Introduction
Lymphoepithelial carcinoma is a nasopharyngeal
carcinoma with lymphoid stroma and nonkeratinizing squamous cells.
Lymphoepithelioma-like carcinomas (LELCs) arise on the exterior of
the nasopharynx; however, they resemble lymphoepithelial carcinomas
histologically. LELCs commonly occur close to the nasopharynx,
while they have also been detected in other sites, including the
salivary glands (1), lungs
(2–4), skin (5), liver, cervix, urinary bladder
(6), breast (7), thymus and stomach (8). Certain LELC types are associated with
Epstein-Barr virus (EBV) infection (particularly LELCs of the
stomach, salivary glands, lungs, skin and thymus) (4,5,9).
Pulmonary LELCs are rare malignancies, usually detected in
nonsmokers (10–13). A total of 9,851 patients with NSCLC
were identified. Among these patients, 37 (0.4%) were diagnosed
with lung LELC. These 37 patients were all from Southern China
(14). Chang et al (11) estimated that pulmonary LELC
represents ~0.92% of all lung cancers, further illustrating the
rarity of pulmonary LELCs. Primary pulmonary LELC exhibits no
significant gender predisposition and a minimal association with
smoking history, however, it exhibits a strong association with EBV
in Asian populations, and a predisposition for early or locally
advanced stages of the disease. In a previous study, the mean age
of patients with lung LELC was reported to be 10 years younger than
that of patients with other histological types of lung carcinoma
(14). Currently, the youngest
pulmonary LELC patient reported in the literature is an
eight-year-old child (15). The
majority of patients undergo complete resection, as well as
chemotherapy and radiotherapy for the treatment of pulmonary LELC.
Recently, a study of 52 primary pulmonary LELC patients
demonstrated that the two- and five-year overall survival rates
were 88 and 62%, respectively, with the majority of patients
diagnosed at early or locally advanced stages of the disease
(16). The present study
investigated the case of a female nonsmoker with pulmonary LELC.
Written informed consent was obtained from the patient.
Case report
A mass was detected on the middle lobe of the right
lung of a 58-year-old female, during a medical check-up at the
West-China Hospital (Chengdu, China) in January 2013. The patient
was asymptomatic and physical examination identified no positive
findings. The female had no history of smoking and alcohol use.
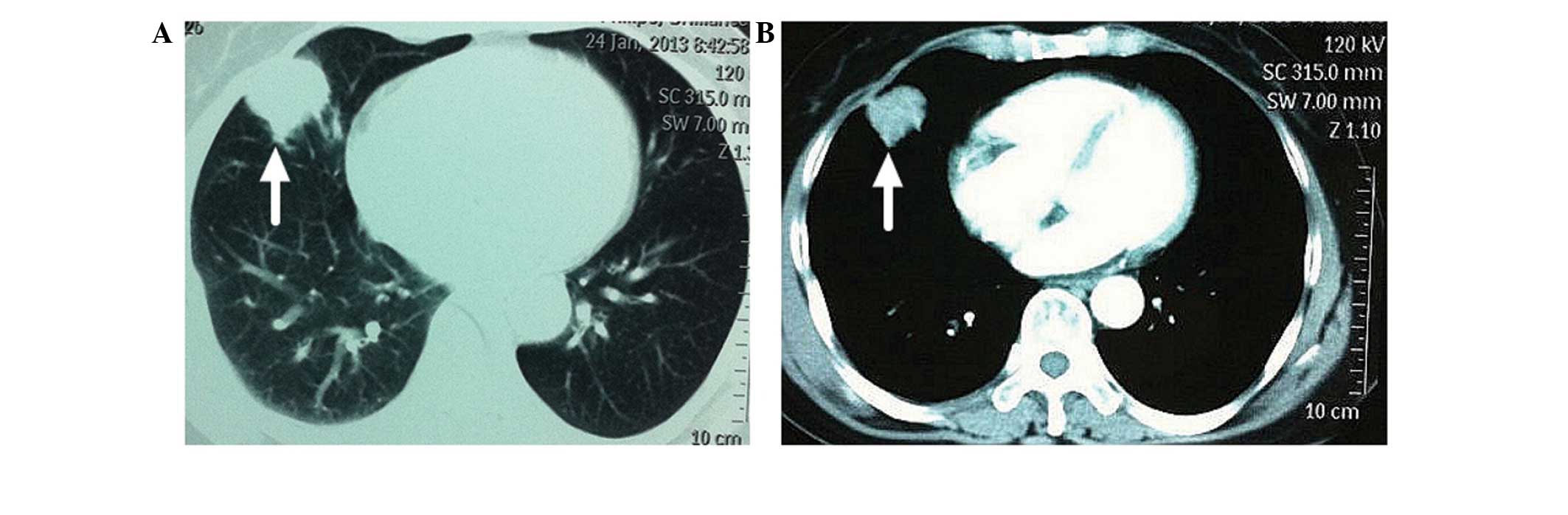
Enhancement computed tomography (CT) of the thorax
revealed a mass in the middle lobe of the right lung, which was
considered to be a possible lung tumor (Fig. 1). In addition, another small lesion
was detected in the same lung lobe; however, this was not
considered to be a metastatic lesion. Fibrobronchoscopic brushing
(17) demonstrated the presence of
squamous metaplasia with severe hyperplasia at the middle lobe of
the right lung. A bone scan and a CT scan of the skull indicated no
metastasis.
