Introduction
Hepatocellular carcinoma is a common type of
malignant liver tumor with >600,000 cases diagnosed per year,
particularly in Asia and Africa (1,2). The
five-year survival rate of this disease is <9% (3) and the number of mortalities associated
with hepatocellular carcinoma is >500,000 patients per year
(4). Due to the lack of effective
techniques and therapeutic strategies, hepatocellular carcinoma is
an important health problem. The current treatment strategies for
hepatocellular carcinoma, including the use of chemotherapy,
radiotherapy and surgery, are relatively limited. In addition,
liver cancer recurrence and metastasis following treatment with the
aforementioned strategies are common (5,6).
Therefore, it is desirable to develop novel and effective agents
with minimum adverse effects for the prevention and treatment of
hepatocellular carcinoma.
Apoptosis or programmed cell death plays a
significant role in the growth regulation of normal and neoplastic
tissues, since it balances cell proliferation (7–9). Cells
undergoing apoptosis usually present cell shrinkage, chromatin
condensation, nuclear fragmentation and apoptotic bodies (10,11).
Apoptosis usually occurs in a well-designed sequence of
morphological events mediated by intrinsic and extrinsic pathways,
and has been demonstrated to activate the programmed cell death
pathway to exert anticancer functions (12,13).
Numerous antitumor drugs are known to trigger cell death by
inducing apoptosis. In particular, traditional Chinese medicine has
been widely used in the treatment of cancer patients, having an
effective function (14,15). Solamargine (SM), a member of the
Solanaceae (or nightshade) family, is extracted from the Chinese
herb Solanum incanum L. and is a major steroidal
glycoalkaloids (16). Previous
studies have demonstrated that SM strongly inhibits the growth and
induces the apoptosis of several human tumor cells, including
breast, prostate, colon, lung and hepatoma cancer cells (17–19).
Therefore, its marked antitumor activity has received increasing
attention. Certain studies have demonstrated the underlying
mechanisms of apoptosis induced by SM in human cancer cell lines,
including through tumor necrosis factor receptors and mitochondrial
release of cytochrome c (20).
However, the underlying mechanisms of the effect of SM in hepatoma
cancer lines remain unclear. Therefore, the aim of the present
study was to observe and detect the effect of SM on the human
hepatocellular carcinoma cell lines, SMMC7721 and HepG2.
Materials and methods
Materials
SM with a purity of >98% was purchased from Yilin
Biotechnology Co., Ltd. (Shanghai, China). In addition, MTT and
DAPI were purchased from Beyotime Institute of Biotechnology
(Shanghai, China). Dulbecco's modified eagle's medium (DMEM) and
fetal bovine serum (FBS) were purchased from Gibco Life
Technologies (Grand Island, NY, USA). The Annexin V/propidium
iodide (PI) Apoptosis Detection kit and the Cell Cycle Analysis kit
were obtained from BD Biosciences (San Diego, CA, USA). Rabbit
anti-human polyclonal B-cell lymphoma-2 (Bcl-2; 1:1,000; cat. no.
2876S), rabbit anti-human polyclonal Bcl-2-associated X protein
(Bax; 1:1,000; cat. no. 2274S), rabbit anti-human monoclonal
caspase-3 (1:1,000; cat. no. 9664S), rabbit anti-human polyclonal
caspase-9 (1:1,000; cat. no. 9502S), mouse anti-human monoclonal
proliferating cell nuclear antigen (pcna; 1:1,000; cat. no. 2586S)
and mouse anti-human monoclonal β-actin (1:1,000; cat. no. 3700S)
primary antibodies were obtained from Cell Signaling Technology,
Inc. (Beverly, MA, USA). Rabbit anti-human polyclonal Ki67 primary
antibody (1:500; cat. no. BA1508) was purchased from Wuhan Boster
Biotechnology, Ltd., (Wuhan, China). Horseradish peroxidase
(HRP)-conjugated goat anti-rabbit (1:2,000; cat. no. 7071S) and
goat anti-mouse (1:2,000; cat. no. 7072S) IgG secondary antibodies,
were obtained from Cell Signaling Technology, Inc. All other
chemicals used were commercial products of reagent grade.
Cell lines and culture
Human hepatoma cells (SMMC7721 and HepG2) were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The two human hepatoma cell lines were
maintained in DMEM supplemented with 10% FBS, 100 U/ml penicillin
(Gibco Life Technologies) and 100 µg/ml streptomycin (Gibco Life
Technologies) in a 37°C incubator containing 5% CO2.
