). The
first is fluorescence-activated cell sorting (FACS) according to
CSC-specific cell surface markers, such as cluster of
differentiation 44 (CD44) or CD133 (8,9). The
second is that side populations (SP) of tumor cells, which exhibit
intracellular Hoechst 33342 exclusion in vitro and also
preferentially express adenosine triphosphate-binding cassette
transporter G2 (ABCG2) (10–12),
are isolated and characterized as CSCs (13–15).
The third is the spheroid body formation assay in which cells are
cultured in non-adherent conditions in a serum-free medium
supplemented with basic fibroblast growth factor (bFGF) and
epidermal growth factor (EGF). The latter approach has been
suggested as a practical approach for individual solid tumor
tissues or cancer cells (16,17).
The present study aimed to develop spheroid body-forming cells in
the MKN-45 GC cell line and to analyze the expression of two
putative candidate stem cell markers, octamer-binding transcription
factor-4 (OCT4) and ABCG2, in spheroid body-forming cells.
Materials and methods
Culture of parental cells and spheroid
body-forming cells
The human MKN-45 GC cell line was purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and
cultured in RPMI-1640 medium containing 10% fetal bovine serum
(FBS), then plated at a density of 1×106 live cells per
75-cm2 flask. Once the cells had become attached they
were subsequently passaged upon confluence. Spheroid bodies were
derived by placing the parental cells into serum-free RPMI-1640
culture medium containing 1% N-2 supplement, 2% B-27 supplement
(both Invitrogen, Carlsbad, CA, USA), 1% antibiotic mixture (Gibco,
Carlsbad, CA, USA), 20 ng/ml human FGF-2 and 100 ng/ml EGF (both
Chemicon, Temecula, CA, USA). The parental cells were plated in
96-well ultra-low attachment plates (Corning Inc., Corning, NY,
USA) at 100 cells per well. Two weeks later, the plates were
analyzed for spheroid body formation and quantified using an
inverted microscope (Olympus, Tokyo, Japan) at ×40 and ×100
magnification. Once the primary spheroid bodies had reached a size
of ~200–500 cells per spheroid body, they were dissociated at a
density of 1,000 cells per ml and 100 μl single cell suspension was
seeded in each well of the 96-well ultra-low attachment plates
(Corning) in serum-free medium, as described previously. Two weeks
later, the wells were analyzed for subspheroid body formation.
qPCR
Total RNA was extracted from the parental and
spheroid body-forming cells using Qiagen RNeasy mini kits (Qiagen,
Hilden, Germany) according to the manufacturer's instructions. RNA
was treated with DNase I (Qiagen) to eliminate genomic DNA
contamination. The integrity and purification of the RNA samples
were monitored by agarose gel electrophoresis. The concentration of
RNA was determined by repeated OD measurements of aliquots at a
wavelength of 260 nm. A reverse-transcription reaction to
transcribe 1 μg total RNA into complementary DNA was performed
using reagents of an Omniscript RT kit (Qiagen).
To determine the fold changes in the expression of
each gene, qPCR was performed using an Eppendorf
Mastercycler® ep realplex (2S; Eppendorf, Hamburg,
Germany). EvaGreen® (Biotium Inc., Hayward, CA, USA)
served as a dye that bound to the amplified DNA to emit
fluorescence during the reactions. EvaGreen has emerged as an
optimal green fluorescent DNA dye for qPCR, of equal or better
sensitivity compared with SYBR Green I (18). The 25-μl reaction mixture contained
12.5 μl Evagreen qPCR Master Mix (Biotium Inc.), 1 μl primers (10
mM), 1 μl template cDNA and 10.5 μl double distilled water
(ddH2O). The glyceraldehyde-3 phosphate dehydrogenase
(GAPDH) gene served as an internal control for the expression
levels of the target apoptosis genes. The primer sequences are
shown in Table I. After an initial
incubation for 2 min at 96°C, the reactions were performed for 40
cycles of 96°C for 15 sec and 60°C for 45 sec (florescence
collection). Fluorescence was measured during the extension step of
each cycle. A melting curve analysis was performed to ensure the
amplification of a single PCR product. Reactions with no template
were included as a negative control. By setting the threshold at
the level of the middle steady portion of the reaction cycles
versus florescence curve, the Ct values of the target genes were
calculated using Mastercycler ep realplex analysis software
(Eppendorf) and the 2−ΔΔCT method. Finally, the PCR
products were separated by 1.5% agarose gel electrophoresis in the
presence of ethidium bromide, prior to being visualized on an
ultraviolet illuminator to verify product sizes and then recorded.