Surgery was performed under induction with midazolam
(0.05–0.1 mg/kg) followed by the subsequent use of intravenous
anesthesia (2 μg/kg, sufentanil; 2 mg/kg/h, propofol) with tracheal
intubation. The patient underwent a lobectomy of the middle lobe
(including sequential resection of the right pulmonary middle lobe
vein, artery and trachea) and systematic mediastinal
lymphadenectomy (group 2–4, 7 and 9–11 lymph nodes were swollen).
The resected tissue and lymph nodes were frozen and biopsy was
performed, revealing evidence of carcinoma. The surgery was
completed following careful hemostasis and washing of the pleural
cavity with warm saline solution. The patient did not present any
complications, such as cough, chest pain and hemoptysis. No
adjuvant chemotherapy and radiotherapy were performed. The patient
was discharged a week after surgery and follow-up visits were
scheduled. The resected specimen was 10×5×3 cm in size, containing
a 3×2.5×2 cm tumor. Histologically, the tumor was solid and
off-white in color, with a clear demarcation between the
surrounding normal lung tissues, while pleural invasion was
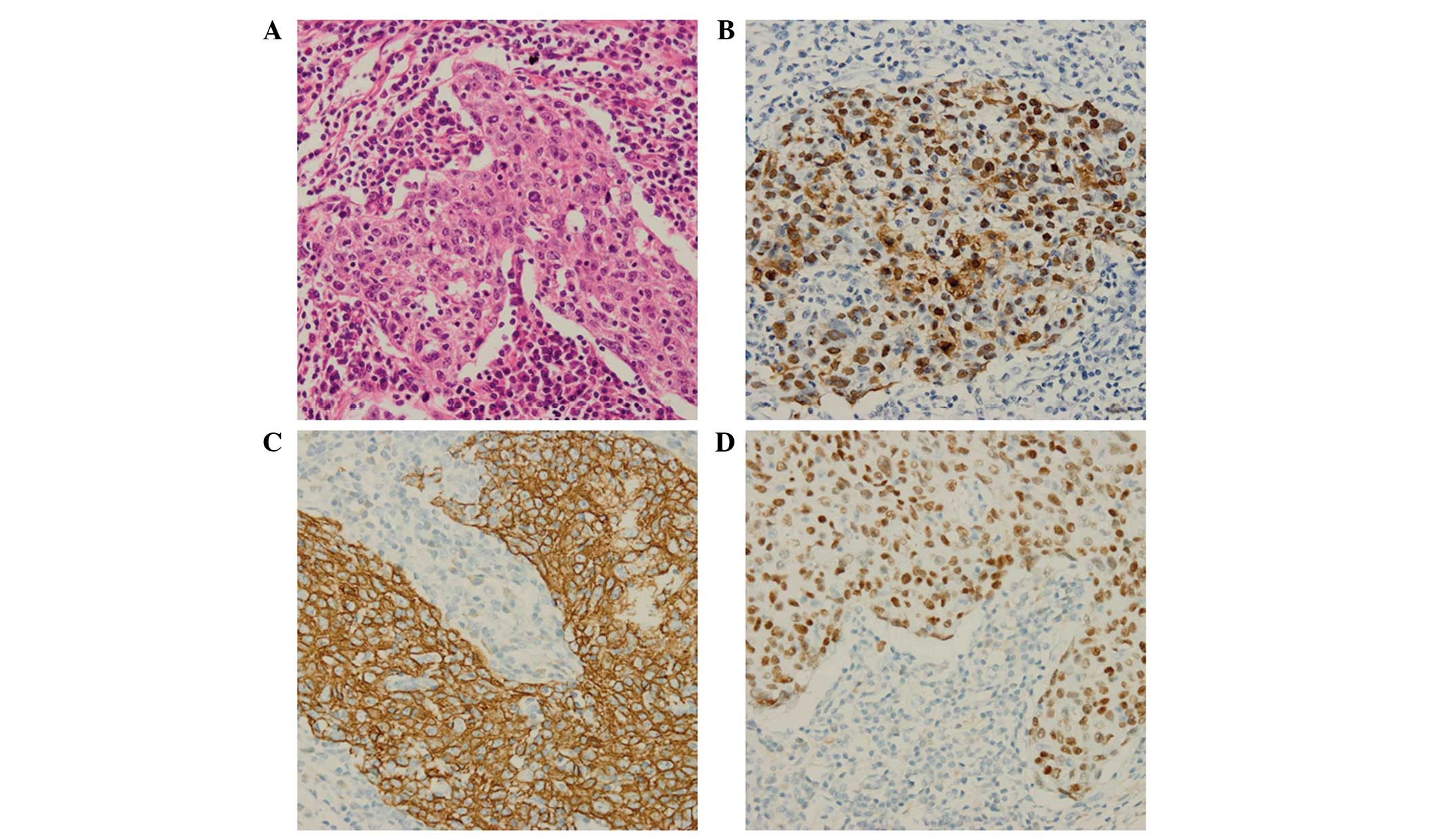
observed. Immunohistochemical analysis revealed that the tumor
cells were positive for protein kinase C, p63, cytokeratin 5/6 (CK
5/6) and EBV-encoded small RNA (EBER), whereas the cells were
negative for CK 7 and thyroid transcription factor-1 (Fig. 2). Lymph nodes collected during the
surgery revealed no metastasis. Furthermore, the histological and