Cytotoxicity and colony formation
assay
The cytopathic effects of SM were evaluated in the
SMMC7721 and HepG2 cells using an MTT assay, which is a common
colorimetric technique used to detect the number of viable cells,
cytotoxicity and cell proliferation. IC50 is defined as
the concentration of drug causing 50% inhibition of cell growth
compared with the control group. The MTT assay was performed
according to the manufacturer's instructions. In the colony-forming
assay, the cells were seeded into 6-well culture plates at a low
density of 500 cells/well, treated with various concentrations of
SM (5, 10 or 20 µM) and incubated for two weeks. Subsequently, the
cells were fixed with 4% paraformaldehyde and stained with Giemsa
(Beyotime Institute of Biotechnology). Images were then captured
using a fluorescence microscope (Eclipse TS100; Nikon Corporation,
Tokyo, Japan) and the clonogenicity was determined.
Detection of apoptosis
Cell and cell nucleus morphological changes
SMMC7721 and HepG2 cells (1×106/well)
were seeded in 6-well plates and then treated with SM (20 µM) for
24 h. The cell morphological changes were observed using a light
microscope (CHK-213; Olympus Corporation, Tokyo, Japan). For
fluorescent staining, the samples were treated with 20 µM SM for 24
h, fixed with ice-cold 4% paraformaldehyde and stained with 1 µg/ml
DAPI for 10 min. Subsequently, images were captured using a
fluorescence microscope (Eclipse TS100; Nikon Corporation).
Apoptosis ratio of SM-treated cells
The apoptotic ratio was detected using an Annexin
V/PI method (21). Briefly, the cells
were treated with various concentrations of SM (0, 5, 10 or 20 µM)
for 24 h, trypsinized (Gibco Life Technologies) and resuspended in
100 µl binding buffer, followed by addition of 5 µl Annexin V and
PI in each tube. Next, 400 µl binding buffer was added to each
reaction tube and the cells were collected for further
analysis.
Cell cycle analysis
Detection of the cell cycle distribution was
performed following the addition of 20 µM SM for 24 h. The cells
were harvested using trypsinization, fixed with 4% paraformaldehyde
for 30 min and then washed with phosphate-buffered saline (PBS).
Next, the samples were centrifuged for 10 min at a speed of 91 × g.
Subsequently, the cells were stained with 0.5 mg/ml PI containing
0.5 mg/ml RNase (BD Biosciences, Franklin Lakes, NJ, USA) at 4°C
for 30 min. The DNA content was then measured with a FACScan flow
cytometer (BD Biosciences).
Western blot analysis
Following SM treatment for 24 h, the cells were
washed with ice-cold PBS twice. Cell lysates were collected using a
lysis buffer (Beyotime Institute of Biotechnology) and the cell
lysate proteins were loaded and separated using SDS-PAGE. Next, the
samples were transferred onto polyvinylidene difluoride membranes
and incubated in PBS containing 3% bovine serum albumin (BSA) for 1
h at room temperature. Antibodies in Tris-buffered saline/Tween 20
(TBST) with 1% BSA were then added and the membranes were incubated
at 4°C overnight. Subsequently, the membranes were washed with TBST
and incubated with the secondary antibody for 1 h at room
temperature. Immunoreactive bands were detected using an enhanced
chemiluminescence reagent (EMD Millipore, Billerica, MA, USA) and
all the blots were quantified using LANE 1D software (Sage Creation
Science Co., Ltd., Beijing, China).
Statistical analysis
All the data are expressed as the mean ± standard
deviation and the differences between two groups were analyzed
using Student's t-test. All statistical analyses were performed
using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant difference.
All the experiments were performed at least in triplicate.