qPCR was performed independently three times in triplicate.
 | Table IBase sequences of primers for
qPCR. |
Table I
Base sequences of primers for
qPCR.
| Primer name | Sequence |
|---|
| OCT4-Forward |
AACGACCATCTGCCGCT |
| OCT4-Reverse |
CGATACTGGTTCGCTTTCTCT |
| ABCG2-Forward |
TGAGGGTTTGGAACTGTGG |
| ABCG2-Reverse |
GATTCTGACGCACACCTGG |
| GAPDH-Forward |
GGCATCCTGGGCTACACT |
| GAPDH-Reverse |
CCACCACCCTGTTGCTGT |
Immunofluorescence staining for candidate
CSC markers
In brief, the cells plated onto poly-L-lysine-coated
glass coverslips were fixed with 4% paraformaldehyde, then washed
with phosphate-buffered saline (PBS). The cells were permeabilized
with 0.1% Triton X-100/PBS for 10 min and subsequently incubated
with primary antibodies (anti-OCT4 rabbit polyclonal and anti-ABCG2
mouse monoclonal antibodies; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). The cells were further probed with fluorescein
isothiocyanate or rhodamine-tagged secondary antibodies.
4′,6-Diamidino-2-phenylindole (DAPI), which is a fluorescent stain
that binds strongly to A-T rich regions in DNA, was used for the
nuclear counterstain. The fluorescence was recorded using an
inverted fluorescence microscope (Leica, Mannheim, Germany).
Western blot analysis
For the western blot analyses, proteins were
harvested from the cells plated to between 70 and 80% confluence.
Spheroid body-forming or parental cells were lysed directly in
lysis buffer to collect whole cell extracts. Protein samples for
western blotting were prepared by boiling the cell extracts
following the addition of denaturing sample buffer. Subsequently,
the proteins were separated using SDS-PAGE on an 8 or 15% gel, then
transferred onto PVDF membranes. The membranes were incubated at
4°C overnight with primary antibody and subsequently incubated with
horseradish peroxidase-conjugated secondary antibodies for 1 h at
room temperature. Finally, protein bands were visualized using
chemiluminescence (Santa Cruz) exposure on BioMax film (Kodak,
Rochester, NY, USA). A 1:200 concentration was used for the
anti-OCT4 and anti-ABCG2 primary antibodies (Santa Cruz
Biotechnology, Inc.).
In vivo tumorigenicity experiments
Male, six to eight-week-old athymic nude mice
(nu/nu) were obtained from the Shanghai Laboratory Animal Center of
the Chinese Academy of Sciences (Shanghai, China) and housed under
pathogen-free conditions in a barrier animal facility. All animal
procedures were performed with the approval of the Animal Ethics
Committee of Nantong University.
For the in vivo tumorigenicity experiments,
equal numbers (1×104, 2×104, 2×105
and 2×106) of freshly dissociated cells were suspended
in 200 μl PBS and then the spheroid body-forming cells were
injected subcutaneously into the right rear flank of each mouse
(six mice per group). The parental cells were injected
subcutaneously into the left rear flank of each mouse and the
tumorigenic capacity of the spheroid body-forming and parental
cells was evaluated. The mice were observed for tumor growth every
10 days over eight weeks, then sacrificed by cervical dislocation.
The grafts were removed, fixed with 10% buffered formalin and
stained with hematoxylin and eosin.
Statistical analysis
All experiments were repeated at least three times
and representative results are presented. All values in the figures
and text are shown as the mean ± SD. Statistical analyses were
performed using the SPSS statistical software package (SPSS/PC+;
SPSS Inc., Chicago, IL, USA). Significant differences among mean
values were evaluated by Student's t-test. A two-sided value of
P<0.05 was considered to indicate a significant difference.