immunohistochemical analyses confirmed the diagnosis of pulmonary
LELC. The patient was healthy and asymptomatic following surgery.
Thoracic enhancement CT revealed no signs of metastasis at three,
six and 23 months following surgery.
Discussion
A network database search of PubMed and Web of
Science was conducted using the keywords “pulmonary” and “LELC” for
studies reported in the English language between 1987 and 2015. A
total of 196 such cases (male, 96; female, 100) were described in
the literature (Table I), and
patient age ranged between 8 and 83 years. Among the 196 patients,
111 were smokers (56.63%) and 45 were non-smokers (22.96%),
however, information regarding smoking status was unavailable for
40 (20.41%) patients. The first pulmonary LELC case was described
by Bégin et al in 1987 (4).
Of the 196 cases reported, the majority of cases involved Asian
patients, with approximately two-thirds of cases arising in
southern China (12,40), Taiwan (11) and Hong Kong (29), illustrating the geographical
distribution characteristics of pulmonary LELC. A close association
exists between pulmonary LELCs and EBV infection, which is absent
in other types of lung carcinomas. Among the 196 patients reported
in the literature (Table I), 145
patients (73.98%) tested positive for EBV infection, 42 patients
(21.43%) patients tested negative for EBV infection and information
was unavailable for nine patients (4.59%). Previous studies have
identified the presence of EBV infection in the tumor cells or
serum of LELC patients (19,26,41).