Results
Proliferation of hepatoma cells is
inhibited by SM
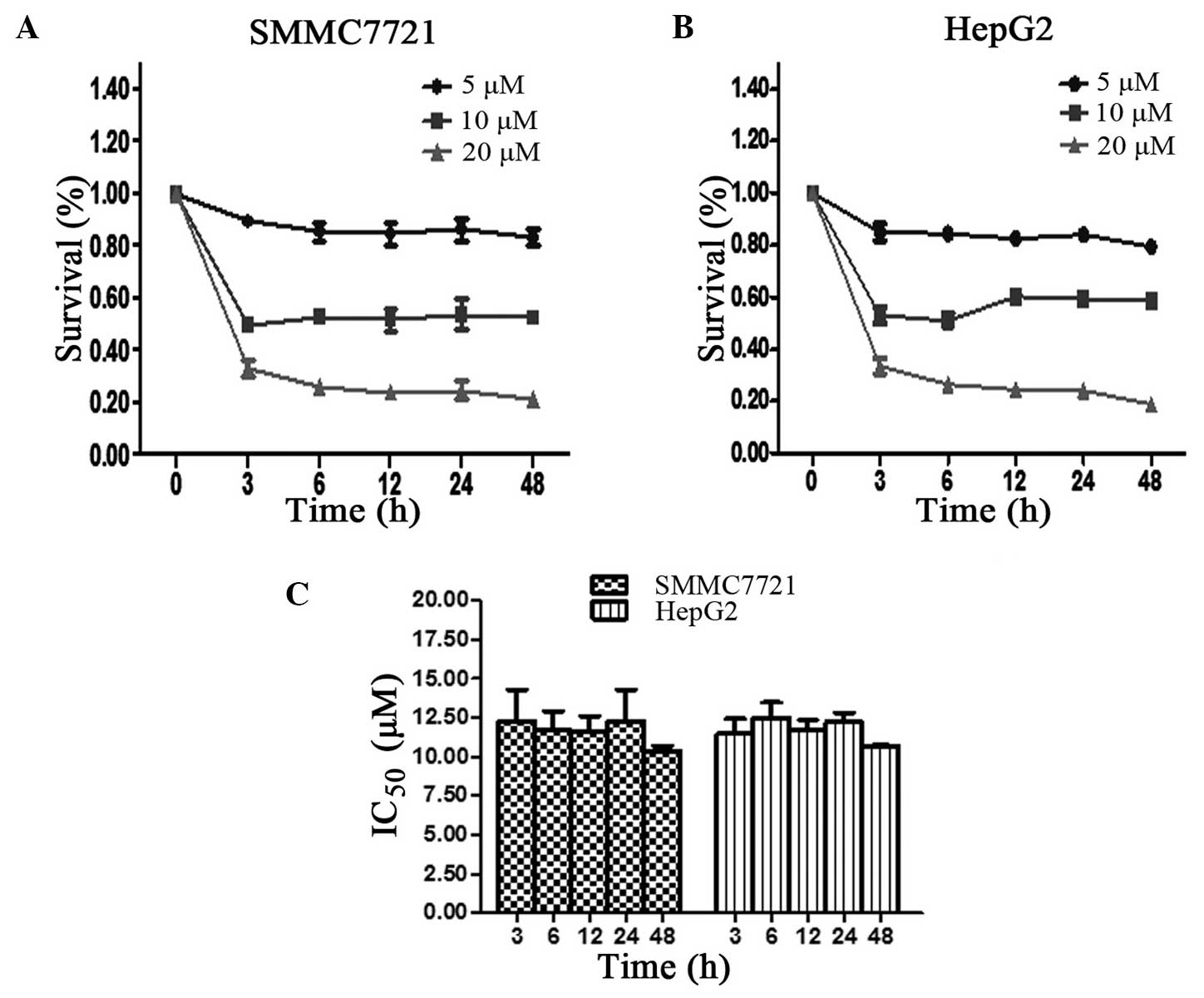
The inhibition of hepatoma cell proliferation was
determined using an MTT assay. As shown in Fig. 1A and B, the time-survival curve was
constructed following treatment of each cell line with 5, 10 or 20
µM SM. At the 3, 6, 12, 24 and 48 h time points, the ratio of cell
survival was found to slightly change with time; however, cell
survival was mainly dose-dependent. The minimum survival rate was
detected at 20 µM SM treatment for 48 h in the SMMC7721 and HepG2
cells. The IC50 values of the SMMC7721 cells were 12.27,
11.86, 11.57, 12.17 and 10.48 µM following treatment for 3, 6, 12,
24 and 48 h, respectively. However, no statistically significant
differences were detected among the different time points
(P>0.05; Fig. 1C). Similar results
were obtained for the HepG2 cell line, which confirmed the results
of the SMMC7721 cells.
Clonogenicity of hepatoma cells is
decreased by SM
For the colony formation assay, 500 SMMC7721 cells
were seeded onto a 6-well plate and incubated for two weeks; next,
images were captured and the cell spheres were counted, as shown in
Fig. 2A. Compared with the untreated
group (0 µM SM), the survival fractions were 52.6, 42.1 and 26.3%
of cells following 24-h exposure to 5, 10 and 20 µM SM,
respectively. The results demonstrated that the density and number
of cell spheres significantly decreased with increasing SM
concentration (P<0.001; Fig. 2B).
The HepG2 cells exhibited a similar pattern following SM
treatment.