Results
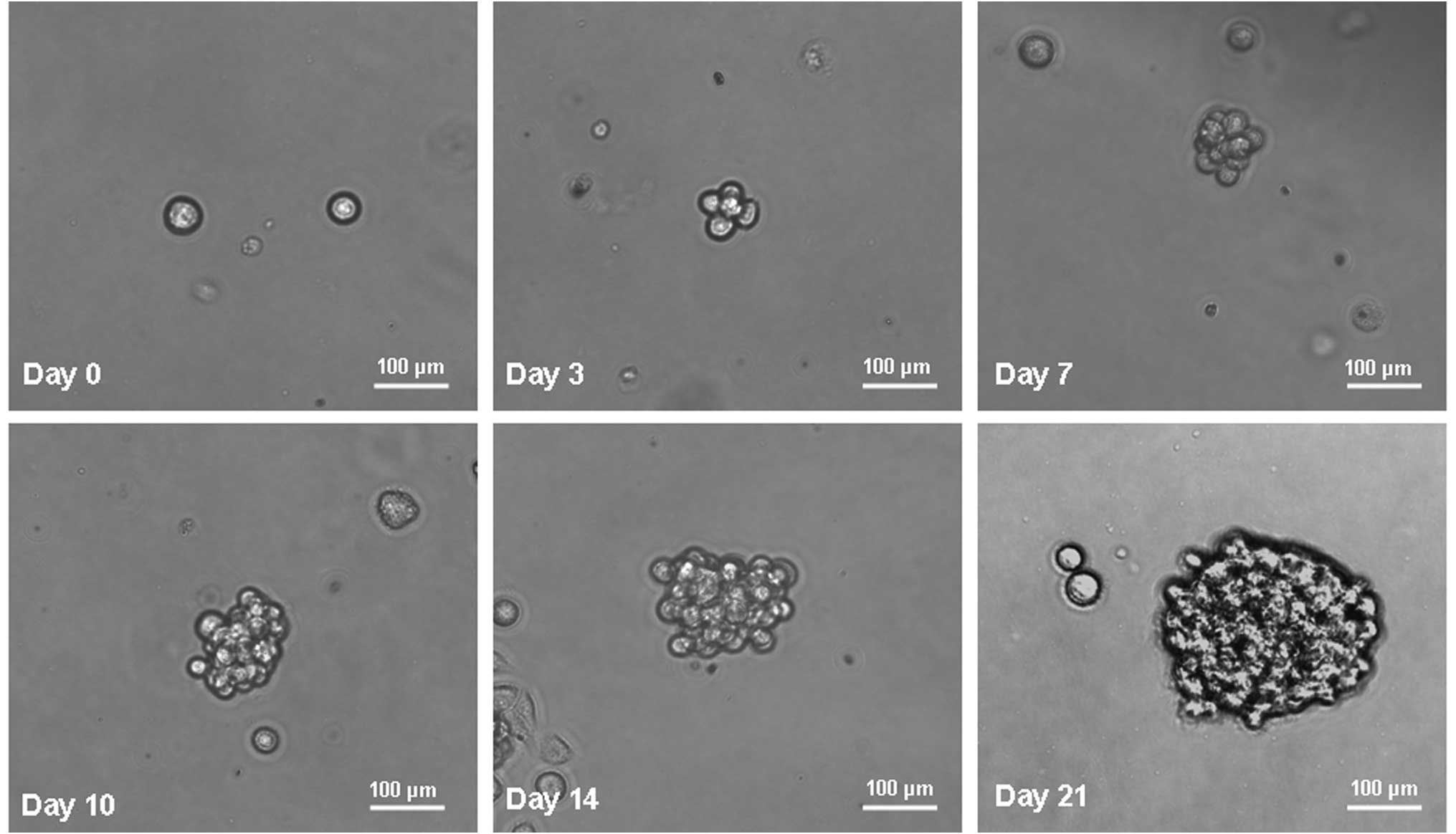
GC cells form anchorage-independent
spheroid bodies
MKN-45 parental cells were cultured in serum-free
medium as described in the methods section. Under these conditions,
the cells grew in non-adherent, three-dimensional spheroid clusters
known as spheroid bodies. The self-renewing capacity of these
spheroid body-forming cells was assessed by dissociation into
single cells and growth in serum-free medium as described in the
methods section. The spheroid bodies appeared to be taking shape at
day 3. At day 7, the spheroid bodies were formed substantially. At
day 10 and 14, the spheroid bodies were completely formed. At day
21, the spheroid bodies had become well-rounded structures composed
of numerous, compacted cells. Fig.
1 shows the generation of a spheroid body from a single MKN-45
cell. The propagation of a single cell cultured in a 96-well dish
was recorded separately at days 0, 3, 7, 10, 14 and 21.