Circulating serum EBV DNA may be used as a tumor marker in the
clinical management of patients with lung LELC (9,11,40,42). A
study demonstrated that patients with a pretherapy serum EBV DNA
level of >10,000 copies/ml exhibited significantly lower overall
survival rates (18). Accurate
diagnosis is significant and a prerequisite for treatment.
 | Table IPatient characteristics of the 196
cases of pulmonary lymphoepithelioma-like carcinoma published
between 1987 and 2015 in the English literature. |
Table I
Patient characteristics of the 196
cases of pulmonary lymphoepithelioma-like carcinoma published
between 1987 and 2015 in the English literature.
| Author, year
(ref) | Cases, n (F/M) | Age (years) | Smoking status, n
(S/NS) | EBV, n (+/−) |
|---|
| Ma et al, 2013
(19) | 41 (19/22) | 25–74a | 10/31 | 37/4 |
| Jeong et al,
2013 (21) | 1 (1/0) | 60 | 0/1 | 1/0 |
| Dong et al,
2015 (22) | 1 (0/1) | 83 | N/A | N/A |
| Yener et al,
2012 (23) | 1 (0/1) | 62 | 1/0 | 0/1 |
| Tanaka et al,
2012 (24) | 1 (1/0) | 71 | 0/1 | 0/1 |
| Shen et al,
2012 (25) | 1 (1/0) | 75 | 1/0 | 1/0 |
| Hayashi et al,
2012 (20) | 1 (0/1) | 70 | 0/1 | 1/0 |
| Xia et al,
2009 (26) | 21 (8/13) | 40–67a | 7/14 | 12/9 |
| Bildirici et
al, 2005 (27) | 1 (1/0) | 66 | 0/1 | 0/1 |
| Ngan et al,
2004 (18) | 19 (10/9) | 52.7b | 8/11 | 11/8 |
| Kobayashi et
al, 2004 (28) | 1 (1/0) | 67 | N/A | 1/0 |
| Ho et al,
2004 (29) | 10 (5/5) | 38–71a | 2/8 | 6/4 |
| Hernández Vázquez,
et al 2004 (30) | 1 (0/1) | 59 | 1/0 | N/A |
| Abe et al,
2004 (31) | 1 (1/0) | 57 | 0/1 | N/A |
| Morbini et
al, 2003 (32) | 1 (0/1) | 25 | 0/1 | 1/0 |
| Chang et al,
2002 (11) | 23 (16/7) | 42–80a | 6/17 | 23/0 |
| Han et al,
2001 (12) | 32 (10/22) | 39–73a | N/A | 30/2 |
| Barroso et
al, 2000 (33) | 1 (0/1) | 25 | 0/1 | 1/0 |
| Kasai et al,
1999 (34) | 1 (1/0) | 39 | 1/0 | 1/0 |
| Chen et al,
1998 (14) | 5 (3/2) | 43–66a | 0/5 | 5/0 |
| Wöckel et
al, 1997 (35) | 2 (1/1) | 49, 66 | N/A | N/A |
| Curcio et
al, 1997 (15) | 1 (1/0) | 8 | 0/1 | 1/0 |
| Wong et al,
1995 (36) | 9 (1/8) | 33–71a | 4/5 | 9/0 |
| Wöckel et
al, 1995 (37) | 1 (1/0) | 47 | 0/1 | NA |
| Higashiyama et
al, 1995 (9) | 2 (0/2) | 55, 65 | N/A | 2/0 |
| Ferrara and Nappi,
1995 (2) | 2 (1/1) | 64, 78 | 1/1 | 0/2 |
| Chow et al,
1995 (38) | 2 (0/2) | 56, 66 | N/A | N/A |
| Chan et al,
1995 (3) | 11 (5/6) | 38–73a | 2/9 | 11/0 |
| Miller et
al, 1991 (39) | 1 (1/0) | 65 | 1/0 | 0/1 |
| Bégin et al,
1987 (4) | 1 (1/0) | 40 | 0/1 | N/A |
The diagnosis of lung LELC is usually based on the
results of cytopathologic, histopathologic, immunohistochemical and
EBER-positivity analyses, as well as a detailed systemic
examination to exclude a possible extrapulmonary (nasopharyngeal)
origin of the carcinoma and other lung diseases (43). Imaging diagnostic methods, including
CT or magnetic resonance imaging (MRI) scans, are able to identify
nonspecific lesions that resemble other pulmonary carcinomas. On CT
scans, pulmonary LELCs usually appear as large, central,
well-defined and lobulated tumors with vascular or bronchial
encasement and obstructive pneumonia (43). Calcification has been rarely
observed in pulmonary LELCs. In addition, MRI scans of LELCs
usually detect an isointense or low-intensity signal on T1-weighted
images and a slightly increased signal on T2-weighted images, while
enhancement of abnormal tissue is typically observed (19,44).