SM induces cell and cell nucleus
morphological changes due to apoptosis
Following cell treatment with 20 µM SM for 24 h,
changes in the cell morphology were observed using a microscope. As
shown in Fig. 3A, the cells were
evidently reduced in size and their margins were unclear, with
certain parts of the cells appearing smaller and rounder when
compared with the control group. Following DAPI staining, the
SM-treated cells presented nuclear chromatin condensation and
fragmentation, as shown in Fig. 3B.
Similar results were obtained for the two cell lines, SMMC7721 and
HepG2. These results indicated that the SM-treated hepatoma cells
presented cellular features of apoptosis.
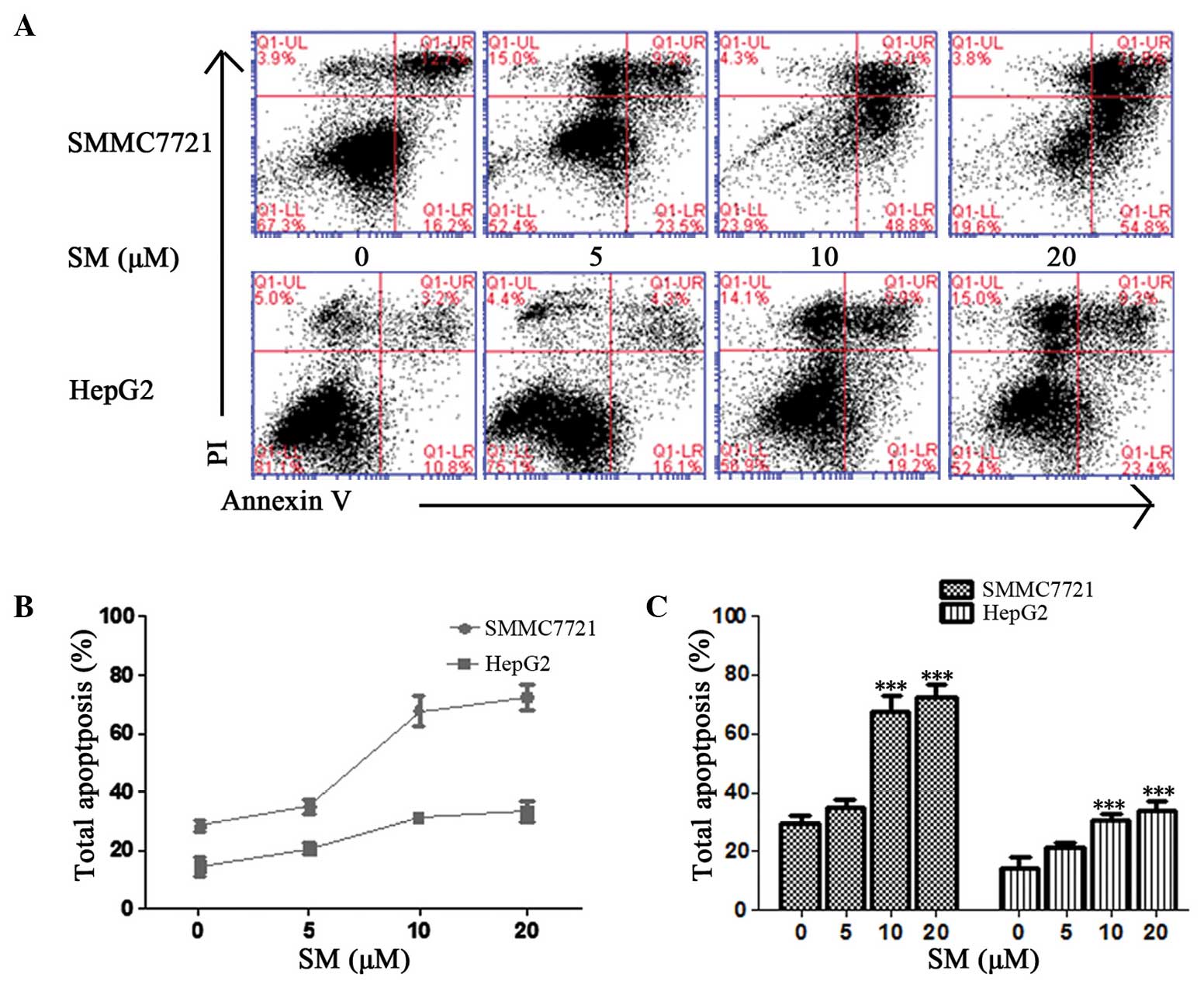
Ratio of SM-induced apoptosis in
hepatoma cells
The ratio of SM-induced apoptosis was detected using
an Annexin V/PI method. Early and late apoptosis percentages were
determined based on the cells present in the lower right (LR) and
upper right (UR) quadrants of the graphs, respectively (Fig. 4A). The total percentages of SM-induced
apoptosis in the SMMC7721 cells were 28.9, 32.7, 71.8 and 76.6%
following exposure to 0, 5, 10 and 20 µM SM, respectively, for 24 h
(Fig. 4A); therefore, the effect of
SM was dose-dependent (Fig. 4B and
C). At a concentration of 5 µM SM, the total apoptosis
percentage was found to be slight increased, but the difference was
not statistically significant (P>0.05; Fig. 4C). By contrast, the apoptosis induced
in the 10 and 20 µM SM-treated cells was evidently increased when
compared with the untreated cells and demonstrated statistically
significant differences (P<0.001; Fig.