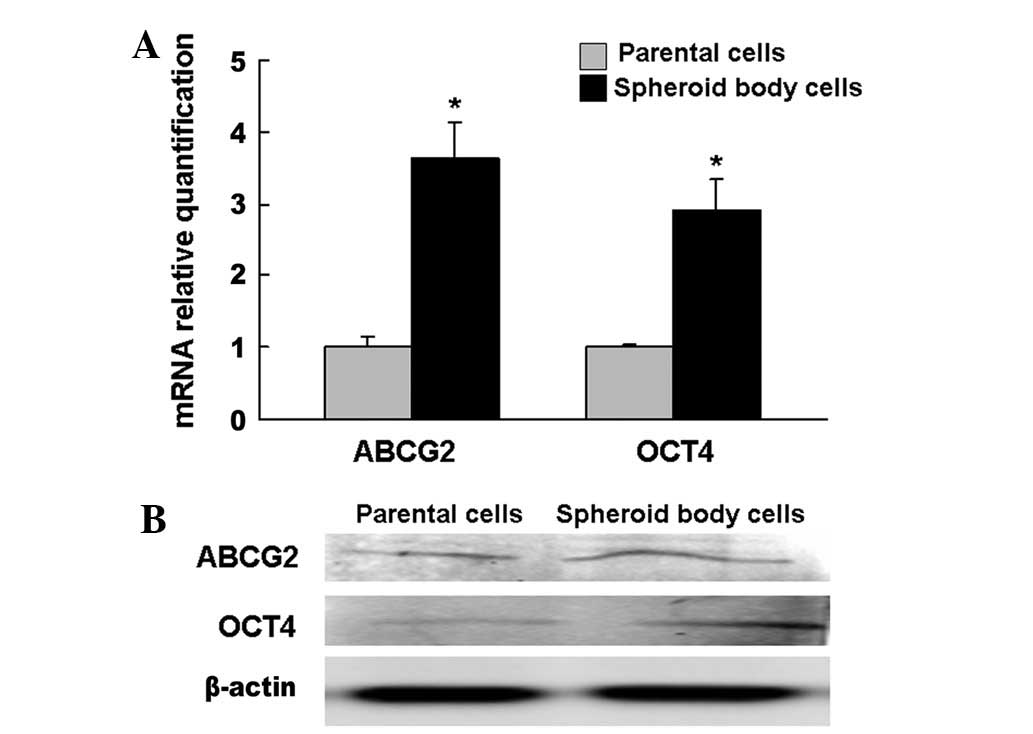
Spheroid body-forming cells overexpress
candidate CSC markers, OCT4 and ABCG2
qPCR and western blotting were performed on the
spheroid body-forming and parental cells. The results showed that
significantly more cells expressed OCT4 and ABCG2 among the
spheroid body-forming cells compared with the parental cells
(Fig. 2).
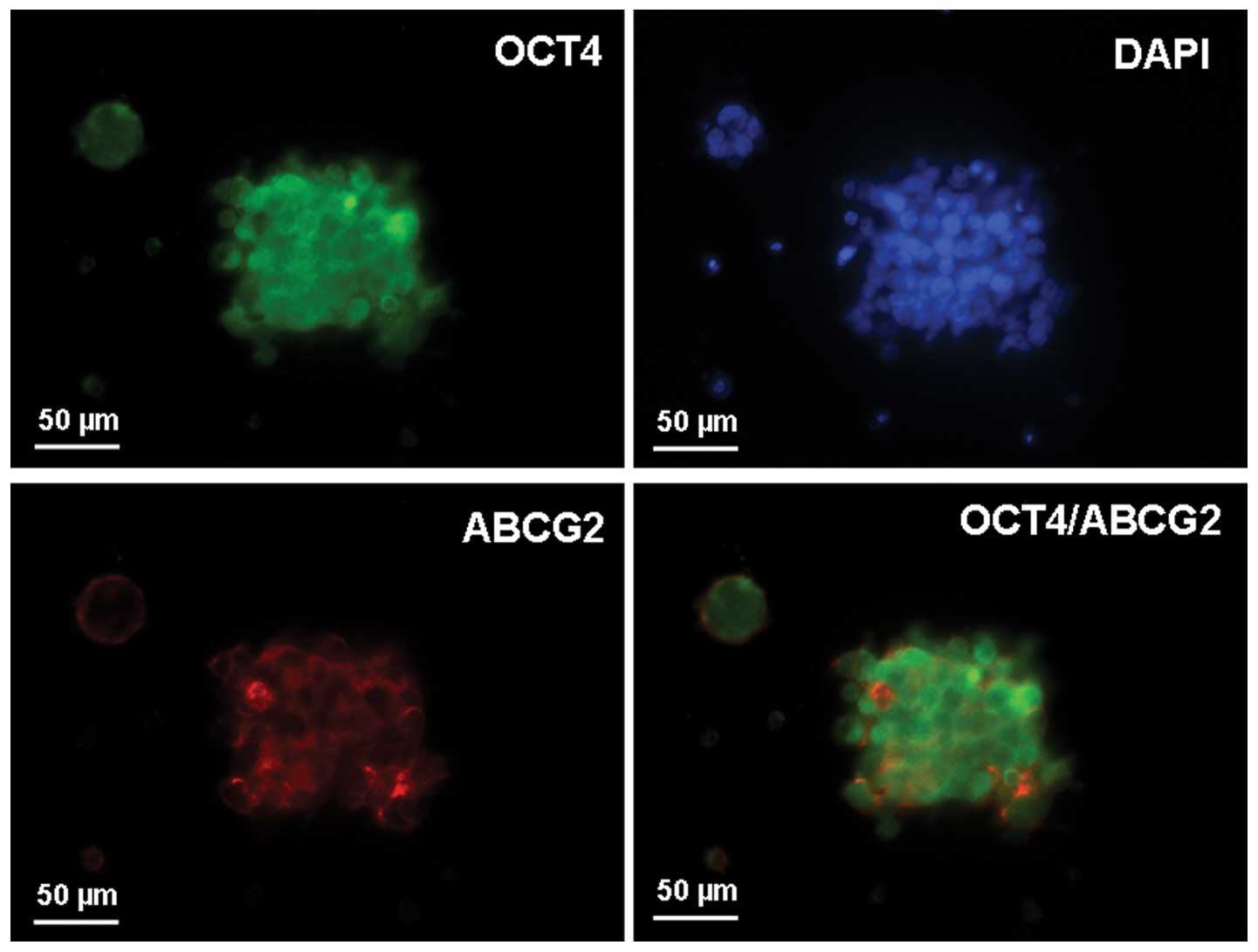
Intracellular localization of OCT4 and
ABCG2 in spheroid body-forming cells
To examine the subcellular localization of OCT4 and
ABCG2 in the spheroid body-forming cells, immunofluorescence
staining of OCT4 and ABCG2 was performed. Positive staining for
OCT4 and ABCG2 was observed, with OCT-4 mainly present within the
perinuclear cytoplasm of the spheroid body-forming cells and ABCG2
mainly present in the membrane. Dual staining for OCT4 and ABCG2
indicated that the candidate CSC markers, OCT4 and ABCG2, were
colocalized in the spheroid body-forming cells (Fig. 3).
Spheroid body-forming cells exhibit high
tumorigenicity in vivo
The tumorigenicity experiments in vivo showed
that as few as 2×104 cells from an MKN-45 spheroid body
were able to form a tumor when subcutaneously injected into nude
mice (Fig. 4A and B), while
2×106 parental cells were required for the same effect.
This value was 100-fold higher than that for the spheroid
body-forming cells. Moreover, the spheroid body-forming cells
generated subcutaneous tumors with larger volumes in shorter times
compared with those generated from the parental cells. The
transplanted tumors were confirmed as GC using hematoxylin and
eosin staining (Fig. 4C).
Discussion
OCT-4, a member of the POU-domain transcription
factor family, is expressed in pluripotent embryonic stem and germ
cells (19). OCT-4 functions as a
master switch during differentiation by regulating the pluripotent
potential in stem cells (20–22).
The expression of OCT-4 has also been shown in human breast cancer
stem-like cells and its expression may be implicated in
self-renewal and tumorigenesis (23). The ABCG2 transporter is a member of
the ATP-binding cassette transporter family responsible for the SP
phenotype in various human cancers and the corresponding
non-malignant tissues, and is widely used to detect and isolate
somatic stem/progenitor cells (24). Fukuda et al demonstrated that
the SP fraction of GC cells had a sphere forming ability and high
tumorigenicity in non-obese diabetic mice, as well as resistance to
anticancer drugs and an immunophenotype similar to that of stem
cells (25).