The cytological features of the specimens are commonly analyzed by
needle aspiration or fibrobronchoscopic brushing, which reveal
abnormal cell morphology that usually appears as large clusters of
neoplastic cells with scant cytoplasm. The nuclei are normally
large and hyperchromatic, with irregular contour and prominent
nucleoli (20). Histologically, the
tumors appear solid and off-white in color, with a clear
demarcation between the surrounding normal pulmonary tissues, while
occasionally pleural invasion is observed. Immunohistochemical
analysis of pulmonary LELCs usually detects positive staining of
membrane tumor markers, including latent membrane protein-1, viral
capsid antigen and CKs (20). In
addition, EBER detection is significant in the diagnosis of
pulmonary LELCs, since EBER is absent in other lung carcinomas,
such as non-small-cell lung carcinomas. Similar to nasopharyngeal
carcinomas, pulmonary LELCs are sensitive to chemotherapy and
radiotherapy (13,31). In early-stage pulmonary LELCs, the
main treatment method is surgical resection, while comprehensive
treatment (surgery, chemotherapy and radiotherapy) is adopted in
patients with advanced or unresectable tumors (31). Previous studies have revealed that
early-stage pulmonary LELC cases present an improved prognosis
compared with advanced cases or other pulmonary carcinoma types in
follow-ups after surgery (10,16).
Fibrobronchoscopic brushing is the most widely used
method with a decisive role in the diagnosis of lung carcinomas. In
the present study, fibrobronchoscopic brushing revealed squamous
metaplasia with severe hyperplasia at the middle lobe of the right
lung. However, immunohistochemical analysis diagnosed the presence
of a pulmonary LELC. Squamous metaplasia, which is the basis of
squamous cell carcinomas, differs from pulmonary LELC in the
therapeutic methods used and the prognostic evaluation. Squamous
metaplasia requires regular follow-up in out-patient clinics, while
pulmonary LELC is treated by surgery and chemotherapy. Therefore,
distinguishing between LELC and other nonmalignant or premalignant
conditions is essential. The present study indicated that despite
the rarity of pulmonary LELC, it should be included as one of the
differential diagnoses for lung malignancies. Therefore, physicians
must consider performing larger biopsies, particularly when
histological examination of tissue removed during surgery remains
unidentified.
Acknowledgements
The authors would like to thank the staff of the
Department of Thoracic Surgery at West-China Hospital of Sichuan
University for their assistance and efforts.
References
|
1
|
Chow TL, Chow TK, Lui YH, et al:
Lymphoepithelioma-like carcinoma of oral cavity: report of three
cases and literature review. Int J Oral Maxillofac Surg.
31:212–218. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrara G and Nappi O:
Lymphoepithelioma-like carcinoma of the lung. Two cases diagnosed
in Caucasian patients. Tumori. 81:144–147. 1995.PubMed/NCBI
|
|
3
|
Chan JK, Hui PK, Tsang WY, et al: Primary
lymphoepithelioma-like carcinoma of the lung. A clinicopathologic
study of 11 cases. Cancer. 76:413–422. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bégin LR, Eskandari J, Joncas J and
Panasci L: Epstein-Barr virus related lymphoepithelioma-like
carcinoma of lung. J Surg Oncol. 36:280–283. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aoki R, Mitsui H, Harada K, et al: A case
of lymphoepithelioma-like carcinoma of the skin associated with
Epstein-Barr virus infection. J Am Acad Dermatol. 62:681–684. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshino T, Ohara S and Moriyama H:
Lymphoepithelioma-like carcinoma of the urinary bladder: a case
report and review of the literature. BMC Res Notes. 7:7792014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abdou AG and Asaad NY:
Lymphoepithelioma-like carcinoma of the breast: Cytological,
histological, and immunohistochemical characteristics. Diagn
Cytopathol. Mar 8–2014.(Epub ahead of print). PubMed/NCBI
|
|
8
|
Bai Y, Gao Q, Ren G, et al: Epstein-Barr
virus-associated lymphoepithelioma-like gastric carcinoma located
on gastric high body: two case reports. Indian J Pathol Microbiol.
