Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer mortality worldwide (1), and one of the most common types of
malignant tumors in China. Hepatic resection for HCC may benefit
the majority of patients with well-preserved liver function and no
evidence of portal hypertension (2).
Due to the biological characteristics of HCC, 20–90% of the
diagnosed cases of HCC present with vascular invasion, particularly
portal vein tumor thrombus (PVTT). Vascular invasion has been
previously identified as a major risk factor for recurrence and
mortality following resection of HCC (3). Although patients with HCC that exhibit
vascular invasion are associated with a poor prognosis, liver
resection provides improved long-term results than palliative
therapies or supportive care (4).
Peng et al (5) reported that
hepatic resection may result in excellent long-term survival in
certain patients. In those studies, the authors observed that the
survival rate of patients with HCC following hepatic resection was
14.1% at 3 years, while the survival rate of patients without
hepatic resection was 7.3%. Zhang et al (6) described numerous factors that may affect
the prognosis of patients affected by HCC with vascular invasion,
following hepatectomy. However, there are limited studies
describing the impact of hepatectomy on the long-term outcome of
these patients.
In the present study, a large cohort of patients
with HCC and PVTT treated with surgical resection, were analyzed
restrospectively. The purpose of the study was to identify the
clinical and histopathological variables that correlate with
recurrence and survival. A prognosis model for patients with HCC
presenting with vascular invasion was suggested, based on the risk
factors observed to be associated with prognosis.
Materials and methods
Ethics
The Ethics Committee of the Cancer Center of Sun
Yat-Sen University (Guangzhou, Guangdong, China) approved the
present study. Written informed consent was obtained from the
participants prior to enrollment in the study.
Patients
Data from a cohort of patients undergoing hepatic
resection for HCC between March 2001 and May 2008 was prospectively
collected and analyzed. Patients were admitted in the study
according to the following selection criteria: i) Patients were not
administered any therapy prior to the hepatectomy; ii) patients
presented with PVTT, including macroscopic and/or microscopic
vascular invasion; iii) patients had been subjected to hepatic
resection, followed by histological examination to confirm negative
surgical margins; and iv) patients did not present extrahepatic
metastases, severe liver dysfunction (Child-Pugh class C) or had
deceased prior to surgery. Those patients who had succumbed to
diseases other than HCC were excluded from the study.
Clinicopathological variables
Among the 952 patients recruited for the study, 245
presented with PVTT. From the corresponding chart reviews, the
following clinicopathological information was obtained for each
patient: Gender; age; ascites; cirrhosis; levels of alanine
aminotransferase (ALT) and α-fetoprotein (AFP); serum levels of
total bilirubin (TB), hepatitis B surface antigen (HBsAg),
hepatitis C virus antibody (HCvAb) and albumin (ALB); tumor
location, number and size; resection margin, macroscopic vascular
invasion; and Edmondson Steiner grade.
Survival time was measured in months. Tumor size was
assessed based on the largest dimension of the tumor specimen. The
histological grades (I–IV) were determined according to the highest
grade observed, using the criteria of Edmondson and Steiner
(7). The width of the surgical margin
was measured as the smallest distance from the tumor edge to the
resection line. Vascular invasion was considered to be macroscopic
if invasion of the vessel was visible macroscopically and by
computed tomography (CT) and magnetic resonance imaging (MRI)
examination. By contrast, microvascular invasion was defined by the
presence of clusters of cancer cells floating in the vascular space
line formed by endothelial cells, following histopathological
examination of the resected specimens by two independent
pathologists.
Patient follow-up
Measurement of AFP levels in serum, enhanced CT scan
of the chest and abdomen or abdominal enhanced MRI in addition to
chest CT/X-ray examination were performed in the first 1–2 months
post-surgery; then, every 2–3 months in the first year, and every
3–6 months thereafter. When tumor recurrence or metastasis was
suspected, further examinations, including MRI, hepatic angiography
and biopsies were performed. Telephone calls were also conducted
when necessary. CT and MRI scanning images were interpreted at the
start of the study by a group of four radiologists, who possessed
≥10 years of experience in image diagnosis, in the Department of
Radiology of the Cancer Center of Sun Yat-Sen University. The
follow-up for each patient data were regularly updated in the
database. The follow-up period was terminated on May 1, 2013 or on
the date of the patient's demise. By the end of the follow-up, 11
of the 245 patients were unable to be contacted or data was
incomplete. The median follow-up of the remaining 234 patients was
20 months (range, 3–99 months).
Statistical analyses
Overall survival (OS) was measured from the date of
hepatectomy until expiration, or to the most recent follow-up time.
Disease-free survival (DFS) was measured from the date of hepatic
resection to the date of the first diagnosis of tumor recurrence or
the most recent follow-up time. Survival curves were calculated
using the Kaplan-Meier method, and compared using the log-rank
test. Variables observed to be significant in univariate analysis
(P<0.05) were entered into a step-down Cox proportional hazard
regression analysis to identify risk factors that correlated with
DFS and OS. SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) was
used to perform the statistical analyses. P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinicopathological
characteristics
Table I summarizes the
clinicopathological characteristics of the patients with HCC
exhibiting PVTT. The median age was 47 years (range, 15–80 years,
mean = 46.96±12.29 years). At the end of the study, 63 patients had
survived, and 171 patients were deceased. The median survival time
was 18.0 months, and the average survival time was 31.39±28.83
months. Patients were randomly divided into the experimental or
validation groups (n=157 and 77, respectively), according to a ~2:1
proportion, and were stratified into low- and high-risk groups by
using AFP levels = 400 ng/ml as cut-off values.
 | Table I.Clinicopathological characteristics of
the 234 patients with HCC presenting PVTT. |
Table I.