Previously, Chen et al demonstrated that
OCT-4 siRNA treatment resulted in a significant downregulation of
ABCG2 expression and an increase in the chemosensitivity of
CD133-positive cells (26). Jia
et al enriched CD90+/CD133+
hepatocellular carcinoma CSCs using spheroid body formation and
observed that OCT4 and ABCG2 were highly expressed in the enriched
CD90+/CD133+ liver CSCs and were closely
associated with chemotherapy drug resistance (27). However, the expression of OCT4 and
ABCG2 has not been reported in the spheroid body-forming cells of
GC.
Spheroid body cultures have increasingly been used
as a method for enriching stem cells, which relies on their
property of anchorage-independent growth. Through the application
of a spheroid body culture, numerous types of potential CSC
subpopulations have been reported to have been isolated and
enriched from primary tumors (28–35).
The spheroid body-forming cells from primary tumors, including
those of ovarian and breast cancer, have demonstrated stem-like
properties and expressed their CSC markers (29,33).
To the best of our knowledge, there have been few reports on the
isolation and characterization of gastric CSCs by the method of
spheroid body culture. Consequently, the present study developed
spheroid body cells by cultivating the human MKN-45 GC cell line
within a defined serum-free medium, and demonstrated that the cells
derived from the spheroid bodies were able to generate greater
numbers of new spheroid bodies and subcutaneous tumors in nude
mice, with larger volumes in shorter times, compared with those
generated from the parental cells. This indicated that the spheroid
body-forming cells were capable of self-renewal and proliferation
and possessed higher tumorigenicity, which are key characteristics
of CSCs.
To further investigate the CSC properties of
spheroid body-forming cells, the MKN-45 spheroid body-forming cells
were evaluated for the expression of the putative candidate CSC
markers, OCT4 and ABCG2. The present study observed that OCT4 and
ABCG2 were overexpressed in the MKN-45 spheroid body-forming cells
compared with the parental cells. More significantly, a focus was
first placed on the question of whether there is a physical linkage
between OCT4 and ABCG2 in spheroid body-forming cells. The
OCT4-positive spheroid body-forming cells were observed to be
co-stained with ABCG2, indicating the co-expression of OCT4 and
ABCG2 in MKN-45 spheroid bodies, which may represent a
subpopulation of gastric CSCs.
In conclusion, the study demonstrated that
non-adherent spheroid body-forming cells from the human MKN-45 GC
cell line that are cultured in a defined serum-free medium possess
gastric CSC properties. Since these cells co-expressed OCT4 and
ABCG2 in the MKN-45 spheroid bodies, they may represent a
subpopulation of gastric CSCs. The correlation between OCT4 and
ABCG2 in GC stem cells requires further investigation.
Acknowledgements
The present study was supported by a grant from the
Bureau of Science and Technology of Nantong City (HS2012067).
Abbreviations:
|
CSC
|
cancer stem cell
|
|
GC
|
gastric cancer
|
|
OCT4
|
octamer-binding transcription
factor-4
|
|
ABCG2
|
adenosine triphosphate binding
cassette transporter G2
|
References
|
1
|
Yasui W, Sentani K, Sakamoto N, Anami K,
Naito Y and Oue N: Molecular pathology of gastric cancer: research
and practice. Pathol Res Pract. 207:608–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Samson PS, Escovidal LA, Yrastorza SG,
Veneracion RG and Nerves MY: Re-study of gastric cancer: analysis
of outcome. World J Surg. 26:428–433. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu C and Alman BA: Side population cells
in human cancers. Cancer Lett. 268:1–9. 2008. View Article : Google Scholar
|
|
5
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar
|
|
6
|
Burkert J, Wright NA and Alison MR: Stem
cells and cancer: an intimate relationship. J Pathol. 209:287–297.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimano K, Satake M, Okaya A, Kitanaka J,
Kitanaka N, Takemura M, Sakagami M, Terada N and Tsujimura T:
Hepatic oval cells have the side population phenotype defined by
expression of ATP-binding cassette transporter ABCG2/BCRP1. Am J
Pathol. 163:3–9. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lechner A, Leech CA, Abraham EJ, Nolan AL
and Habener JF: Nestin-positive progenitor cells derived from adult
human pancreatic islets of Langerhans contain side population (SP)
cells defined by expression of the ABCG2 (BCRP1) ATP-binding
cassette transporter. Biochem Biophys Res Commun. 293:670–674.