57:463–466. 2014.PubMed/NCBI
|
|
9
|
Higashiyama M, Doi O, Kodama K, et al:
Lymphoepithelioma-like carcinoma of the lung: analysis of two cases
for Epstein-Barr virus infection. Hum Pathol. 26:1278–1282. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang CJ, Feng AC, Fang YF, et al:
Multimodality treatment and long-term follow-up of the primary
pulmonary lymphoepithelioma-like carcinoma. Clin Lung Cancer.
13:359–362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang YL, Wu CT, Shih JY and Lee YC: New
aspects in clinicopathologic and oncogene studies of 23 pulmonary
lymphoepithelioma-like carcinomas. Am J Surg Pathol. 26:715–723.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han AJ, Xiong M, Gu YY, et al:
Lymphoepithelioma-like carcinoma of the lung with a better
prognosis. A clinicopathologic study of 32 cases. Am J Clin Pathol.
115:841–850. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ho JC, Wong MP and Lam WK:
Lymphoepithelioma-like carcinoma of the lung. Respirology.
11:539–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen FF, Yan JJ, Lai WW, et al:
Epstein-Barr virus-associated nonsmall cell lung carcinoma:
undifferentiated “lymphoepithelioma-like” carcinoma as a distinct
entity with better prognosis. Cancer. 82:2334–2342. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Curcio LD, Cohen JS, Grannis FW Jr, et al:
Primary lymphoepithelioma-like carcinoma of the lung in a child.
Report of an Epstein-Barr virus-related neoplasm. Chest.
111:250–251. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang Y, Wang L, Zhu Y, et al: Primary
pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with
long-term follow-up. Cancer. 118:4748–4758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bolgova LS, Gordienko TM, Mantsurov NE, et
al: Exfoliative cytological diagnosis of lung cancer with
bronchoscopic material. Klin Lab Diagn. 15–17. 2009.(In
Russian).
|
|
18
|
Ngan RK, Yip TT, Cheng WW, et al: Clinical
role of circulating Epstein-Barr virus DNA as a tumor marker in
lymphoepithelioma-like carcinoma of the lung. Ann N Y Acad Sci.
1022:263–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma H, Wu Y, Lin Y, Cai Q, Ma G and Liang
Y: Computed tomography characteristics of primary pulmonary
lymphoepithelioma-like carcinoma in 41 patients. Eur J Radiol.
82:1343–1346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayashi T, Haba R, Tanizawa J, et al:
Cytopathologic features and differential diagnostic considerations
of primary lymphoepithelioma-like carcinoma of the lung. Diagn
Cytopathol. 40:820–825. 2012. View
Article : Google Scholar
|
|
21
|
Jeong JS, Kim SR, Park SY, et al: A Case
of Primary Pulmonary Lymphoepithelioma-like Carcinoma Misdiagnosed
as Adenocarcinoma. Tuberc Respir Dis (Seoul). 75:170–173. 2013.
View Article : Google Scholar
|
|
22
|
Dong A, Zhang J, Wang Y, et al: FDG PET/CT
in Primary Pulmonary Lymphoepithelioma-like Carcinoma. Clin Nucl
Med. 40:134–137. 2015. View Article : Google Scholar
|
|
23
|
Yener NA, Balikçi A, Çubuk R, et al:
Primary lymphoepithelioma-like carcinoma of the lung: report of a
rare case and review of the literature. Turk Patoloji Derg.
28:286–289. 2012.PubMed/NCBI
|
|
24
|
Tanaka S, Chen F and Date H: Pulmonary
lymphoepithelioma-like carcinoma with rapid progression. Gen Thorac
Cardiovasc Surg. 60:164–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen DH, Cheng CY, Lin LF, et al:
Conversion from FDG-negative to -positive during follow-up in a
rare case of pulmonary lymphoepithelioma-like carcinoma. Clin Nucl
Med. 37:679–681. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia J, Jiang L, Zhang J, et al: The
Clinical Analysis of 21 Patients with Lymphoepithelioma-like
Carcinoma after Operation. Zhongguo Fei Ai Za Zhi. 12:1169–1173.
