Introduction
Renal cell carcinoma (RCC) is the most common type
of cancer of the adult kidney, accounting for ~3% of all adult
malignancies worldwide (1). As a
result, RCC is an important cause of cancer morbidity and
mortality. Complete surgical excision of the tumor remains the only
curative treatment for RCC (2).
Radical and partial nephrectomy are alternative treatments with
equivalent long-term oncological and renal functional outcomes
(3). Xp11.2 translocation RCC
(Xp11.2 RCC), a recently classified distinct subtype of RCC, is a
rare tumor that typically affects children and young adults. As it
is difficult to differentiate Xp11.2 RCCs from conventional RCCs
radiologically, the preoperative diagnosis of Xp11.2 RCCs remains
challenging, and Xp11.2 RCC is usually treated in the same manner
as conventional RCC.
Case report
A 30-year-old female was referred to The Affiliated
Drum Tower Hospital of the Medical College of Nanjing University
(Nanjing, Jiangsu, China) due to a right renal mass, found
incidentally during a routine physical examination. The patient
exhibited no symptoms and the medical history was uneventful. A
computed tomography (CT) scan (Fig. 1A
and B) showed a 3.0×3.2-cm mass, with cystic and solid
components, at the upper pole of the right kidney.
Contrast-enhanced ultrasonography showed focal enhancement in the
cystic zone, which was different from a renal cyst (Fig. 1D).
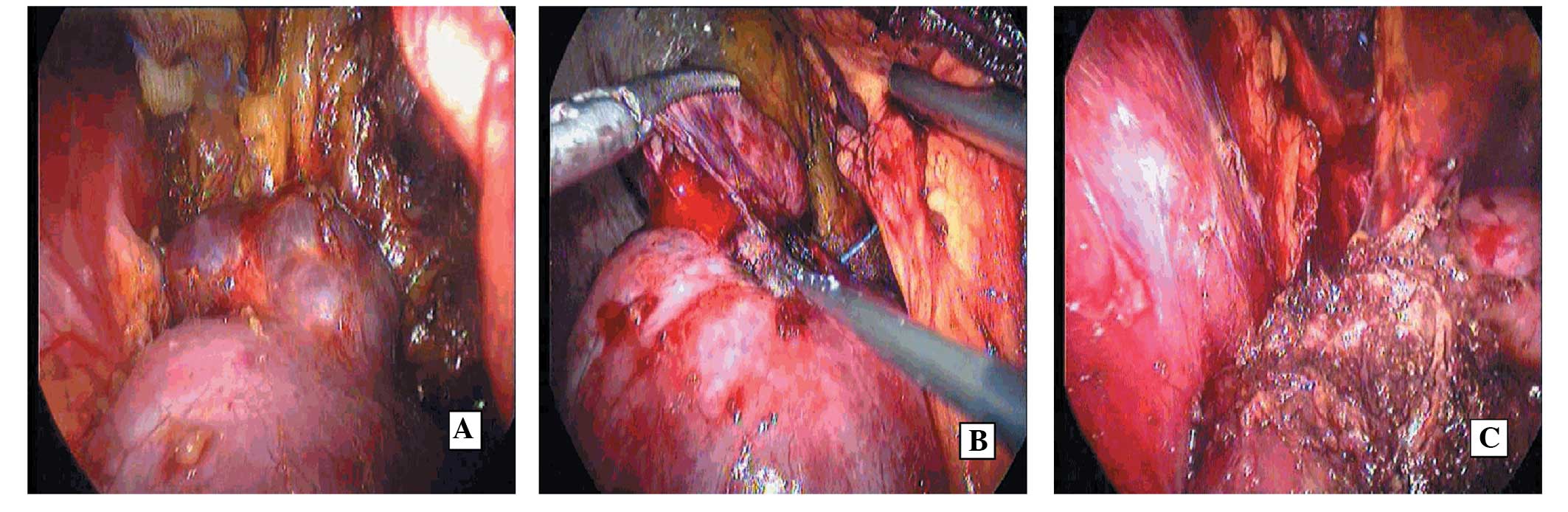
Laparoscopic tumor enucleation (TE) assisted by
radiofrequency ablation (RFA) was performed. The renal tumor, which
was cystoid and positioned at the upper pole of the right kidney,
was exposed via retroperitoneal laparoscopy (Fig. 2A). First, a cooled electrode was
inserted in the kidney between the tumor and normal renal tissue
under ultrasound guidance (Fig.