4C). HepG2 cells exhibited a similar pattern following SM
treatment.
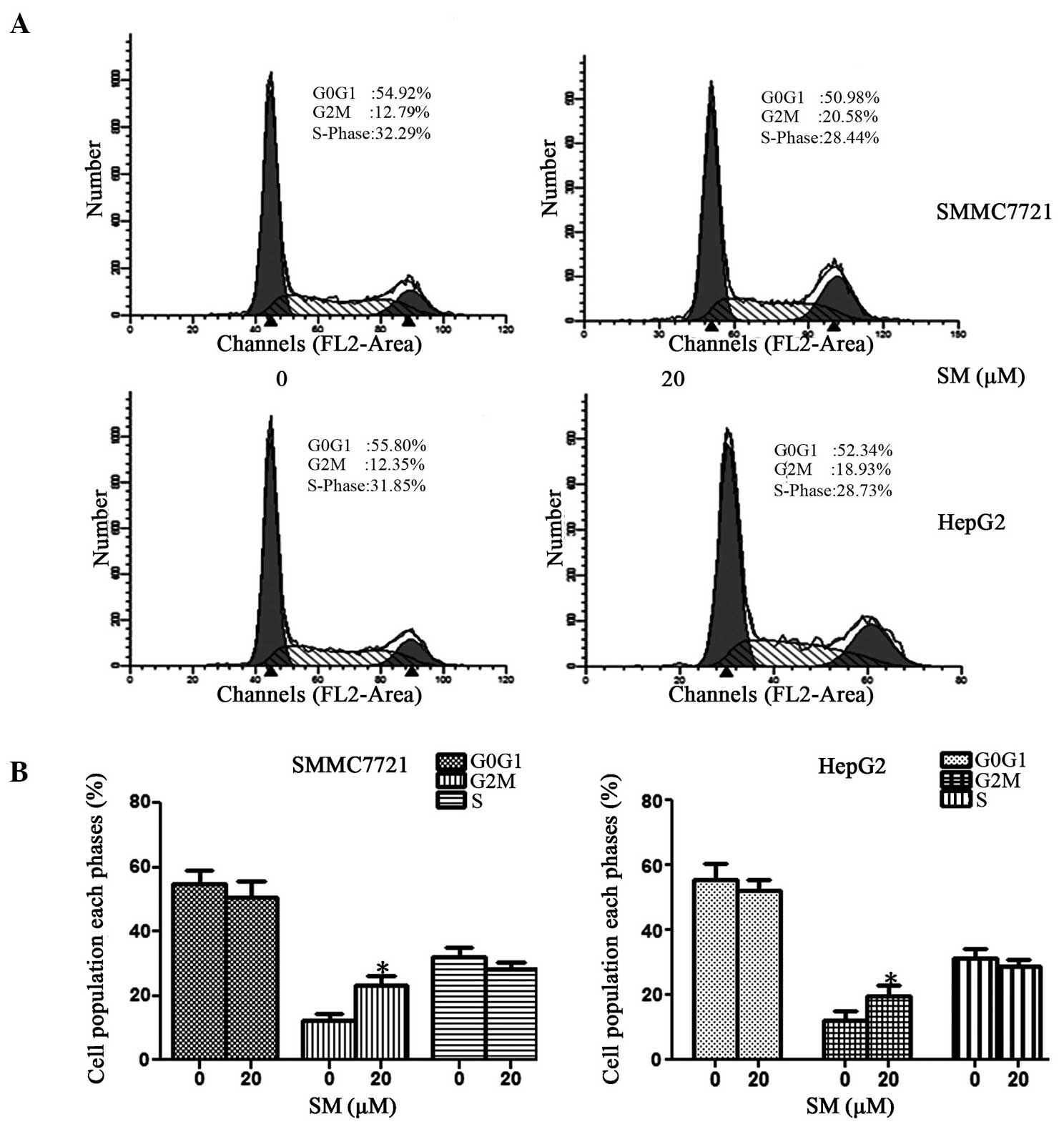
SM arrests cell cycle at
G2/M phase
The distribution of the cell cycle was detected
using a FACScan flow cytometer and is shown in Fig. 5A. In SMMC7721 cells treated with 20 µM
SM, the percentage of cells at the G0/G1
phase ranged between 54.92% (0 µM SM) and 50.98% (20 µM SM), while
at the S phase it varied between 32.29 and 28.44%. By contrast, the
percentage of cells at the G2/M phase increased from
12.79% (0 µM SM) to 20.57% (20 µM SM). The results revealed that SM
may induce G2/M phase arrest, with almost no effect on
G0/G1 and S phases, as shown in Fig. 5B. Similar results were also achieved
in the HepG2 cell line.