2009.(In Chinese).
|
|
27
|
Bildirici K, Ak G, Peker B, et al: Primary
lymphoepithelioma-like carcinoma of the lung. Tuberk Toraks.
53:69–73. 2005.PubMed/NCBI
|
|
28
|
Kobayashi M, Ito M, Sano K, et al:
Pulmonary lymphoepithelioma-like carcinoma: predominant
infiltration of tumor-associated cytotoxic T lymphocytes might
represent the enhanced tumor immunity. Intern Med. 43:323–326.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ho JC, Lam WK, Wong MP, et al:
Lymphoepithelioma-like carcinoma of the lung: experience with ten
cases. Int J Tuberc Lung Dis. 8:890–895. 2004.PubMed/NCBI
|
|
30
|
Hernández Vázquez J, de Miguel Díez J,
Llorente Iñigo D, et al: Large cell lymphoepithelioma-like
carcinoma of the lung. Arch Bronconeumol. 40:381–383. 2004.(In
Spanish). View Article : Google Scholar
|
|
31
|
Abe T, Tanabe Y, Watanabe S, et al: A case
of recurrent pulmonary lymphoepithelioma-like carcinoma responding
to treatment with CBDCA/paclitaxel combined chemotherapy. Gan To
Kagaku Ryoho. 31:1215–1217. 2004.(In Japanese). PubMed/NCBI
|
|
32
|
Morbini P, Riboni R, Tomaselli S, et al:
Eber- and LMP-1-expressing pulmonary lymphoepithelioma-like
carcinoma in a Caucasian patient. Hum Pathol. 34:623–625. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barroso A, Nogueira R, Lencastre H, et al:
Primary lymphoepithelioma-like carcinoma of the lung. Lung Cancer.
28:69–74. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kasai K, Kon S, Sato N, et al: Case report
of lymphoepithelioma-like carcinoma of the lung - lymphoid
population consisting of cytotoxic T cells in resting state. Pathol
Res Pract. 195:773–779. 1999. View Article : Google Scholar
|
|
35
|
Wöckel W, Höfler G, Popper HH and
Morresi-Hauf A: Lymphoepithelioma-like lung carcinomas. Pathologe.
18:147–152. 1997.(In German). View Article : Google Scholar
|
|
36
|
Wong MP, Chung LP, Yuen ST, et al: In situ
detection of Epstein-Barr virus in non-small cell lung carcinomas.
J Pathol. 177:233–240. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wöckel W, Höfler G, Popper HH and Morresi
A: Lymphoepithelioma-like carcinoma of the lung. Pathol Res Pract.
191:1170–1174. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chow LT, Chow WH, Tsui WM, et al:
Fine-needle aspiration cytologic diagnosis of
lymphoepithelioma-like carcinoma of the lung. Report of two cases
with immunohistochemical study. Am J Clin Pathol. 103:35–40.
1995.PubMed/NCBI
|
|
39
|
Miller B, Montgomery C, Watne AL, et al:
Lymphoepithelioma-like carcinoma of the lung. J Surg Oncol.
48:62–68. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han AJ, Xiong M and Zong YS: Association
of Epstein-Barr virus with lymphoepithelioma-like carcinoma of the
lung in southern China. Am J Clin Pathol. 114:220–226. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hsu JL and Glaser SL: Epstein-barr
virus-associated malignancies: epidemiologic patterns and etiologic
implications. Crit Rev Oncol Hematol. 34:27–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Han A, Xiong M and Zong Y: Association of
epstein-barr virus with lymphoepithelioma-like carcinoma of the
lung. Zhonghua Bing Li Xue Za Zhi. 26:222–224. 1997.(In Chinese).
PubMed/NCBI
|
|
43
|
Mo Y, Shen J, Zhang Y, et al: Primary
lymphoepithelioma-like carcinoma of the lung: distinct computed
tomography features and associated clinical outcomes. J Thorac
Imaging. 29:246–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hoxworth JM, Hanks DK, Araoz PA, et al:
Lymphoepithelioma-like carcinoma of the lung: radiologic features
of an uncommon primary pulmonary neoplasm. AJR Am J Roentgenol.
186:1294–1299. 2006. View Article : Google Scholar : PubMed/NCBI
|