Clinicopathological characteristics of
the 234 patients with HCC presenting PVTT.
| Variables | No. of patients
(%) |
|---|
| Gender |
|
| Male | 202 (86.3) |
|
Female | 32 (13.7) |
| HBsAg |
|
|
Positive | 217 (92.7) |
|
Negative | 17 (7.3) |
| HCVAb |
|
|
Positive | 2 (0.9) |
|
Negative | 232 (99.1) |
| Ascites |
|
| No | 188 (80.3) |
| Yes | 46 (19.7) |
| Cirrhosis |
|
| No | 38 (16.2) |
| Yes | 196 (83.8) |
| AFP (ng/ml) |
|
|
<400 | 93 (39.7) |
|
≥400 | 141 (60.3) |
| Tumor location |
|
| Left
lobe | 79 (33.8) |
| Right
lobe | 129 (55.1) |
| Left
and right lobes | 26 (11.1) |
| Tumor number |
|
|
Single | 158 (67.5) |
|
Multiple | 76 (32.5) |
| Tumor rupture |
|
| No | 181 (77.4) |
|
Yes | 53 (22.6) |
| Vascular
invasion |
|
|
Micro | 144 (61.5) |
|
Secondary branch | 43 (18.4) |
| Primary
branch | 47 (20.1) |
| Edmonson grade |
|
| I +
II | 123 (52.6) |
| III +
IV | 111 (47.4) |
| Adjuvant TACE |
|
| No | 187 (79.9) |
|
Yes | 47 (20.1) |
| Mean
age ± SD (years) | 46.9±10.32 |
| TB
(µmol/l) | 16.9±10.1 |
| ALT
(U/l) | 50.47±34.48 |
| ALB
(g/l) | 41.7±4.1 |
| Tumor
size (cm) | 9.12±4.60 |
|
Resection margin (cm) | 1.0±0.8 |
Univariate analysis of survival for
patients in the experimental group
Macrovascular invasion includes trunk, primary
and/or secondary branch vascular invasion. In the present study,
macrovascular invasion of the primary or secondary branches were
solely considered. Table II contains
the results of the survival analysis conducted on patients affected
by primary and secondary branches vascular invasion within the
experimental group. According to these results, no significant
differences were observed in DFS and OS analysis between the
patients with vascular invasion of the primary branches, compared
to those patients with vascular invasion of the secondary branches
within the experimental group (Table
II). Therefore, to facilitate the subsequent statistical and
clinical analyses, primary and secondary branches vascular invasion
were combined together into a single category termed macrovascular
invasion.
 | Table II.Survival analysis of patients with
vascular invasion of primary and secondary branches in the
experimental group. |
Table II.
Survival analysis of patients with
vascular invasion of primary and secondary branches in the
experimental group.
| Macrovascular | No. of | DFS rate (%)
|
| OS rate (%)
|
|---|
| invasion | patients | 1-year | 3-years | 5-years | P-value | 1-year | 3-years | 5-years | P-value |
|---|
| Secondary
branch | 28 | 28.6 | 10.7 | 10.7 | 0.067 | 53.6 | 25.0 | 25.0 | 0.098 |
| Primary branch | 38 | 15.8 | 5.3 | 5.3 |
| 39.5 | 15.8 | 5.3 |
|
Table III summarizes
the results of the univariate analysis for survival rate following
hepatic resection of patients with HCC affected by PVTT. For the
analysis, parameters including patient age, tumor size and levels
of ALT, TB and ALB, were divided into two subgroups depending on
the average values, whereas resection margin was divided into three
subgroups by using 0.5 and 1.0 cm as cut-off values.
 | Table III.Survival analysis of the patients in
the experimental group. |
Table III.
Survival analysis of the patients in
the experimental group.
|
| No. of | DFS rate (%)
|
| OS rate (%)
|
|---|
| Variables | patients | 1-year | 3-years | 5-years | P-value | 1-year | 3-years | 5-years | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
|
|
|
Male | 136 | 35.3 | 22.8 | 18.4 | 0.126 | 66.9 | 33.8 | 27.2 | 0.278 |
|
Female | 21 | 52.4 | 28.6 | 23.8 |
| 61.9 | 47.6 | 38.1 |
|
| Age (years) |
|
|
|
|
|
|
|
|
|
|
<46.9 | 79 | 39.2 | 24.1 | 21.5 | 0.754 | 64.6 | 35.4 | 27.8 | 0.801 |
|
≥46.9 | 78 | 37.2 | 24.4 | 17.9 |
| 57.0 | 35.9 | 29.5 |
|
| Ascites |
|
|
|
|
|
|
|
|
|
| No | 125 | 39.2 | 23.2 | 19.2 | 0.946 | 64.0 | 34.4 | 27.2 | 0.973 |
|
Yes | 32 | 34.4 | 28.1 | 21.9 |
| 50.0 | 40.6 | 34.4 |
|
| Cirrhosis |
|
|
|
|
|
|
|
|
|
| No | 24 | 29.2 | 20.8 | 16.7 | 0.498 | 54.2 | 33.3 | 25.0 | 0.580 |
|
Yes | 133 | 39.8 | 24.8 | 20.3 |
| 66.7 | 36.1 | 29.3 |
|
| ALT (U/l) |
|
|
|
|
|
|
|
|
|
|
<50.4 | 99 | 42.4 | 26.3 | 20.2 | 0.282 | 60.6 | 36.4 | 30.3 | 0.491 |
|
≥50.4 | 58 | 31.0 | 20.7 | 19.0 |
| 62.1 | 34.5 | 25.9 |
|
| TB (µmol/l) |
|
|
|
|
|
|
|
|
|
|
<16.9 | 87 | 40.2 | 24.1 | 19.5 | 0.464 | 67.8 | 39.1 | 28.7 | 0.361 |
|
≥16.9 | 70 | 35.7 | 24.3 | 20.0 |
| 52.9 | 31.4 | 28.6 |
|
| HBsAg |
|
|
|
|
|
|
|
|
|
|
Negative | 11 | 45.5 | 27.3 | 18.2 | 0.844 | 45.5 | 36.4 | 18.2 | 0.545 |
|
Positive | 146 | 37.7 | 24.0 | 19.9 |