Expression of apoptosis- and
proliferation-associated proteins in SM-treated cells
The apoptosis-associated proteins were detected in
order to determine the signaling pathway responsible for the
apoptosis in SM-treated cells. Western blot analysis revealed that
the expression levels of Bax, caspase-3 and caspase-9 in SM-treated
cells were evidently upregulated when compared with the control
group. By contrast, the Bcl-2 protein expression levels were
significantly downregulated when compared with the control group.
The Bcl-2, Bax, caspase-3 and caspase-9 protein expression levels
were affected in a dose-dependent manner in the HepG2 cell lines
(Fig. 6A and B). In addition, the
expression levels of Bcl-2 were signficantly downregulated and the
expression levels of Bax, caspase-3 and caspase-9 were evidently
upregulated in the SM-treated SMMC7721 cells when compared with the
control group (Fig. 6A and B). The
results revealed that SM increased the activity of the
apoptosis-associated proteins. In addition, the
proliferation-associated proteins, Ki67 and pcna, were also
examined and the two cell lines demonstrated a decrease in the
protein expression levels in a dose-dependent manner (Fig. 6C and D). Therefore, SM inhibited the
cell proliferation and assisted the promotion of apoptosis.
Discussion
Chinese herbs consist of various active components
and have a particular function in the regulation of cell behavior,
with their effectiveness proven in a number of in vivo
studies and clinical trials (22,23).
Solamargine (SM), which is purified from Solanum incanum,
has been demonstrated to possess a number of pharmacological
properties and is one of the most promising agents for the
treatment of various cancer types. However, the molecular mechanism
through which SM affects liver cancer cells remains to be
clarified. The present study demonstrated that SM may inhibit cell
proliferation and promote apoptosis in human hepatoma cells.
In the current study, the results of the MTT assay
confirmed that SM effectively reduced the cell viability and
proliferation in a dose-dependent manner in the SMMC7721 and HepG2
cell lines. A colony formation assay further demonstrated that SM
inhibited the growth and cell clonogenicity of hepatoma cells in a
concentration-dependent manner. In addition, certain studies have
previously reported that SM inhibited cell proliferation in
different cancer cell lines in a dose-dependent manner (24–26), which
is consistent with the results of the present study.
Cell cycle is a vital biological process that
regulates the growth and metabolism of the human body (27–29). In
the present study, the effect of SM on the cell cycle was
investigated. SM was identified to affect the cell cycle
distribution and arrest the cell cycle at the G2/M
phase, with only a limited effect on the
G0/G1 and S phases. According to these
results, the SM-induced proliferation inhibition in hepatoma cells
was hypothesized to be associated with the induction of
G2/M arrest. Furthermore, the expression of
proliferation-associated proteins, Ki67 and pcna, was investigated.
Ki67 is a nuclear protein expressed during cellular proliferation,
particularly during the mitosis phase (30), while pcna has been identified as a
nuclear antigen observed in the S phase (31). Ki67 and pcna are effective markers
used to determine the growth capacity of a cell population
(32). In the present study, the
western blot analysis results demonstrated that Ki67 and pcna were
downregulated following exposure to SM, which indicated that the
hepatoma cell capacity of proliferation was inhibited following
treatment with SM, when compared with the untreated group.
Furthermore, the present study proposed that SM suppressed cell
growth, assisting the promotion of apoptosis.