2B). The electrode was internally cooled with a peristaltic
pump circulating chilled water, and the tip temperature of the
electrode was kept ~12°C. The electrical power was elevated to 100
W. RFA lasted for 10 min. Next, the ablation was performed again on
an area 2 cm away from the first electrode. Finally, the tumor was
removed along the pseudocapsule (Fig.
2C).
Gross examination showed a cystoid tumor of 3.0 cm
in diameter, with solid tissue and septa inside. The hematoxylin
and eosin-stained histopathological specimen revealed predominant
tumor cells showing papillary architectures with calcification. A
fibrous capsule surrounded the tumor. The pathological stage was
classified as pT1aNxM0. Immunochemistry for transcription factor E3
(TFE3) showed intense nuclear staining in the majority of the tumor
cells.
Subsequent to 2.5 years of follow-up, the patient
showed no evidence of disease on contrast-enhanced CT and
ultrasonography (Fig. 1C and
E).
Discussion
Xp11.2 RCCs have been present in the World Health
Organization classification of kidney tumors for nine years
(4). Xp11.2 RCCs are relatively
rare tumors that typically occur in children and young adults.
Approximately one-third of pediatric RCCs are estimated to be
Xp11.2 RCCs associated with TFE3 gene fusion. The incidence in
adults has recently been evaluated as 1.5% of all types of RCCs
(5). The exact frequency of Xp11.2
RCCs is actually underestimated in patients >40 years old, as
its histological features often mimic clear cell RCCs or papillary
RCCs.
Xp11.2 RCCs can present with macroscopic hematuria,
flank pain, colic, masses and even metastatic symptoms first. It is
more common for patients with Xp11.2 RCCs to exhibit symptoms than
those with clear cell RCCs. The current case presented with only a
right renal mass, without any symptoms.
Xp11.2 RCCs originate from the renal medulla,
manifesting as cystic or solid masses. Zhu et al (6) suggested that the density of Xp11.2
RCCs was greater than that of the normal renal cortex and medulla
on unenhanced CT. The enhancement was higher than that observed in
the renal medulla during the cortical and medullary phases, but
lower than that in the normal renal medulla during the delayed
phase. However, it is not uncommon to manifest multilocular cystic
RCC-like CT images for Xp11.2 RCCs (7,8). This
case appeared as a cystoid mass that contained low attenuating
necrotic or hemorrhagic foci on unenhanced images and a
well-defined mass with focal enhanced solid portions on enhanced
images.
As Xp11.2 RCCs resemble conventional RCCs
radiologically, the pre-operative diagnosis of Xp11.2 RCCs remains
challenging, and this type of RCC is usually treated in the same
manner as conventional RCC.
At present, the treatment for RCCs remains as
surgical excision. Radical nephrectomy and partial nephrectomy (PN)
are alternative treatments with equivalent long-term oncological
and renal functional outcomes. The enucleation technique has
recently been developed following attempts to further spare the
renal parenchyma. TE has equivalent oncological outcomes to partial
nephrectomy, particularly for small renal masses (9). TE has been associated with a 16%
adverse event rate, and of those events, only 3% required
re-intervention (10). Minervini
et al (11) reported that
three out of 164 (1.8%) patients exhibited local recurrence; one
(0.6%) presented with true local recurrence at the enucleation site
detected at 35 months post-surgery, while two presented with kidney
recurrence elsewhere that was associated with concurrent systemic
metastases diagnosed at 16 and 13 months post-surgery.
RFA, as a minimally invasive treatment, can assist
surgical procedures. A needle is introduced into the tumor and
produces an increase in temperature high enough to destroy the
tumor cells, while transmitting minimal collateral damage to the
surrounding renal parenchyma (12).
Prior to TE, radiofrequency coagulation can be used to make the
surrounding parenchymal vessel occlusive via a cooled electrode
inserted in the kidney between the tumor and normal renal tissue.
TE is relatively bloodless, obviating the requirement for hilar
clamping (13), and the ablation
ensures that the surviving tumor cells are killed in the tumor bed.
So RFA-assisted TE can protect the renal unit whilst eliminating
residual tumor cells. To the best of our knowledge, the present
case is the first Xp11.2 RCC treated with laparoscopic RFA-assisted
enucleation.
The clinical course of this tumor type is
heterogeneous. While certain cases behave indolently, such as the
present case, other cases may behave quite aggressively (14). An age of >50 years may be
associated with a poor prognosis (15).