| 62.3 | 35.6 | 29.5 |
|
| HCVAb |
|
|
|
|
|
|
|
|
|
|
Negative | 155 | 37.4 | 23.2 | 19.4 | 0.316 | 60.6 | 34.8 | 28.4 | 0.360 |
|
Positive | 2 | 100 | 100 | 50 |
| 100 | 100 | 50 |
|
|
| ALB (g/l) |
|
|
|
|
|
|
|
|
|
|
<41.7 | 74 | 37.8 | 27.0 | 23.0 | 0.752 | 56.8 | 37.8 | 32.4 | 0.750 |
|
≥41.7 | 83 | 38.6 | 21.7 | 16.9 |
| 65.1 | 33.3 | 25.0 |
|
| Tumor location |
|
|
|
|
|
|
|
|
|
| Left
lobe | 49 | 36.7 | 26.5 | 20.7 | 0.822 | 63.3 | 38.8 | 34.7 | 0.697 |
| Right
lobe | 89 | 38.2 | 22.5 | 20.2 |
| 61.8 | 33.7 | 27.0 |
|
| Left
and right lobes | 19 | 42.1 | 26.3 | 21.1 |
| 52.6 | 36.8 | 21.1 |
|
| Edmonson grade |
|
|
|
|
|
|
|
|
|
| I +
II | 80 | 41.3 | 27.5 | 26.3 | 0.284 | 68.8 | 41.3 | 30.0 | 0.260 |
| III +
IV | 77 | 35.1 | 20.8 | 19.5 |
| 53.2 | 29.9 | 27.3 |
|
| Adjuvant TACE |
| No | 126 | 42.1 | 27.0 | 21.4 | 0.161 | 61.9 | 38.1 | 29.4 | 0.635 |
|
Yes | 31 | 22.6 | 12.9 | 12.9 |
| 58.1 | 25.8 | 25.8 |
|
| Resection margin
(cm) |
|
|
|
|
|
|
|
|
|
|
≤0.5 | 28 | 35.7 | 25.0 | 14.3 | 0.123 | 60.7 | 32.1 | 25.0 | 0.182 |
|
0.5–1.0 | 52 | 28.8 | 17.3 | 13.5 |
| 57.7 | 34.6 | 19.2 |
|
|
≥1.0 | 77 | 45.5 | 28.6 | 24.7 |
| 63.6 | 37.7 | 36.4 |
|
| AFP (ng/ml) |
|
|
|
|
|
|
|
|
|
|
<400 | 64 | 48.4 | 28.1 | 20.3 | 0.034 | 75.0 | 46.9 | 35.9 | 0.014 |
|
≥400 | 93 | 31.2 | 21.5 | 19.4 |
| 51.6 | 28.0 | 23.7 |
|
| Tumor number |
|
|
|
|
|
|
|
|
|
|
Single | 104 | 45.2 | 27.9 | 23.1 | 0.001 | 68.3 | 39.4 | 32.7 | 0.010 |
|
Multiple | 53 | 24.5 | 17.0 | 11.3 |
| 47.2 | 28.3 | 20.8 |
|
| Tumor size
(cm) |
|
|
|
|
|
|
|
|
|
|
<9.1 | 94 | 45.7 | 26.6 | 20.2 | 0.026 | 68.1 | 43.6 | 33.0 | 0.037 |
|
≥9.1 | 63 | 27.0 | 20.6 | 19.0 |
| 47.6 | 23.8 | 22.2 |
|
| Tumor rupture |
|
|
|
|
|
|
|
|
|
|
No | 120 | 44.2 | 28.3 | 22.5 | 0.001 | 67.5 | 42.5 | 34.2 | <0.001 |
|
Yes | 37 | 18.9 | 10.8 | 10.8 |
| 40.5 | 13.5 | 10.8 |
|
| Vascular
invasion |
|
|
|
|
|
|
|
|
|
|
Microscopic | 91 | 50.5 | 36.3 | 28.6 | <0.001 | 72.5 | 47.0 | 38.5 | <0.001 |
|
Macroscopic | 66 | 19.7 | 6.1 | 6.1 |
| 43.9 | 18.2 | 15.2 |
|
According to the results of the univariate analysis,
levels of AFP ≥400 ng/ml, presence of multiple tumors, tumor size
≥9.1 cm, tumor rupture and macrovascular invasion were identified
as significant prognostic factors (P<0.05) associated with poor
prognosis of DFS and OS.
Multivariate analysis of survival for
patients in the experimental group
The parameters that were demonstrated to be
significant in univariate analysis were entered in a Cox
proportional hazards model for multivariate analysis, which
identified the presence of multiple tumors, tumor rupture and
macrovascular invasion as independent risk factors of recurrence
and survival (Tables IV and V).
 | Table IV.Multivariate analysis of DFS for the
experimental group. |
Table IV.
Multivariate analysis of DFS for the
experimental group.
| Variables | B | SE | Hazard ratio | P-value |
|---|
| AFP | 0.249 | 0.206 | 1.283 | 0.227 |
| Tumor number | 0.336 | 0.102 | 1.399 | 0.001 |
| Tumor size | 0.133 | 0.211 | 1.143 | 0.527 |
| Tumor rupture | 0.489 | 0.221 | 1.631 | 0.027 |
| Macrovascular
invasion | 0.261 | 0.119 | 1.299 | 0.027 |
 | Table V.Multivariate analysis of OS for the
experimental group. |
Table V.
Multivariate analysis of OS for the
experimental group.
| Variables | B | SE | Hazard ratio | P-value |
|---|
| AFP | 0.385 | 0.207 | 1.470 | 0.063 |
| Tumor number | 0.236 | 0.1045 | 1.266 | 0.024 |
| Tumor size | 0.033 | 0.2105 | 1.034 | 0.877 |
| Tumor rupture | 0.646 | 0.224 | 1.907 | 0.004 |
| Macrovascular
invasion | 0.246 | 0.120 | 1.279 | 0.040 |
Establishment and validation of a
prognostic model
According to the results of the multivariate
analysis, the presence of multiple tumors, tumor rupture and
macrovascular invasion appeared to be independent risk factors of
recurrence and survival for patients with HCC and PVTT who had been
subjected to hepatic resection. Thus, group standards were
established based on these three independent risk factors. Patients
were divided into low-, medium- and high-risk groups (designated as
group 1, 2 and 3, respectively), according to the number of risk
factors that the patients presented. Thus, patients in the low-risk
group presented a single tumor, no tumor rupture and no
macrovascular invasion. Patients belonging to the medium-risk group
exhibited one of the following characteristics: Multiple tumors,
tumor rupture or macrovascular invasion, whereas patients
classified as high-risk presented two or three of these
characteristics.