The cell morphology was observed and DAPI staining
indicated that SMMC7721 and HepG2 cells treated with SM
demonstrated cellular features of apoptosis. Subsequently, the
percentages of early and late apoptosis were distinguished using an
Annexin V and PI method, which identified an increasing trend
(Fig. 4B). Upon treatment with 5 µM
SM, the percentage of total apoptosis increased, but the difference
was not statistically significant compared with the untreated group
(P>0.05). Therefore, this SM concentration was hypothesized to
be relative low and unable to trigger further cell apoptosis that
would be detected by Annexin V and PI. By contrast, at 10 and 20 µM
SM, the apoptosis rate was identified to be significantly different
compared with the control group (P<0.001). In order to clarify
the exact mechanism of apoptosis in hepatoma cells, the expression
levels of Bcl-2 family proteins and caspases were examined
(33,34). Bcl-2 family proteins play a crucial
role in the mitochondrial apoptosis pathway, and the Bax/Bcl-2
ratio reflects a close association to the extent of apoptosis
(35,36). According to the results of the present
study, SM downregulated the expression of Bcl-2 and upregulated the
expression of Bax, which indicated that Bcl-2 family proteins are
involved in the SM triggered apoptosis in SMMC7721 and HepG2 cells.
Furthermore, caspases are key factors in the execution of apoptosis
(37–39), with the present results demonstrating
that the expression levels of caspase-3 and caspase-9 were elevated
following SM treatment. These results revealed that the activation
of the Bcl-2/Bax and caspase signaling pathways is involved in the
SM-induced apoptosis of hepatoma cells.
In conclusion, SM plays a crucial role in triggering
hepatoma cell death through apoptosis. In addition, SM was
identified to effectively reduce the growth and induce cell cycle
arrest at the G2/M phase, which promoted apoptosis. The
present study aimed to provide a novel therapeutic agent for the
treatment of hepatocellular carcinoma. However, to the best of our
knowledge, numerous mechanisms are involved in SM-induced apoptosis
and further research is required to elucidate these.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Jiangsu Province (no. BK2011487), the
National Natural Science Foundation of China (no. 81170573) and the
Social Development Foundation of Zhenjiang City (no.
SZC201130128).
References
|
1
|
Di Bisceglie AM, Rustgi VK, Hoofnagle JH,
Dusheiko GM and Lotze MT: NIH conference, Hepatocellular carcinoma.
Ann Intern Med. 108:390–401. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen JG and Zhang SW: Liver cancer
epidemic in China: past, present and future. Semin Cancer Biol.
21:59–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poupon R, Fartoux L and Rosmorduc O:
Therapeutic advances in hepatocellular carcinoma. Bull Acad Natl
Med. 192:23–31. 2008.(In French). PubMed/NCBI
|
|
4
|
Bosch FX, Ribes J and Borràs J:
Epidemiology of primary liver cancer. Semin Liver Dis. 19:271–285.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forner A, Hessheimer AJ, Isabel Real M and
Bruix J: Treatment of hepatocellular carcinoma. Crit Rev Oncol
Hematol. 60:89–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jakobs TF, Hoffmann RT, Tatsch K, Trumm C
and Reiser MF: Therapy response of liver tumors after selective
internal radiation therapy. Radiologe. 48:839–849. 2008.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fadeel B and Orrenius S: Apoptosis: a
basic biological phenomenon with wide-ranging implications in human
disease. J Intern Med. 258:479–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jacobson MD, Weil M and Raff MC:
Programmed cell death in animal development. Cell. 88:347–354.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kerr JF: Shrinkage necrosis: a distinct
mode of cellular death. J. Pathol. 105:13–20. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Searle J, Kerr JF and Bishop CJ: Necrosis
and apoptosis: distinct modes of cell death with fundamentally
different significance. Pathol Annu. 17:229–259. 1982.PubMed/NCBI
|
|
12
|
Rich T, Watson CJ and Wyllie A: Apoptosis:
the germs of death. Nat Cell Biol. 1:E69–E71. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu W and Kavanagh JJ: Anticancer therapy
targeting the apoptotic pathway. Lancet Oncol. 4:721–729. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCulloch M, See C, Shu XJ, et al:
Astragalus-based Chinese herbs and platinum-based chemotherapy for
advanced non-small-cell lung cancer: meta-analysis of randomized
trials. J Clin Oncol. 24:419–430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mok TS, Yeo W, Johnson PJ, et al: A
double-blind placebo-controlled randomized study of Chinese herbal
medicine as complementary therapy for reduction of
chemotherapy-induced toxicity. Ann Oncol. 18:768–774. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lorey S, Porzel A and Ripperger H: Steroid
alkaloid glycosides from Solanum coccineum. Phytochemistry.