The urological and radiological outcomes of this
case were satisfactory, which may be attributed to the tumor
dimension and the initial presentation. Although this type of renal
cancer is prone to lymph node metastasis prior to surgical
intervention, a few XP11.2 RCCs treated with partial nephrectomy
have been found with no recurrence or metastasis in the limited
studies available. In the studies by Argani et al (1/28
cases; 6-month follow-up) (16) and
Komai et al (2/7 cases; 96- and 132-month follow-up,
respectively) (17), Xp11.2 RCC
patients with small tumors (<4 cm) and no symptoms were shown to
have usually favorable outcomes subsequent to PN. More time is
required to further observe this type of RCC, which may belong to a
special subtype of Xp11.2 RCCs.
In conclusion, laparoscopic RFA-assisted enucleation
may be an effective method for Xp11.2 RCC patients with small
tumors (<4 cm) and no symptoms. The patient in the present study
had a favorable clinical course. More data and longer follow-up
times are required to determine the optimal treatment methods and
outcomes for this type. The rare and sporadic nature of the cases
restricts currently restricts multi-sample research.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. CA Cancer J Clin. 3:11–30. 2013.
|
|
2
|
Ljungberg B, Cowan NC, Hanbury DC, et al:
EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol.
58:398–406. 2010.
|
|
3
|
Van Poppel H, Becker F, Cadeddu JA, et al:
Treatment of localised renal cell carcinoma. Eur Urol. 60:662–672.
2011.
|
|
4
|
Eble JN, Sauter G, Epstein JI, et al:
Renal carcinomas associated with Xp112 translocations/TFE3 gene
fusions. Pathology and Genetics of Tumors of the Urinary System and
Male Genital Organs. IARC Press; Lyon: pp. 372004
|
|
5
|
Komai Y, Fujiwara M, Fujii Y, et al: Adult
Xp11 translocation renal cell carcinoma diagnosed by cytogenetics
and immunohistochemistry. Clin Cancer Res. 15:1170–1176. 2009.
|
|
6
|
Zhu QQ, Wang ZQ, Zhu WR, et al: The
multislice CT findings of renal carcinoma associated with XP11.2
translocation/TFE gene fusion and collecting duct carcinoma. Acta
Radiol. 54:355–362. 2013.
|
|
7
|
Suzigan S, Drut R, Faria P, et al: Xp11
translocation carcinoma of the kidney presenting with multilocular
cystic renal cell carcinoma-like features. Int J Surg Pathol.
15:199–203. 2007.
|
|
8
|
Jing H, Tai Y, Xu D, Yang F and Geng M:
Renal cell carcinoma associated with Xp11.2 translocations, report
of a case. Urology. 76:156–158. 2010.
|
|
9
|
Laryngakis NA and Guzzo TJ: Tumor
enucleation for small renal masses. Curr Opin Urol. 22:365–371.
2012.
|
|
10
|
Minervini A, Vittori G, Lapini A, et al:
Morbidity of tumour enucleation for renal cell carcinoma (RCC):
results of a single-centre prospective study. BJU Int. 109:372–378.
2012.
|
|
11
|
Minervini A, Serni S, Tuccio A, et al:
Local recurrence after tumour enucleation for renal cell carcinoma
with no ablation of the tumour bed: results of a prospective
single-centre study. BJU Int. 107:1394–1399. 2011.
|
|
12
|
Matlaga BR, Zagoria RJ, Woodruff RD, et
al: Phase II trial of radio frequency ablation of renal cancer:
evaluation of the kill zone. J Urol. 168:2401–2405. 2002.
|
|
13
|
Zhao X, Zhang S, Liu G, et al: Zero
ischemia laparoscopic radio frequency ablation assisted enucleation
of renal cell carcinoma: experience with 42 patients. J Urol.
188:1095–1101. 2012.
|
|
14
|
Meyer PN, Clark JI, Flanigan RC and Picken
MM: Xp11.2 translocation renal cell carcinoma with very aggressive
course in five adults. Am J Clin Pathol. 128:70–79. 2007.
|
|
15
|
Arnoux V, Long JA, Fiard G, et al: Xp11.2
translocation renal carcinoma in adults over 50 years of age: about
four cases. Prog Urol. 22:932–937. 2012.(In French).
|
|
16
|
Argani P, Olgac S, Tickoo SK, et al: Xp11
translocation renal cell carcinoma in adults: expanded clinical,
pathologic, and genetic spectrum. Am J Surg Pathol. 31:1149–1160.
2007.
|
|
17
|
Komai Y, Fujiwara M, Fujii Y, et al: Adult
Xp11 translocation renal cell carcinoma diagnosed by cytogenetics
and immunohistochemistry. Clin Cancer Res. 15:1170–1176. 2009.
|