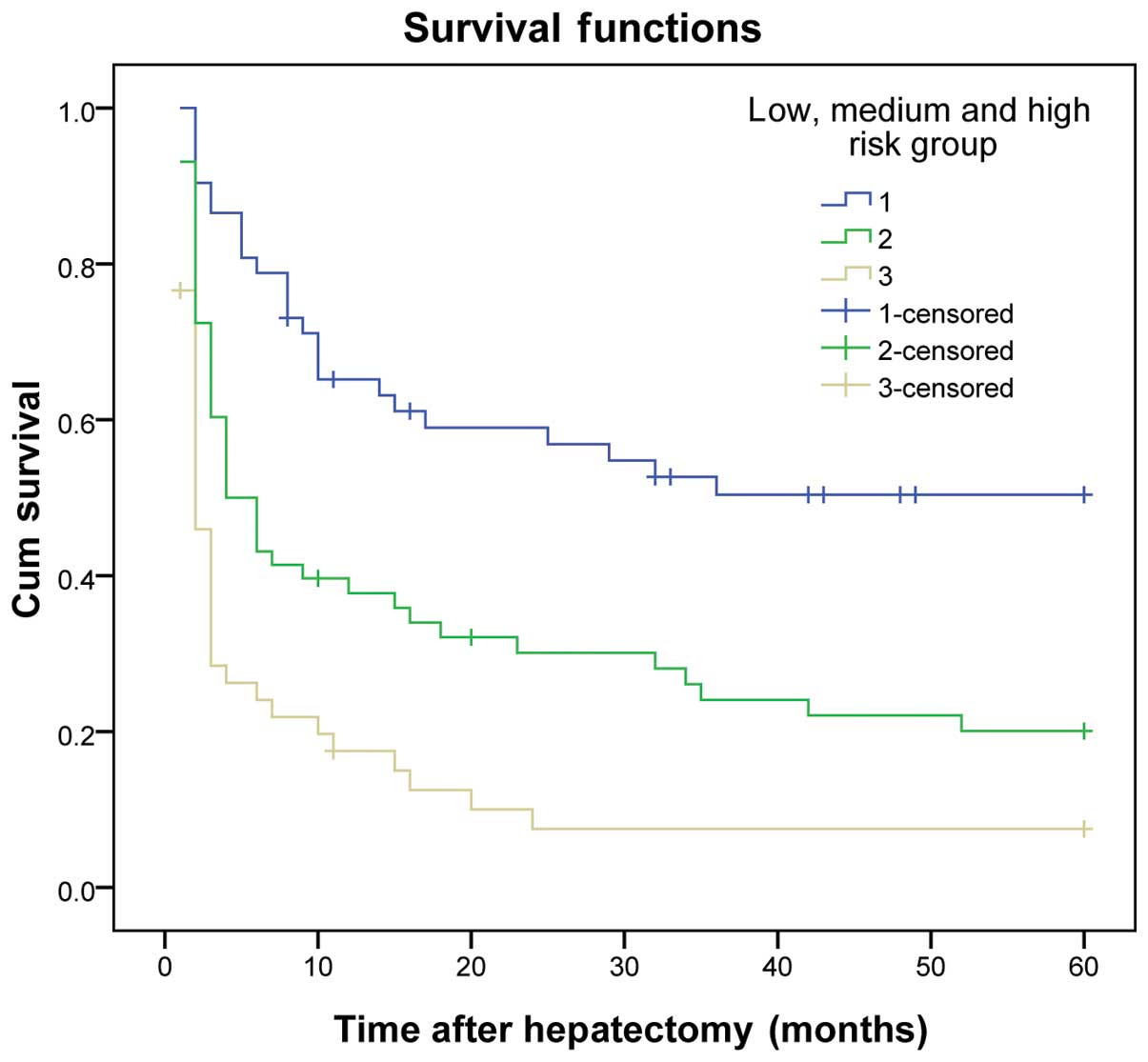
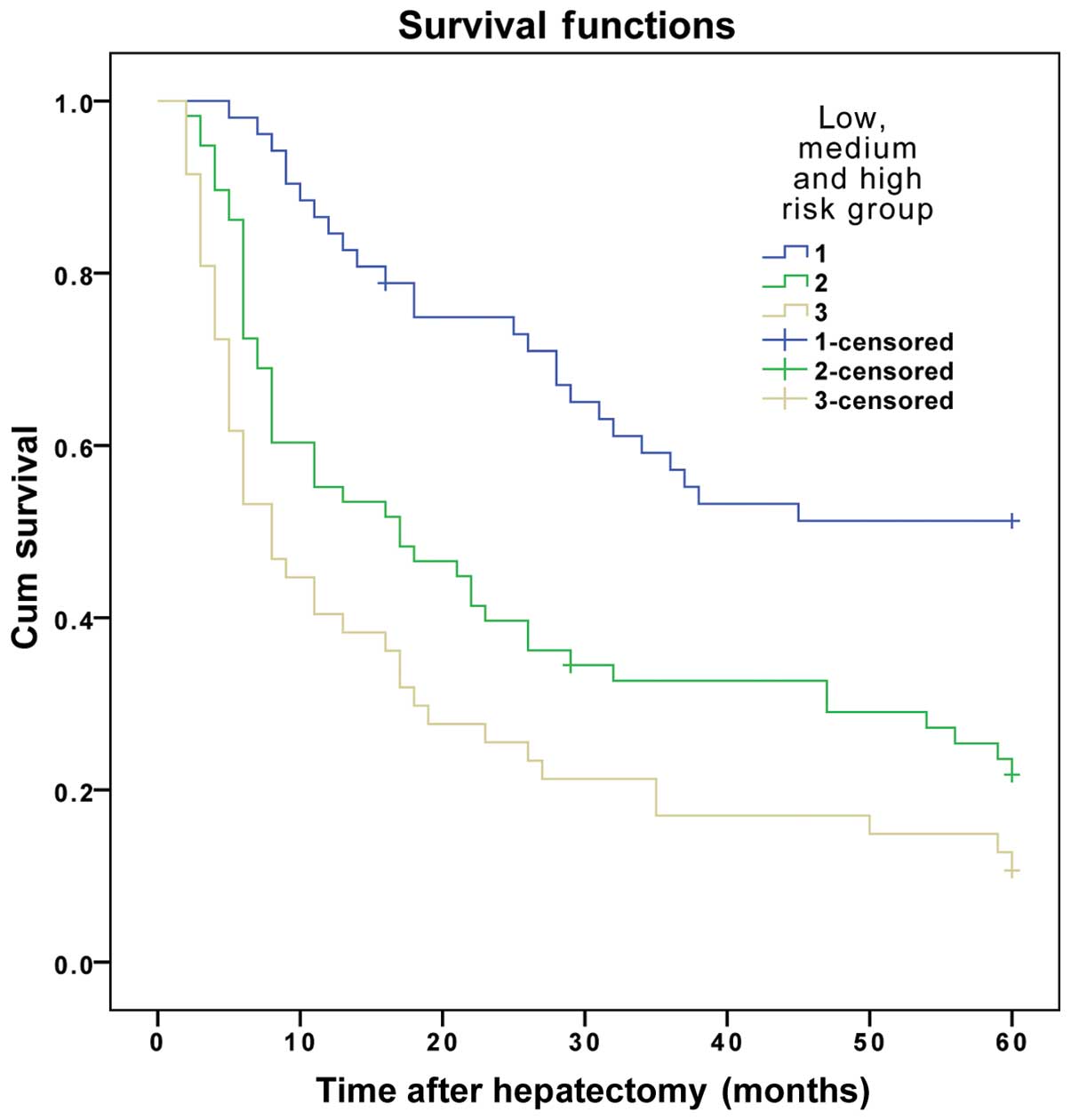
The results demonstrated that the DFS and OS rates
reduced gradually as the number of risk factors increased. The DFS
and OS rates of patients in the three subgroups within the
experimental group were significantly different (P<0.01)
(Table VI and Figs. 1 and 2).