41:1633–1635. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Son YO, Kim J, Lim JC, Chung Y, Chung GH
and Lee JC: Ripe fruit of Solanum nigrum L. inhibits cell growth
and induces apoptosis in MCF-7 cells. Food Chem Toxicol.
41:1421–1428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Li Q, Feng T and Li K: Aqueous
extract of Solanum nigrum inhibit growth of cervical carcinoma
(U14) via modulating immune response of tumor bearing mice and
inducing apoptosis of tumor cells. Fitoterapia. 79:548–556. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu K, Kobayashi H, Dong A, Jing Y, Iwasaki
S and Yao X: Antineoplastic agents. III: Steroidal glycosides from
Solanum nigrum. Planta Med. 65:35–38. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shiu LY, Chang LC, Liang CH, Huang YS,
Sheu HM and Kuo KW: Solamargine induces apoptosis and sensitizes
breast cancer cells to cisplatin. Food Chem Toxicol. 45:2155–2164.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu T, Zhu W, Yang X, et al: Detection of
apoptosis based on the interaction between annexin V and
phosphatidylserine. Anal Chem. 81:2410–2413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lam W, Bussom S, Guan F, et al: The
four-herb Chinese medicine PHY906 reduces chemotherapy-induced
gastrointestinal toxicity. Sci Trans Med. 2:45ra592010.
|
|
23
|
Chen W, Lim CE, Kang HJ and Liu J: Chinese
herbal medicines for the treatment of type A H1N1 influenza: a
systematic review of randomized controlled trials. PLoS One.
6:e280932011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding X, Zhu FS, Li M and Gao SG: Induction
of apoptosis in human hepatoma SMMC-7721 cells by solamargine from
Solanum nigrum L. J Ethnopharmacol. 139:599–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun L, Zhao Y, Li X, Yuan H, Cheng A and
Lou H: A lysosomal-mitochondrial death pathway is induced by
solamargine in human K562 leukemia cells. Toxicol In Vitro.
24:1504–1511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Zhao Y, Wu WK, Liu S, Cui M and Lou
H: Solamargine induces apoptosis associated with p53
transcription-dependent and transcription-independent pathways in
human osteosarcoma U2OS cells. Life Sci. 88:314–321. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baggett D, Nakaya MA, McAuliffe M,
Yamaguchi TP and Lockett S: Whole cell segmentation in solid tissue
sections. Cytometry A. 67:137–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dormann D, Libotte T, Weijer CJ and
Bretschneider T: Simultaneous quantification of cell motility and
protein-membrane-association using active contours. Cell Motil
Cytoskeleton. 52:221–230. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu B, Cheng HD, Huang J, et al:
Probability density difference-based active contour for ultrasound
image segmentation. Pattern Recognit. 43:2028–2042. 2010.
View Article : Google Scholar
|
|
30
|
Beresford MJ, Wilson GD and Makris A:
Measuring proliferation in breast cancer:practicalities and
applications. Breast Cancer Res. 8:2162006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leonardi E, Girlando S, Serio G, et al:
PCNA and Ki67 expression in breast carcinoma: correlations with
clinical and biological variables. J Clin Pathol. 45:416–419. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Scholzen T and Gerdes J: The Ki-67
protein: from the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang N, Wang X, Huo Q, et al: The
oncogene metadherin modulates the apoptotic pathway based on the
tumor necrosis factor superfamily member TRAIL (Tumor Necrosis
Factor-related Apoptosis-inducing Ligand) in breast cancer. J Biol
Chem. 288:9396–9407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chan SL and Yu VC: Proteins of the bcl-2
family in apoptosis signalling: from mechanistic insights to
therapeutic opportunities. Clin Exp Pharmacol Physiol. 31:119–128.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reyes-Zurita FJ, Rufino-Palomares EE,
Lupiáñez JA and Cascante M: Maslinic acid, a natural triterpene
from Olea europaea L, induces apoptosis in HT29 human colon-cancer
cells via the mitochondrial apoptotic pathway. Cancer Lett.
273:44–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang N, Kong X, Yan S, Yuan C and Yang Q:
Huaier aqueous extract inhibits proliferation of breast cancer
cells by inducing apoptosis. Cancer Sci. 101:2375–2383. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Antonsson B: Bax and other pro-apoptotic
Bcl-2 family ‘killer-proteins’ and their victim the mitochondrion.
Cell Tissue Res. 306:347–361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Crompton M: Bax, Bid and the
permeabilization of the mitochondrial outer membrane in apoptosis.
Curr Opin Cell Biol. 12:414–419. 2000. View Article : Google Scholar : PubMed/NCBI
|