2002. View Article : Google Scholar
|
|
12
|
Hirschmann-Jax C, Foster AE, Wulf GG,
Nuchtern JG, Jax TW, Gobel U, Goodell MA and Brenner MK: A distinct
‘side population’ of cells with high drug efflux capacity in human
tumor cells. Proc Natl Acad Sci USA. 101:14228–14233. 2004.
|
|
13
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dean M, Fojo T and Bates S: Tumor stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
15
|
Chiba T, Kita K, Zheng YW, Yokosuka O,
Saisho H, Iwama A, Nakauchi H and Taniguchi H: Side population
purified from hepatocellular carcinoma cells harbors cancer stem
cell-like properties. Hepatology. 44:240–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee J, Kotliarova S, Kotliarov Y, Li A, Su
Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK
and Fine HA: Tumor stem cells derived from glioblastomas cultured
in bFGF and EGF more closely mirror the phenotype and genotype of
primary tumors than do serum-cultured cell lines. Cancer Cell.
9:391–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radio-resistance by preferential activation of the
DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao F, Leung WY and Xin X:
Characterization of EvaGreen and the implication of its
physicochemical properties for qPCR applications. BMC Biotechnol.
7:762007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosner MH, Vigano MA, Ozato K, Timmons PM,
Poirier F, Rigby PW and Staudt LM: A POU-domain transcription
factor in early stem cells and germ cells of the mammalian embryo.
Nature. 345:686–692. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okita K, Ichisaka T and Yamanaka S:
Generation of germline-competent induced pluripotent stem cells.
Nature. 448:313–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park IH, Zhao R, West JA, Yabuuchi A, Huo
H, Ince TA, Lerou PH, Lensch MW and Daley GQ: Reprogramming of
human somatic cells to pluripotency with defined factors. Nature.
451:141–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu J, Vodyanik MA, Smuga-Otto K,
Antosiewicz-Bourget J, Frane JL, Tian S, et al: Induced pluripotent
stem cell lines derived from human somatic cells. Science.
318:1917–1920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Olempska M, Eisenach PA, Ammerpohl O,
Ungefroren H, Fandrich F and Kalthoff H: Detection of tumor stem
cell markers in pancreatic carcinoma cell lines. Hepatobiliary
Pancreat Dis Int. 6:92–97. 2007.PubMed/NCBI
|
|
25
|
Fukuda K, Saikawa Y, Ohashi M, Kumagai K,
Kitajima M, Okano H, Matsuzaki Y and Kitagawa Y: Tumor initiating
potential of side population cells in human gastric cancer. Int J
Oncol. 34:1201–1207. 2009.PubMed/NCBI
|
|
26
|
Chen YC, Hsu HS, Chen YW, Tsai TH, How CK,
et al: Oct-4 expression maintained cancer stem-like properties in
lung cancer-derived CD133-positive cells. PLoS One. 3:e26372008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia Q, Zhang X, Deng T and Gao J: Positive
correlation of Oct4 and ABCG2 to chemotherapeutic resistance in
CD90(+)CD133(+) liver cancer stem cells. Cell Reprogram.
15:143–150. 2013.PubMed/NCBI
|
|
28
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks P: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
29
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gibbs CP, Kukekov VG, Reith JD,
Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN and
Steindler DA: Stem-like cells in bone sarcomas: implications for
tumorigenesis. Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fang D, Nguyen TK, Leishear K, Finko R,
Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE and Herlyn M: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gou S, Liu T, Wang C, Yin T, Li K, Yang M
and Zhou J: Establishment of clonal colony-forming assay for
propagation of pancreatic cancer cells with stem cell properties.
Pancreas. 34:429–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang S, Balch C, Chan MW, Lai HC, Matei
D, Schilder JM, Yan PS, Huang TH and Nephew KP: Identification and
characterization of ovarian cancer-initiating cells from primary
human tumors. Cancer Res. 68:4311–4320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fujii H, Honoki K, Tsujiuchi T, Kido A,
Yoshitani K and Takakura Y: Sphere-forming stem-like cell
populations with drug resistance in human sarcoma cell lines. Int J
Oncol. 34:1381–1386. 2009.PubMed/NCBI
|
|
35
|
Rappa G, Mercapide J, Anzanello F,
Prasmickaite L, Xi Y, Ju J, Fodstad O and Lorico A: Growth of
cancer cell lines under stem cell-like conditions has the potential
to unveil therapeutic targets. Exp Cell Res. 314:2110–2122. 2008.
View Article : Google Scholar : PubMed/NCBI
|