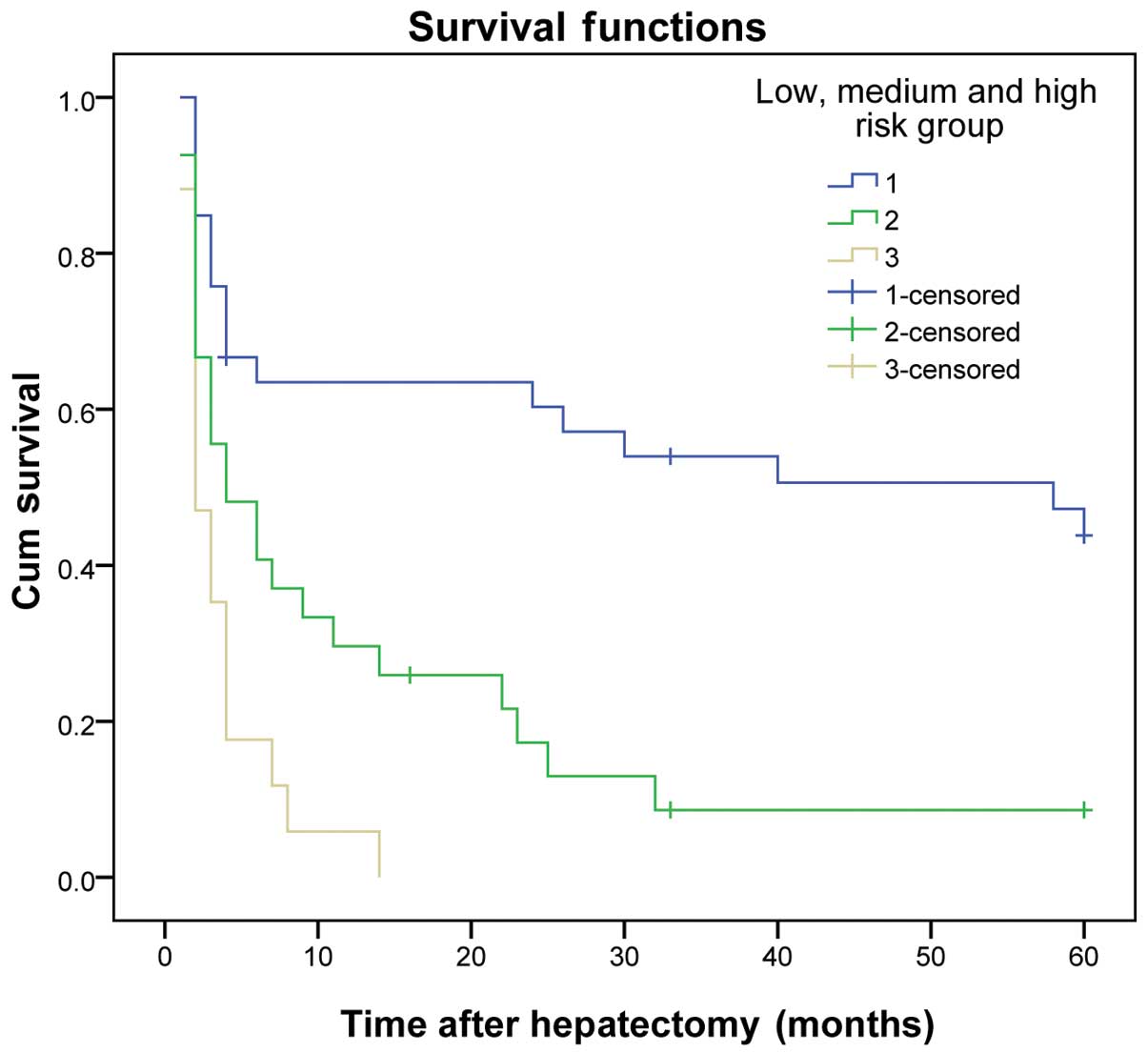
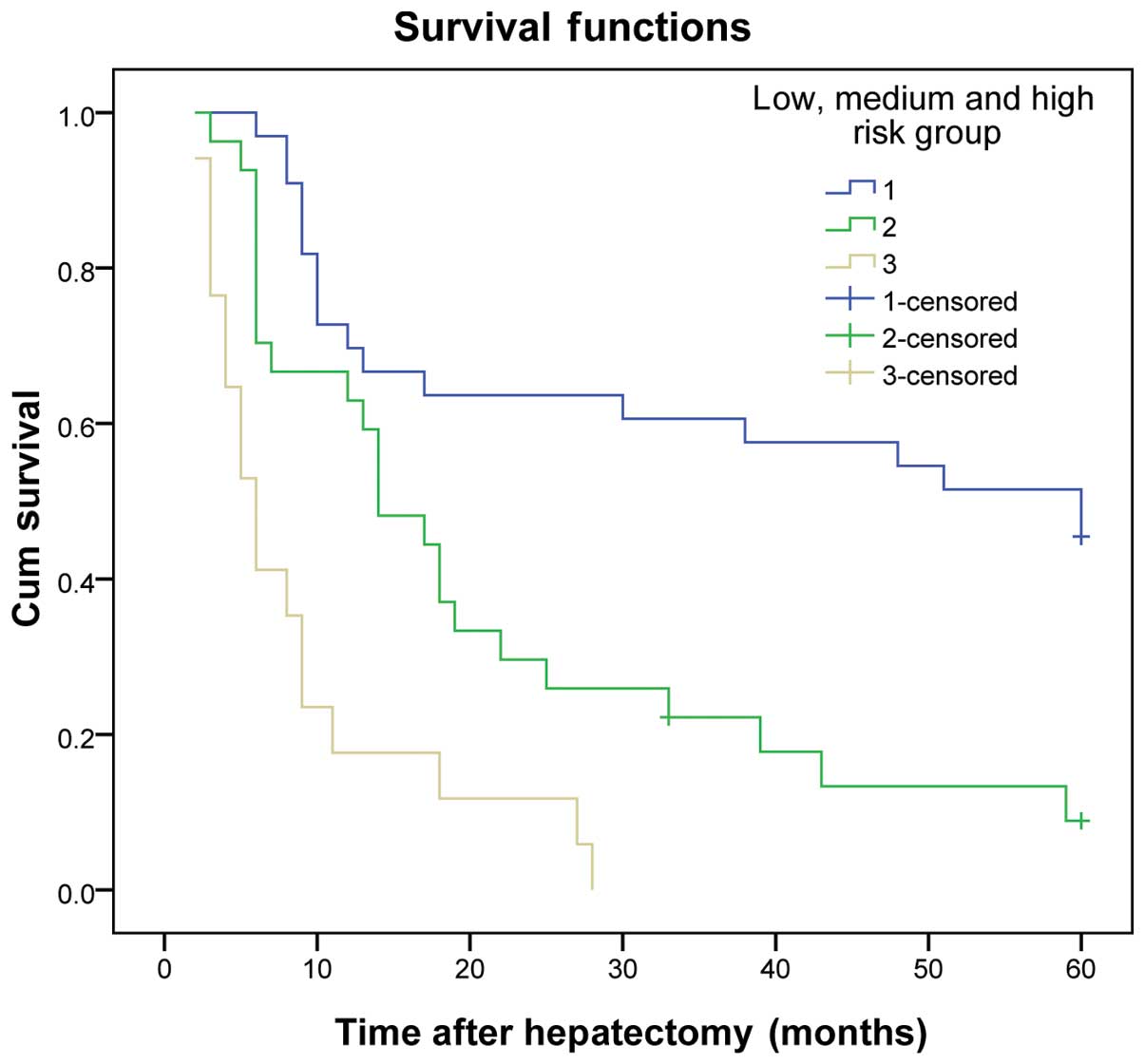
The same results were obtained for the validation group (P<0.01)
(Table VII and Figs. 3 and 4).
 | Table VI.Survival analysis of patients in the
low, medium and high-risk groups within the experimental group. |
Table VI.
Survival analysis of patients in the
low, medium and high-risk groups within the experimental group.
|
| No. of | DFS rate (%)
|
| OS rate (%)
|
|
|---|
| Groups | patients | 1-year | 3-years | 5-years | P-value | 1-year | 3-years | 5-years | P-value |
|---|
| 1 | 52 | 61.5 | 44.2 | 34.6 | ﹤0.001 | 86.5 | 57.7 | 50.0 | ﹤0.001 |
| 2 | 58 | 34.5 | 20.7 | 17.2 |
| 55.2 | 31.0 | 22.4 |
|
| 3 | 47 | 14.9 | 6.4 | 6.4 |
| 40.4 | 17.0 | 12.8 |
|
 | Table VII.Survival analysis of patients in the
low-, medium- and high-risk groups within the validation group. |
Table VII.
Survival analysis of patients in the
low-, medium- and high-risk groups within the validation group.
|
| No. of | DFS rate (%)
|
| OS rate (%)
|
|
|---|
| Groups | patients | 1-year | 3-years | 5-years | P-value | 1-year | 3-years | 5-years | P-value |
|---|
| 1 | 33 | 60.6 | 48.5 | 42.4 | ﹤0.001 | 72.7 | 60.6 | 51.5 | ﹤0.001 |
| 2 | 27 | 34.8 | 3.7 | 3.7 |
| 63.0 | 18.5 | 7.4 |
|
| 3 | 17 | 5.9 | 0.0 | 0.0 |
| 17.6 | 0.0 | 0.0 |
|
Discussion
Hepatic resection is an accepted first-line
treatment for patients with HCC that exhibit well-preserved liver
function and no evidence of portal hypertension, which is possibly
curative and may result in excellent long-term survival (8,9). However,
patients affected by HCC that have been subjected to hepatic
resection have been observed to experience a high rate of tumor
recurrence, with the majority of recurrence and metastasis
following resection of HCC occurring within the remaining liver
(10). This is primarily due to
intrahepatic dissemination of the tumor via the intrahepatic
vascular system (11). Therefore,
vascular invasion is considered to be an important risk factor for
tumor recurrence and patient survival following resection of HCC
(12). For patients with vascular
invasion, the value of hepatic resection is controversial (13). In clinical practice, certain patients
presenting with vascular invasion may achieve satisfactory survival
following surgical comprehensive treatment (5). The purpose of the present study was to
investigate the prognosis factors of patients with HCC and vascular
invasion, in order to establish a classification criteria based on
these factors that would aid the identification of patients
suitable for surgical treatment.
In the present study, the number of tumors was
demonstrated to be an independent risk factor of recurrence and
survival. Due to its invasive nature, HCC tends to form multiple
nodules that lead to postoperative recurrence (14). Zhong et al (15) suggested that in patients with HCC and
preserved liver function, the presence of multinodular tumors was
not a contraindication for hepatic resection. However, previous
studies have suggested that patients with multiple tumors have a
higher probability of worse survival prognosis than those with a
single tumor, due to the significant risk of recurrence following
hepatic resection for HCC (16). This
hypothesis is supported by the results of the studies by Wang et
al (17), who observed that the
prognosis of patients with multinodular HCC was poor, compared to
those with single-nodular HCC.
The present study revealed that the incidence of
spontaneous tumor rupture in patients with HCC affected by PVTT was
higher than in patients with HCC overall (22.6 vs. 12.1%,
respectively). Tumor spontaneous rupture was observed to be an
independent risk factor of recurrence and survival for patients
with HCC affected by PVTT, and patients with tumor rupture
exhibited a higher probability of poor prognosis than those without
tumor rupture. The mechanism of spontaneous tumor rupture in HCC
has been proposed to be associated with rapid tumor growth that may
lead to tumor necrosis; tumor invasion of the venous, resulting in
venous occlusion; or increased macrophages and neutrophils-mediated
vascular endothelial cell permeability, which may damage the walls
of the blood vessels (18).
Previous studies have confirmed that macrovascular
invasion was associated with the malignant degree of HCC, and that
the degree of vascular invasion affected the prognosis of patients
with HCC (19). These studies also
suggested that DFS and OS rates in patients with HCC presenting
macrovascular invasion were lower than those in patients without
macrovascular invasion (20).
Previous studies have suggested that the levels of
AFP were associated with survival following resection (21,22). By
contrast, the levels of AFP were not observed to be a prognostic
predictor for survival in other studies (23). In the present study, the levels of AFP
were not able to predict the survival of patients with HCC and
PVTT, following hepatic resection. The fact that the levels of AFP
were observed to be important prognostic factors for survival but
did not directly correlate with tumor size or number, suggests that
AFP may display other properties within the complex biology of HCC
(20).
Previous studies indicated that the extent of liver
resection margin was an independent prognostic factor for OS
(24,25). However, the present study demonstrated
that the extent of liver resection margin did not influence the
postoperative OS, in agreement with previous reports (26,27). This
apparent discrepancy may be explained by the fact that HCC has a
propensity to disseminate via vascular invasion. Thus, intrahepatic
metastasis is likely to be present beyond 1 cm in the majority of
patients prior to operation. The results derived from the present
study suggest that a resection margin ≥1 cm had little beneficial
effect on prolonging the OS rate for patients with HCC and PVTT who
underwent hepatic resection.
Cheng et al (28) proposed that the type of tumor thrombi
may aid to determine the treatment plan and assess the prognosis of
patients with HCC presenting PVTT. In their studies, the authors
observed that there was no significant difference between the DFS
and OS rates of patients with HCC affected by primary branch of
PVTT, compared to those exhibiting secondary branch of PVTT.
However, the results from the present study differ from other
reports in the literature (29),
possibly due to the relatively small size of the sample. Thus,
further studies are required to confirm these preliminary
observations.
Chung et al (30) compared the outcomes of patients
treated with transarterial chemoembolization (TACE) with those of
patients receiving supportive care according to their Child-Pugh
class. The authors demonstrated that TACE may be performed safely
and improve the OS of patients with HCC. However, the resuls from
the present study indicate that TACE was not capable of benefiting
patients with HCC and PVTT, in regards to their DFS and OS rates.
This may be associated with the small number of cases used in the
study, and the fact that the majority of patients were not treated
with TACE, but only those in poor condition.
Several staging methods or liver staging systems for
HCC have been proposed, including those described by Okuda, Cancer
of the Liver Italian Program, Barcelona Clinic Liver Cancer, Model
for End-Stage Liver Disease, Chinese University Prognostic Index,
Japanese Integrated System, Tumor Node Metastasis, Groupe d'Etude
de Traitement du Carcinoma Hepatocellulaire and Liver Cancer Study
Group of Japan (31). However, the
predictive performance of the existing prognostic systems is
non-ideal, due to their inherent limitations, the non-universal
reproducibility and transportability of the results in different
populations and other key factors that must be also considered
(31). Previous studies (32,33) have
reported that vascular invasion is a useful parameter in the
grading and staging system of patients with HCC. However, this was
not observed in the present study.
In the present study, patients with HCC presenting
PVTT were divided into low-, medium- or high-risk groups, according
to the number of risk factors exhibited by the patients. The DFS
and OS rates for these groups were observed to be significantly
different. These results suggest that an increase in the number of
risk factors leads to worse DFS and OS rates. Thus, the prognostic
model proposed in the present study, based on the above risk
factors, may be used to guide the treatment, predict the prognosis,
enhance the therapeutic efficiency and improve the survival rates
of patients with HCC affected by PVTT.
In conclusion, the results of the present study have
demonstrated that parameters such as the number of tumors, tumor
rupture and macrovascular invasion may affect the postoperative
outcomes of patients with HCC and PVTT following hepatectomy. In
addition, the prognostic model established, based on the number of
risk factors exhibited by the patient, may be used to guide the
treatment and predict the prognosis of these patients.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81172037/H1606),
Guangzhou Municipal Science and Technology Project of China (grant
no. 2012J4100078), and Guangdong Province Science and Technology
Project of China (grant no. 2013B021800159).
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Faber W, Sharafi S, Stockmann M, Denecke
T, Sinn B, Puhl G, Bahra M, Malinowski MB, Neuhaus P and Seehofer
D: Long-term results of liver resection for hepatocellular
carcinoma in noncirrhotic liver. Surgery. 153:510–517. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ikai I, Arii S, Kojiro M, Ichida T,
Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K
and Yamaoka Y: Reevaluation of prognostic factors for survival
after liver resection in patients with hepatocellular carcinoma in
a Japanese nationwide survey. Cancer. 101:796–802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guglielmi A, Ruzzenente A, Conci S,
Valdegamberi A, Vitali M, Bertuzzo F, De Angelis M, Mantovani G and
Iacono C: Hepatocellular carcinoma: Surgical perspectives beyond
the barcelona clinic liver cancer recommendations. World J
Gastroenterol. 20:7525–7533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS
and Lau WY: Hepatic resection versus transcatheter arterial
chemoembolization for the treatment of hepatocellular carcinoma
with portal vein tumor thrombus. Cancer. 118:4725–4736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang T, Huang JW, Bai YN, Wu H and Zeng
Y: Recurrence and survivals following hepatic resection for
hepatocellular carcinoma with major portal/hepatic vein tumor
thrombus. Hepatol Res. 44:761–768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vauthey JN, Dixon E, Abdalla EK, et al:
Pretreatment assessment of hepatocellular carcinoma: Expert
consensus statement. HPB (Oxford). 12:289–299. 2010.PubMed/NCBI
|
|
9
|
Buck AK, Herrmann K, Eckel F and Beer AJ:
Pancreatic and hepatobiliary cancers. Methods Mol Biol.
727:243–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Regimbeau JM, Abdalla EK, Vauthey JN,
Lauwers GY, Durand F, Nagorney DM, Ikai I, Yamaoka Y and Belghiti
J: Risk factors for early death due to recurrence after liver
resection for hepatocellular carcinoma: results of a multicenter
study. J Surg Oncol. 85:36–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masuda T, Beppu T, Ishiko T, et al:
Intrahepatic dissemination of hepatocellular carcinoma after local
ablation therapy. J Hepatobiliary Pancreat Surg. 15:589–595. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan T, Zhao JJ, Bi XY, et al: Prognosis of
hepatocellular carcinoma: A study of 832 cases. Zhonghua Zhong Liu
Za Zhi. 35:54–58. 2013.(In Chinese). PubMed/NCBI
|
|
13
|
Duseja A: Staging of hepatocellular
carcinoma. J Clin Exp Hepatol. 4:S74–S79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poon RT, Fan ST, Lo CM, Liu CL and Wong J:
Difference in tumor invasiveness in cirrhotic patients with
hepatocellular carcinoma fulfilling the Milan criteria treated by
resection and transplantation: Impact on long-term survival. Ann
Surg. 245:51–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L,
Ye XP, Peng T, Xie GS and Li LQ: Hepatic resection associated with
good survival for selected patients with intermediate and
advanced-stage hepatocellular carcinoma. Ann Surg. 260:329–340.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishizawa T, Hasegawa K, Aoki T, Takahashi
M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N and Makuuchi M:
Neither multiple tumors nor portal hypertension are surgical
contraindications for hepatocellular carcinoma. Gastroenterology.
134:1908–1916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang BW, Mok KT, Liu SI, Chou NH, Tsai CC,
Chen IS, Yeh MH and Chen YC: Is hepatectomy beneficial in the
treatment of multinodular hepatocellular carcinoma? J Formos Med
Assoc. 107:616–626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai ECH and Lau WY: Spontaneous rupture of
hepatocellular carcinoma: A systematic review. Arch Surg.
141:191–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matono R, Yoshiya S, Motomura T, Toshima
T, Kayashima H, Masuda T, Yoshizumi T, Taketomi A, Shirabe K and
Maehara Y: Factors linked to longterm survival of patients with
hepatocellular carcinoma accompanied by tumour thrombus in the
major portal vein after surgical resection. HPB (Oxford).
14:247–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sorrentino P, Tarantino L, D'Angelo S,
Terracciano L, Ferbo U, Bracigliano A, Panico L, De Chiara G,
Lepore M, De Stefano N, et al: Validation of an extension of the
international non-invasive criteria for the diagnosis of
hepatocellular carcinoma to the characterization of macroscopic
portal vein thrombosis. J Gastroenterol Hepatol. 26:669–677. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou L, Rui JA, Wang SB, Chen SG and Qu Q:
Prognostic factors of solitary large hepatocellular carcinoma: The
importance of differentiation grade. Eur J Surg Oncol. 37:521–525.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santambrogio R, Opocher E, Costa M,
Barabino M, Zuin M, Bertolini E, De Filippi F and Bruno S: Hepatic
resection for ‘BCLC stage A’ hepatocellular carcinoma. The
prognostic role of α-fetoprotein. Ann Surg Oncol. 19:426–434. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HS, Park JW, Jang JS, Kim HJ, Shin WG,
Kim KH, Lee JH, Kim HY and Jang MK: Prognostic values of
alpha-fetoprotein and protein induced by vitamin K absence or
antagonist-II in hepatitis B virus-related hepatocellular
carcinoma: A prospective study. J Clin Gastroenterol. 43:482–488.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS,
Zhang CQ, Lau WY and Li JQ: Partial hepatectomy with wide versus
narrow resection margin for solitary hepatocellular carcinoma: A
prospective randomized trial. Ann Surg. 245:36–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Xu LB, Liu C, Pang HW, Chen YJ and
Ou QJ: Prognostic factors and outcome of 438 Chinese patients with
hepatocellular carcinoma underwent partial hepatectomy in a single
center. World J Surg. 34:2434–2441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Miao R, Yang H, Lu X, Zhao Y, Mao
Y, Zhong S, Huang J, Sang X and Zhao H: Prognostic factors after
liver resection for hepatocellular carcinoma: A single-center
experience from China. Am J Surg. 203:741–750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giuliante F, Ardito F, Pinna AD, Sarno G,
Giulini SM, Ercolani G, Portolani N, Torzilli G, Donadon M,
Aldrighetti L, et al: Liver resection for hepatocellular carcinoma
≤3 cm: Results of an Italian multicenter study on 588 patients. J
Am Coll Surg. 215:244–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng SQ, Wu MC, Chen H, Shen F, Yang JH,
Cong WM, Wang PJ and Zhao YX: Significance of typing of tumor
thrombi in determination of treatment and assessment of prognosis
of hepatocellular carcinoma with tumor thrombi in the portal vein.
Zhonghua Yi Xue Za Zhi. 84:3–5. 2004.(In Chinese). PubMed/NCBI
|
|
29
|
Shi J, Lai EC, Li N, Guo WX, Xue J, Lau
WY, Wu MC and Cheng SQ: A new classification for hepatocellular
carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat
Sci. 18:74–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chung GE, Lee JH, Kim HY, Hwang SY, Kim
JS, Chung JW, Yoon JH, Lee HS and Kim YJ: Transarterial
chemoembolization can be safely performed in patients with
hepatocellular carcinoma invading the main portal vein and may
improve the overall survival. Radiology. 258:627–634. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maida M, Orlando E, Cammà C and Cabibbo G:
Staging systems of hepatocellular carcinoma: A review of
literature. World J Gastroenterol. 20:4141–4150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kinoshita A, Onoda H, Imai N, Iwaku A,
Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H, Matsushima M, et
al: The Glasgow Prognostic Score, an inflammation based prognostic
score, predicts survival in patients with hepatocellular carcinoma.
BMC Cancer. 13:522013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu C, Xiao GQ, Yan LN, Li B, Jiang L, Wen
TF, Wang WT, Xu MQ and Yang JY: Value of α-fetoprotein in
association with clinicopathological features of hepatocellular
carcinoma. World J Gastroenterol. 19:1811–1819. 2013. View Article : Google Scholar : PubMed/NCBI
|